Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.33 no.4 Bogotá oct./dic. 2018
Letter to Editor
Clinical pathway for the treatment of chronic Hepatitis C, a look and a complement from the perspective of pharmaceutical services
1Grupo Promoción y Prevención Farmacéutica, Universidad de Antioquia. Medellín, Colombia
2Fundación IFARMA. Bogotá D.C., Colombia
Dear editor:
When the Statutory Law on Health (Law 1751 of 2015) went into effect, 1 health became enshrined as an autonomous fundamental right. Its provision has been guaranteed and regulated, and protective mechanisms have been established. In addition, a change of focus was generated in health care policy that has defined Primary Health Care as the basis of Comprehensive Health Care Policy (PAIS - Política de Atención Integral en Salud) within the framework of the General System of Social Security in Health (SGSSS - Sistema General de Seguridad Social en Salud). PAIS implementation guidelines have been defined in the Comprehensive Health Care Model and in the Comprehensive Health Care Pathways which aim to articulate various actors in the SGSSS including the Benefit Plan Insurance Entities, the Health Promotion Institutions, and the secretariats of health in order to guarantee timely and quality access to the health services required by citizens. 2
In accordance with the guidelines established in the PAIS in mid-2017, the Ministry of Health and Social Protection made its first centralized purchase of direct-acting antivirals for the treatment of hepatitis C. It purchased sofosbuvir/ledipasvir, sofosbuvir and daclatasvir. In addition, a clinical pathway for treatment of chronic hepatitis C was established. It seeks to “standardize care based on the best available evidence, improve health outcomes and make the use of the most efficient resources.” 3 Undoubtedly, this innovative strategy for management of high-priced medicines within the framework of the SGSSS has improved access to treatment and the consequent cure of patients with hepatitis C. This was demonstrated in the High Cost Account published in July, 2018. 4 However, there are still difficulties regarding timely and continuous access to treatment and provision of health services especially for patients in the subsidized regime and for vulnerable people such as street people, IV drug users, and the incarcerated population who are not generally affiliated with the SGSSS.
In this context, it is necessary to look for options that can address the problem of hepatitis C on several fronts with actions that strengthen Primary Health Care and prevent the progression of chronic liver disease. These should include hospitalization for decompensation and provisions for subsequent health expenditures. In this sense, the implementation of Promotion and Prevention activities focused on populations that have been identified as being at risk for hepatitis C would allow for timely screening and improve rates of diagnosis and early treatment. These populations include people over 60 years of age, people who received transfusions or donations of organs before 1996, IV drug users, and people who have been tattooed or had piercings in places that do not meet basic sanitary requirements. 3 Recently, the publication of the new Comprehensive Care Pathway for Promotion and Maintenance of Health by the Ministry of Health has taken a step in this direction. It includes a paraclinical package for people 60 years and over that has tests for hepatitis C. 5
Since patients with hepatitis C are highly susceptibility to problems related to medications due to their ages, multiple pathologies, polypharmacy and social characteristics, the inclusion of pharmaceutical care within Primary Health Care could support medical activity to monitor the safety and effectiveness of treatments. It would also help provide continuity to health care provision. Pharmaceutical care provides a comprehensive view of the patient that allows identification of her/his needs related to hepatitis C or underlying diseases, and communicates them to the health care personnel responsible for the patient’s care. 6 It also allows for strict monitoring from screening/ confirmation of infection to cure. Evidence of successful implementation of care programs for patients with chronic hepatitis C exists in the experiences of pharmacists in the United States and Spain. 7-11 These experiences could serve as models to be integrated into the clinical pathway established by the Ministry of Health and Social Protection.
In an effort to contribute to achievement of the best health care outcomes for patients with hepatitis C, we have presented a proposal to complement the clinical pathway with interventions and activities of pharmaceutical services (Figure 1). Prospective studies aimed at evaluating the benefits of this complement to the health care pathway could provide evidence of improvement of access to treatment, demonstrate the clinical outcomes of patients with chronic hepatitis C, and compare those outcomes with outcomes currently achieved.
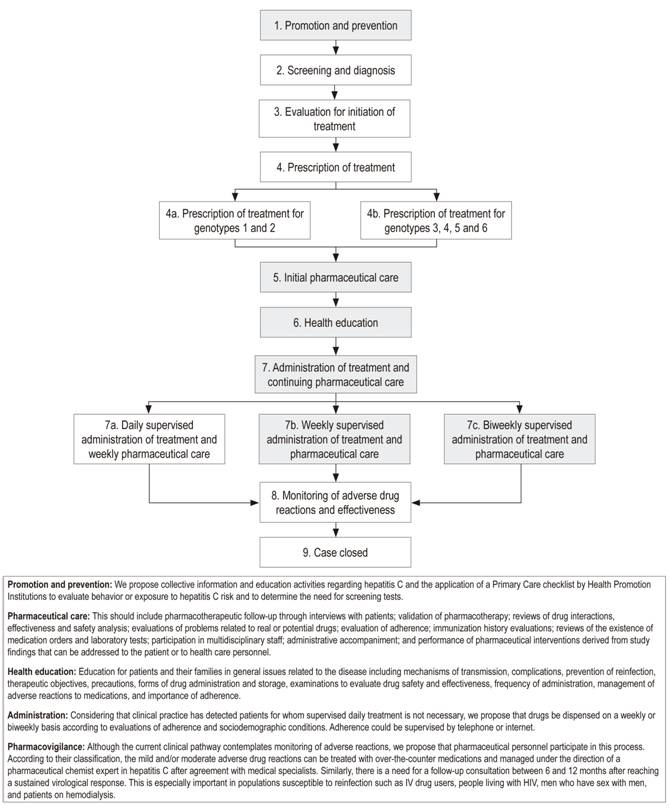
Figura 1 Vía clínica para el tratamiento de la hepatitis C crónica, complementada con intervenciones y actividades del servicio farmacéutico (los recuadros grises son los procesos que se propone complementar en la actual vía clínica). Fuente: adaptación de: Ministerio de Salud y Protección Social. Vía clínica para el tratamiento de hepatitis C. Bogotá, Colombia: Instituto de Evaluación Tecnológica en Salud Ministerio de Salud y Protección Social; 2017.
REFERENCES
1. Congreso de la República. Ley Estatutaria en Salud. Ley 1751/2015 de 16 de febrero. Diario Oficial, no 49.427, (17-02-2015). [ Links ]
2. Ministerio de Salud y Protección Social. Política de Atención Integral en Salud. Bogotá, Colombia: Ministerio de Salud y Protección Social; 2016. [ Links ]
3. Ministerio de Salud y Protección Social. Vía clínica para el tratamiento de hepatitis C. Bogotá, Colombia: Instituto de Evaluación Tecnológica en Salud Ministerio de Salud y Protección Social; 2017. [ Links ]
4. Fondo Colombiano de Enfermedades de Alto Costo. Boletín de información técnica especializada de la Cuenta Alto Costo. Volumen 4, Número 11. Bogotá, Colombia; 2018. [ Links ]
5. Ministerio de Salud y Protección Social. Lineamiento técnico y operativo ruta integral de atención para la promoción y mantenimiento de la salud. Bogotá, Colombia: Dirección de Promoción y Prevención Ministerio de Salud y Protección Social; 2018. [ Links ]
6. Mohammad RA, Bulloch MN, Chan J, Deming P, Love B, Smith L, et al. Provision of clinical pharmacist services for individuals with chronic hepatitis C viral infection: Joint Opinion of the GI/Liver/Nutrition and Infectious Diseases Practice and Research Networks of the American College of Clinical Pharmacy. Pharmacotherapy. 2014;34(12):1341-54. doi: 10.1002/phar.1512. [ Links ]
7. Galewitz P. VA Shifts To Clinical Pharmacists To Help Ease Patients’ Long Waits [Internet]. Kaiser Health News. 2016 [citado 28 de abril de 2018]. Disponible en: Disponible en: https://khn.org/news/va-treats-patients-impatience-with-clinical-pharmacists/ . [ Links ]
8. Zaepfel M, Cristofaro L, Trawinski A, McCarthy K, Rightmier E, Khadem T. Evaluation of a Hepatitis C Patient Management Program at a University Specialty Pharmacy. Ann Pharmacother. 2017;51(4):307-14. doi: 10.1177/1060028016683495. [ Links ]
9. Yang S, Britt RB, Hashem MG, Brown JN. Outcomes of Pharmacy-Led Hepatitis C Direct-Acting Antiviral Utilization Management at a Veterans Affairs Medical Center. J Manag Care Spec Pharm. 2017;23(3):364-9. doi: 10.18553/jmcp.2017.23.3.364. [ Links ]
10. Martin MT, Faber DM. Patient satisfaction with the clinical pharmacist and prescribers during hepatitis C virus management. J Clin Pharm Ther. 2016;41(6):645-9. [ Links ]
11. Chamorro-de-Vega E, Rodriguez-Gonzalez CG, Gimenez-Manzorro A, de Lorenzo-Pinto A, Iglesias-Peinado I, Herranz A, et al. Improving pharmacotherapy outcomes in patients with hepatitis C virus infection treated with direct-acting antivirals: The GRUviC project. Int J Clin Pract. 2017;71(8):e12988. doi: 10.1111/ijcp.12988. [ Links ]
Received: October 03, 2018; Accepted: November 09, 2018











 texto en
texto en 


