Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.34 no.1 Bogotá Jan./Mar. 2019
https://doi.org/10.22516/25007440.264
Original articles
Clinical Pathology Characterization of Eosinophilic Esophagitis in Children and Adolescents at Hospital Universitario Fundación Santa Fe de Bogotá
1Hospital Universitario Fundación Santa Fe de Bogotá, Departamento de Patología y Laboratorios. Bogotá D. C., Colombia.
2Universidad de los Andes, Facultad de Medicina, Grupo de Investigación PediAFe. Bogotá D. C., Colombia.
3Universidad de los Andes, Facultad de Medicina, Grupo de Investigación PediAFe. Hospital Universitario Fundación Santa Fe de Bogotá, Sección de Gastroenterología Pediátrica. Bogotá D. C., Colombia.
4Hospital Universitario Fundación Santa Fe de Bogotá, Departamento de Patología y Laboratorios. Universidad de los Andes, Facultad de Medicina. Bogotá D. C., Colombia
Introduction:
Eosinophilic esophagitis (EoE) is an emerging, chronic and immune-mediated disease. Clinically it is characterized by symptoms associated with esophageal dysfunction, and histologically by predominantly inflammatory eosinophil infiltrate.
Objective:
The aim of this study was to describe the clinical, endoscopic and histopathological characteristics of children and adolescents diagnosed with EoE at the Hospital Universitario Fundación Santa Fe de Bogotá between 2007 and 2017.
Methods:
This is a cross-sectional, descriptive and observational study that included patients under 18 years of age with histopathological diagnoses of EoE.
Results:
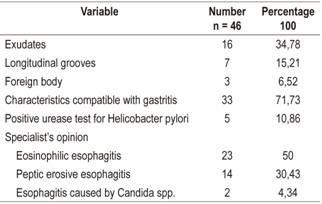
Forty-six patients were included, 31 were male, and the average age was 11.8 years (range 11 months - 18 years). Seventy percent presented abdominal pain, 37% presented heartburn, 28% suffered vomiting, 22% had nausea and dysphagia. The most common antecedents were asthma (41%), allergic rhinitis (37%), gastroesophageal reflux disease (22%) and atopic dermatitis (15%). The most frequent endoscopic finding consisted of whitish exudates found in 35% of the cases. Endoscopic suspicion of EoE was described in 50% of the cases. The histopathological study showed 15 to 40 eosinophils per high power field (HPF) in 52%, 41-60/HPF in 19.5%, and 61-80/HPF in 15.2%. Hyperplasia of the basal lamina was found in 95.6% of the cases.
Conclusions:
The majority of patients were adolescents (69%), the most frequent symptom was abdominal pain (70%), and 40% of cases had histories of atopy. Only 50% had endoscopic findings suggestive of EoE. This study is the first clinical and pathological analysis of EoE cases in children and adolescents in Colombia.
Keywords: Eosinophilic esophagitis; eosinophils; pathology; children; adolescents; Colombia
Introducción:
la esofagitis eosinofílica (EEo) es una enfermedad emergente, crónica e inmunomediada. Clínicamente se caracteriza por síntomas asociados con disfunción esofágica e histológicamente por infiltrado inflamatorio predominante de eosinófilos.
Objetivo:
describir las características clínicas, endoscópicas e histopatológicas de niños y adolescentes con diagnóstico de EEo en el Hospital Universitario Fundación Santa Fe de Bogotá entre 2007 y 2017.
Métodos:
estudio observacional descriptivo de corte transversal que incluyó pacientes menores de 18 años con diagnóstico histopatológico de EEo.
Resultados:
se incluyeron 46 pacientes, 31 de sexo masculino, con una edad promedio de 11,8 años (rango: 11 meses-18 años). El 70 % presentó dolor abdominal, el 37 % pirosis, 28 % emesis y el 22 % náuseas y disfagia. Los antecedentes más referidos fueron asma (41 %), rinitis alérgica (37 %), enfermedad por reflujo gastroesofágico (22 %) y dermatitis atópica (15 %). El hallazgo endoscópico más frecuente fue los exudados blanquecinos, descritos en el 35 %. La sospecha endoscópica de EEo se describió en el 50 % de los casos. El estudio histopatológico mostró de 15 a 40 eosinófilos por campo de alto poder (CAP) en el 52 %, 41-60 en el 19,5 % y 61-80 en el 15,2 %. Se encontró hiperplasia de la lámina basal en el 95,6 % de los casos.
Conclusiones:
los pacientes en su mayoría eran adolescentes (69 %), el síntoma más frecuente fue el dolor abdominal (70 %) y el 40 % de los casos tenía antecedente de atopia. Solamente el 50 % tenía hallazgos endoscópicos sugestivos de EEo. El presente trabajo corresponde al primer análisis clínico y patológico de casos de EEo en niños y adolescentes en Colombia.
Palabras clave: Esofagitis eosinofílica; eosinófilos; patología; niños; adolescentes; Colombia
Introduction
Eosinophilic esophagitis (EoE) is an emerging, immune-mediated, chronic disease that is characterized by infiltration of eosinophils into the esophageal epithelium. In untreated cases it results in fibrosis and esophageal dysfunction. 1,2 Initially considered a rare entity, it is currently one of the most frequently diagnosed conditions in children with feeding problems for in adults with dysphagia and food impaction. 3
When EoE was first described clinically in 1968, 4 it was considered to be a manifestation of gastroesophageal reflux disease (GERD). In the 1990’s, cases of EoE were identified in children and adults who presented other manifestations. In addition, their symptoms and histological alterations failed to improve with suppression of acid production and/or antireflux surgery. Subsequently, other studies reported resolution of EoE resulting from diet management which suggested that it was a single entity that was not always related to GERD. 2,3,5,6,7
Since 2000, there has been an exponential increase in the prevalence of EoE, especially in Western countries. Currently, its annual incidence is comparable to that of Crohn’s disease, 8-11 but it is not clear whether EoE is really a new disease or has simply been recognized recently. 12
EoE has been reported in children and adults around the world with clear predominance among men and boys. Noel and colleagues have reported an incidence of 1/10,000 in children in Ohio between 2000 and 2003 and a prevalence of 4/10,000 for 2003. 13 A 2006 study conducted by Cherian et al. in Australia reported an increase in prevalence from 0.05/10 000 in 1995 to 0.89/10,000 for 2004. 11
More recently, a population-based study in the United States evaluated EoE’s prevalence in more than 35 million clinical records from 2008 to 2011 and found that 16,405 patients had at least one diagnostic code for EoE. Twenty-four percent were under 18 years of age, and the prevalence in children under 20 years of age was calculated at 50.5/100,000. 14 Another study that included around 30 million clinical records from 2010 to 2015 found 7,840 cases of EoE of which 1,250 corresponded to pediatric patients (15.9%), indicating a prevalence of 25.1/100,000. 15
It has become evident that since publication of the recommendations of the first international consensus on EoE, recognition and timely diagnosis of this pathology by physicians has improved substantially. 16 For the first time, this consensus defined the diagnostic criterion for EoE as a finding of more than 15 eosinophils per high power field (HPF) in esophageal tissue. 3,16
As in other immune-mediated diseases such as asthma and eczema, EoE is considered to be a chronic disease. 17,18 Data from randomized clinical trials and from prospective and retrospective cohorts have shown that EoE tends not to resolve spontaneously. 19-23 A study that followed pediatric patients for 14 years found that only 2% of the participants experienced remission of the disease. 24 On the other hand, there is no evidence of that EoE can develop into hypereosinophilic syndrome which compromises other areas of the gastrointestinal tract nor that it can cause neoplasia. 25
Suspicion of EoE begins with symptoms associated with dysfunction or esophageal fibrosis, 2,3 but the presentation and evolution of the disease varies according to the age of the patient. 17 Usually, children have symptoms related to esophageal dysfunction which can mimic GERD. Infants under 5 years of age may have developmental failure and eating difficulties and may choke on solid foods. Children from 6 to 14 years old present vomiting, abdominal pain and dysphagia, while symptoms related to the development of esophageal stenosis and fibrosis, such as food impaction and dysphagia, occur more frequently in those over 15 years of age. 17,26 In addition, it is common for patients diagnosed with EoE to also suffer from other atopic conditions such as rhinitis, dermatitis, asthma, food allergies mediated by immunoglobulin E (IgE) and family histories of atopy. 1,3
In addition to a complete clinical history, tools such as endoscopy and histological study of esophageal tissue are fundamental for diagnosis. 3 The sensitivity of endoscopy for diagnosis of EoE is 15% to 48% while its specificity is 90% to 98%. 27 Key reported endoscopic findings include loss of the normal vascular pattern of the mucosa, corrugated rings, mucosal felinization (concentric rings), white exudates, longitudinal grooves, esophageal stenosis, crepe paper mucosa, and constriction rings in advanced cases.2,28
Histopathological study is currently the gold standard for diagnosis, even though eosinophils in the esophagus are not exclusive to this condition. An eosinophil count is fundamental for diagnosis, 29,30 and a finding of 15 or more eosinophils per HPF has been established as a cut-off point. Once a diagnosis has been made, control of esophageal inflammation is imperative for relief of symptoms and to prevent complications such as an esophageal stenosis.2,3
This study retrospectively describes and analyzes characteristics of clinical presentation and histopathological findings from patients under 18 years of age who were diagnosed with EoE and treated at the Hospital Universitario Fundación Santa Fe (HUFSFB) between January 2007 and October 2017.
Patients and methods
This observational cross-sectional descriptive study began with a search of the pathology and digestive endoscopy databases for patients under 18 years who had had histological diagnoses of EoE (15 or more eosinophils per HPF) at the HUFSFB between January 2007 and October 2017. Once the review was complete, the list of patients was recorded in Excel 2013. Subsequently, pathology and endoscopy reports were reviewed. Pathology reports included eosinophil tissue counst, evidence of microabscesses, papillary elongation, vascular changes, acanthosis, hyperplasia of basement membranes, fibrotic changes and gastric and duodenal eosinophilia. Endoscopy reports included observations of alterations of vascular patterns of the mucosa, whitish exudates, corrugated rings, concentric rings, longitudinal grooves, esophageal stenoses, crepe paper mucosa and constriction rings.
Pertinent clinical information was collected from the clinical records system of the HUFSFB. This information included reasons patients came to the clinic and reports of abdominal pain, nausea, emesis, halitosis, heartburn, dysphagia, regurgitation, cough, diarrhea, early satiety, and food impaction. A descriptive statistical analysis of the population was done with STATA 11.0. The study protocol was reviewed and endorsed by the research ethics committee of the HUFSFB.
Results
Forty-six patients who had been diagnosed with EoE during the study period were found. Their average age was 11.8 years (range: 11 months-18 years), thirty-one (67.39%) were male, and the male: female relationship was 2:1. Clinical characteristics and background of the population are described in Table 1.
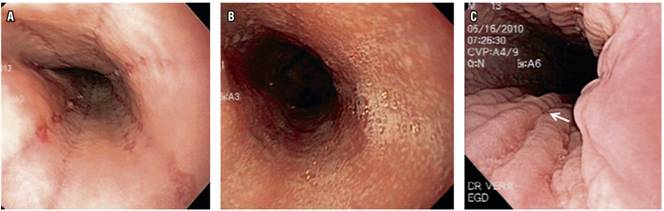
The most frequent esophageal findings of endoscopy were whitish exudates (16 cases, 34.78%) and longitudinal grooves (7 cases, 15.21%) (Figure 1). Based on histories of digestive symptoms associated with atopic disease taken together with endoscopic findings, EoE was suspected in 23 patients (50%). Fourteen other patients (30.43%) were considered to have had erosive peptic esophagitis, and two patients (4.34 %) were suspected of having Candida spp esophagitis (Table 2).
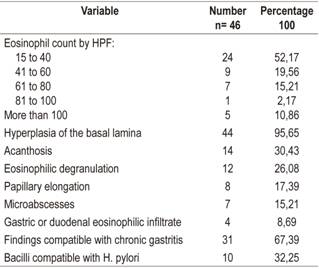
Histological studies of esophageal tissue showed that 24 patients (52.17%) had eosinophil counts from 15 to 40/HPF, 9 patients (19.56%) had eosinophil counts from 41 to 60/HPF, 7 patients (15.21%) had eosinophil counts from 61 to 80, one patient (2.17%) had an eosinophil count from 81 to 100, and five patients (10.86%) had eosinophil counts of more than 100 eosinophils/HPF (10.86%). The most frequent additional histological findings were hyperplasia of the basal lamina (44 patients, 95.65%), acanthosis (14 patients, 30.43%) and eosinophilic degranulation (12 patients, 26.08%) (Figure 2, Table 3).
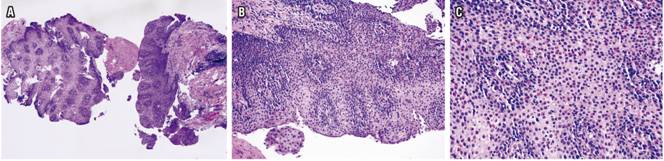
Figure 2 A. H & E 4x shows marked acanthosis, basal cell hyperplasia and papillomatosis. B. H & E 20x shows increased intraepithelial cellularity, predominantly of eosinophils. C. H & E 40x shows infiltrate of eosinophils with presence of more than 80/HPF.
Discussion
In recent decades, great advances have been made in understanding the epidemiology and pathogenesis of EoE. 23 and there has been a notable increase in its incidence and prevalence throughout the world. This is probably related to better recognition of this entity. 2,16
EoE has been reported at all stages of life from lactation to almost 100 years of age. 30,31 Nevertheless, the majority of cases are diagnosed in children, adolescents and adults under 50 years of age. 10,26 There is a constant discrepancy between sexes, and EoE occurs 3 to 4 times more frequently in men than in women, and it is also more frequent among white individuals. 23,32
Seventy-one percent of the 214 cases in a multicenter study from several Latin American countries including Colombia were male. 33 In terms of age, infants account for 2% of cases, preschoolers account for 16%, elementary schoolchildren account for 47%, and adolescents account for 35%. Similarly, in our series the vast majority of patients were male. However, in contrast to the findings of the Latin American cohort, the most frequent age range at diagnosis was 12 to 18 years (69%). 33
According to the accumulated evidence, the natural history of EoE is characterized by two phases: a predominantly inflammatory (usually infantile) form which can evolve into a predominantly fibrosing (adult) form.22 This information partly explains the differences between the clinical presentations of children and adults. 1,16,17
It has been proposed that EoE in children manifests with whitish plaques or exudates, linear grooves, and alteration of the vasculature or edema of the mucosa, but without constriction rings. The resulting symptoms can include pain, heartburn and, in some cases, failure to grow. When pathogenic events progress, usually in the absence of adequate treatment, fibrosis and constriction rings develop. This leads to a clinical pattern characterized by dysphagia in adolescence and adulthood, although fibrous rings can also be seen in children. 1,3,26,28 Our study’s findings match these reports: abdominal pain was found most frequently, followed by heartburn which was most commonly seen in adolescents who constitute the majority of our population. 17
The evidence also shows that patients with EoE have a higher rate of atopic conditions than individuals without EoE. 1,3,17. It has been reported that 30% to 50% of children with EoE have asthma but that only 10% of the population without asthma have EoE. Similarly, 50% to 75% of children with EoE suffer from allergic rhinitis, compared to 30% of the population without EoE. Between 10% and 20% of infants with EoE suffer from food allergies mediated by IgE (urticaria and anaphylaxis) compared to 1% to 5% of normal infants. 2,17
The characteristics of this series are consistent with these data which show that more than 40% of patients had histories of asthma, 37% had allergic rhinitis, and 13% had food allergies. In addition, histories of other immune-mediated conditions such as Hashimoto’s thyroiditis, vitiligo, angioedema and urticaria were found.
Alterations most frequently found through endoscopy in this series were whitish exudates and longitudinal grooves. Gastroenterologists in these cases considered that endoscopic findings and clinical characteristics were key to diagnoses in only half of the cases thus underling the importance of taking biopsies.
EoE is the only form of eosinophilic disease of the gastrointestinal tract for which an objective criterion for diagnosis has been defined. Since eosinophils normally reside in the mucosa of the gastrointestinal tract except for that of the esophagus, 34 eosinophilic diseases are easier to characterize in the esophagus than elsewhere in the gastrointestinal tract. 35 The discovery made in a follow-up study of a group of patients who had been found to have eosinophils in esophageal biopsies 15 years earlier during their childhoods. 25 Initially, it was found that these individuals reported dysphagia more frequently than did controls. In addition, patients who had more than 15 eosinophils per HPF reported medical diagnoses of food allergies, histories of food impaction and requirements for follow-ups by gastroenterology than did patients whose eosinophil count of was less than 15/HPF. In addition, those patients with at least 5 eosinophils per HPF reported more frequent food impaction than did controls suggesting that a lower count than that adopted (15 eosinophils per HPF) also has clinical significance. The findings of that study also show that the intensity of epithelial eosinophilic infiltration is frequently related to tissue alterations in the epithelium and the lamina propria. 25
Histologically, EoE compromises all the tissue components represented in a biopsy. The epithelium may be acanthotic due to expansion of the basal zone which may extend to thickening of the entire epithelium. The presence of intraepithelial inflammatory cells, in this case eosinophils, is due to the response of T helper 2 cells (Th2) against swallowed antigens. 35 In addition to increased numbers of eosinophils, their distribution is usually anomalous with luminal exudate and formation of microabscesses with exudate. When the epithelium is intact, the exudate can cover the surface, but when it is not it can concentrate more predominantly on the lamina propria. It is possible to observe degranulation of eosinophils, possibly secondary to mechanical cell disruption. 35 In contrast to the histology of a normal lamina propria, in cases of EoE, its fibers are usually thickened and dense, 36, it can be compromised by chronic eosinophilic inflammation, 18 and sometimes it has with numerous plasma cells. 35
The histological findings most frequently found among patients in this cohort were hyperplasia of the basal lamina, acanthosis and degranulation of eosinophils. Other changes such as papillary elongation and formation of microabscesses were found less frequently. Interestingly, the gastric biopsies of thirty-one patients (67%) showed histological characteristics compatible with chronic gastritis, and ten patients (32%) had bacilli compatible with H. pylori.
More than half of these patients had eosinophil counts between 15 and 40 eosinophils/HPF and seven patients (two girls and five boys) had counts of 80 or more eosinophils/HPF. It is interesting to note that six of there were preadolescents who were over 12 years of age. This group also included the three patients with histories of Hashimoto’s thyroiditis, and all of them had been diagnosed with an atopic condition or food allergy. Although it is not possible to perform a deeper analysis, we can infer that age at diagnosis influences the severity of presentation and that the atopic or immune-mediated clinical profile of patients diagnosed with EoE is of great relevance.
During the last decade, increased interest in study of the etiology and pathogenesis of EoE has led to greater knowledge of this condition.2,23 Multiple risk factors have been described, but several studies have shown that H. pylori infection could be a protective factor against esophageal eosinophilia and other atopic diseases. 37
One study that analyzed more than 165,000 paired specimens of esophageal and gastric biopsies found a strong inverse association between H. pylori and evidence of esophageal eosinophilia. In other words, those individuals with higher risks of esophageal eosinophilia and those who had been diagnosed with EoE were less likely to be infected with H. pylori. 38 This finding has also been described in other studies of patients diagnosed with of EoE and is consistent with the fact that H. pylori infections are inversely related to development of other atopic diseases such as asthma and eczema. 39,40. The mechanism by which this bacterial infection may protect against EoE is still unknown, but it has been suggested that it is a polarization of the immune system towards response by T helper cells 1 (Th1), whereas the absence of infection favors response by Th2 cells, decreasing tolerance and, therefore, atopy. (38
In this study, no greater differences were found between the eosinophil counts or average eosinophils/HPF (44.5) of the 10 patients in whom H. pylori was evidenced in the gastric biopsy (between 20 and> 100 per HPF) and those of the complete cohort (41 eosinophils per HPF). With regard to histories of atopy, no diagnosis of atopic disease was found in three of the ten patients.
This study is the first published analysis of clinical, endoscopic and histopathological characteristics of EoE in infants and adolescents in this country. Findings such as the predominance of the male sex are consistent with what has been described in other countries of Latin America and elsewhere in the world, but other findings such as the average patient age at the time of diagnosis are dissimilar.
Frequency of symptoms and endoscopic and pathological findings also varied. It was clear that presentation of the EoE is non-specific which is why a high index of suspicion together with good correlation of clinical antecedents, symptoms and endoscopic and histological studies is always necessary.
Findings related to atopic comorbidities, other immune-mediated conditions, and frequent H. pylori infections call for attention and demand more in-depth analyses to gain a better understanding of the way EoE presents in Colombia.
Referencias
1. Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128(1):3-20.e6. doi: 10.1016/j.jaci.2011.02.040. [ Links ]
2. Cianferoni A, Spergel J. Eosinophilic Esophagitis: A Comprehensive Review. Clin Rev Allergy Immunol. 2016;50(2):159-74. doi: 10.1007/s12016-015-8501-z. [ Links ]
3. Furuta GT, Katzka DA. Eosinophilic Esophagitis. N Engl J Med. 2015;373(17):1640-8. doi: 10.1056/NEJMra1502863. [ Links ]
4. Hardy WR, Anderson RE. The hypereosinophilic syndromes. Ann Intern Med. 1968;68(6):1220-9. [ Links ]
5. Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci. 1993;38(1):109-16. [ Links ]
6. Straumann A, Spichtin HP, Bernoulli R, Loosli J, Vögtlin J. Idiopathic eosinophilic esophagitis: a frequently overlooked disease with typical clinical aspects and discrete endoscopic findings. Schweiz Med Wochenschr. 1994;124(33):1419-29. [ Links ]
7. Kelly KJ, Lazenby AJ, Rowe PC, Yardley JH, Perman JA, Sampson HA. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology. 1995;109(5):1503-12. [ Links ]
8. Prasad GA, Alexander JA, Schleck CD, Zinsmeister AR, Smyrk TC, Elias RM, et al. Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clin Gastroenterol Hepatol. 2009;7(10):1055-61. doi: 10.1016/j.cgh.2009.06.023. [ Links ]
9. Straumann A, Simon HU. Eosinophilic esophagitis: escalating epidemiology? J Allergy Clin Immunol. 2005;115(2):418-9. doi: 10.1016/j.jaci.2004.11.006. [ Links ]
10. Hruz P, Straumann A, Bussmann C, Heer P, Simon HU, Zwahlen M, et al. Escalating incidence of eosinophilic esophagitis: a 20-year prospective, population-based study in Olten County, Switzerland. J Allergy Clin Immunol. 2011;128(6):1349-1350.e5. doi: 10.1016/j.jaci.2011.09.013. [ Links ]
11. Cherian S, Smith NM, Forbes DA. Rapidly increasing prevalence of eosinophilic oesophagitis in Western Australia. Arch Dis Child. 2006;91(12):1000-4. doi: 10.1136/adc.2006.100974. [ Links ]
12. Whitney-Miller CL, Katzka D, Furth EE. Eosinophilic esophagitis: a retrospective review of esophageal biopsy specimens from 1992 to 2004 at an adult academic medical center. Am J Clin Pathol. 2009;131(6):788-92. doi: 10.1309/AJCPOMPXJFP7EB4P. [ Links ]
13. Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med. 2004;351(9):940-1. doi: 10.1056/NEJM200408263510924. [ Links ]
14. Dellon ES, Jensen ET, Martin CF, Shaheen NJ, Kappelman MD. Prevalence of eosinophilic esophagitis in the United States. Clin Gastroenterol Hepatol. 2014;12(4):589-96.e1. doi: 10.1016/j.cgh.2013.09.008. [ Links ]
15. Mansoor E, Cooper GS. The 2010-2015 Prevalence of Eosinophilic Esophagitis in the USA: A Population-Based Study. Dig Dis Sci. 2016;61(10):2928-2934. doi: 10.1007/s10620-016-4204-4. [ Links ]
16. Furuta GT, Liacouras CA, Collins MH, Gupta SK, Justinich C, Putnam PE, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133(4):1342-63. doi: 10.1053/j.gastro.2007.08.017. [ Links ]
17. Liacouras CA, Spergel J, Gober LM. Eosinophilic esophagitis: clinical presentation in children. Gastroenterol Clin North Am. 2014;43(2):219-29. doi: 10.1016/j.gtc.2014.02.012. [ Links ]
18. Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA, et al. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol. 2013;108(5):679-92. doi: 10.1038/ajg.2013.71. [ Links ]
19. Konikoff MR, Noel RJ, Blanchard C, Kirby C, Jameson SC, Buckmeier BK, et al. A randomized, double-blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology. 2006;131(5):1381-91. doi: 10.1053/j.gastro.2006.08.033. [ Links ]
20. Dohil R, Newbury R, Fox L, Bastian J, Aceves S. Oral viscous budesonide is effective in children with eosinophilic esophagitis in a randomized, placebo-controlled trial. Gastroenterology. 2010;139(2):418-29. doi: 10.1053/j.gastro.2010.05.001. [ Links ]
21. Straumann A, Conus S, Degen L, Felder S, Kummer M, Engel H, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology. 2010;139(5):1526-37, 1537.e1. doi: 10.1053/j.gastro.2010.07.048. [ Links ]
22. Straumann A, Spichtin HP, Grize L, Bucher KA, Beglinger C, Simon HU. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology. 2003;125(6):1660-9. [ Links ]
23. Dellon ES. Epidemiology of eosinophilic esophagitis. Gastroenterol Clin North Am. 2014;43(2):201-18. doi: 10.1016/j.gtc.2014.02.002. [ Links ]
24. Spergel JM, Brown-Whitehorn TF, Beausoleil JL, Franciosi J, Shuker M, Verma R, et al. 14 years of eosinophilic esophagitis: clinical features and prognosis. J Pediatr Gastroenterol Nutr. 2009;48(1):30-6. doi: 10.1097/MPG.0b013e3181788282. [ Links ]
25. DeBrosse CW, Franciosi JP, King EC, Butz BK, Greenberg AB, Collins MH, et al. Long-term outcomes in pediatric-onset esophageal eosinophilia. J Allergy Clin Immunol . 2011;128(1):132-8. doi: 10.1016/j.jaci.2011.05.006. [ Links ]
26. Orenstein SR, Shalaby TM, Di Lorenzo C, Putnam PE, Sigurdsson L, Mousa H, et al. The spectrum of pediatric eosinophilic esophagitis beyond infancy: a clinical series of 30 children. Am J Gastroenterol. 2000;95(6):1422-30. doi: 10.1111/j.1572-0241.2000.02073.x. [ Links ]
27. Pierre R, Guisande A, Sifontes L, Sosa P, Ninomiya I, González L, et al. Diagnóstico y tratamiento de la esofagitis eosinofílica en niños. Revisión de la literatura y recomendaciones basadas en la evidencia. Acta Gastroenterol Latinoam. 2015;45:263-71. [ Links ]
28. Straumann A, Spichtin HP, Bucher KA, Heer P, Simon HU. Eosinophilic esophagitis: red on microscopy, white on endoscopy. Digestion. 2004;70(2):109-16. doi: 10.1159/000080934. [ Links ]
29. Hurrell JM, Genta RM, Melton SD. Histopathologic diagnosis of eosinophilic conditions in the gastrointestinal tract. Adv Anat Pathol. 2011;18(5):335-48. doi: 10.1097/PAP.0b013e318229bfe2. [ Links ]
30. Grin A, Streutker CJ. Esophagitis: old histologic concepts and new thoughts. Arch Pathol Lab Med. 2015;139(6):723-9. doi: 10.5858/arpa.2014-0132-RA. [ Links ]
31. Kapel RC, Miller JK, Torres C, Aksoy S, Lash R, Katzka DA. Eosinophilic esophagitis: a prevalent disease in the United States that affects all age groups. Gastroenterology . 2008;134(5):1316-21. doi: 10.1053/j.gastro.2008.02.016. [ Links ]
32. Franciosi JP, Tam V, Liacouras CA, Spergel JM. A case-control study of sociodemographic and geographic characteristics of 335 children with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2009;7(4):415-9. doi: 10.1016/j.cgh.2008.10.006. [ Links ]
33. Pierre R, Vieira M, Vázquez-Frías R, Nimoniya I, Messere G, Daza W, et al. Estudio multicéntrico sobre la epidemiologia de la esofagitis eosinofílica pediátrica en América Latina. Rev Gen. 2016;70:125-30. [ Links ]
34. DeBrosse CW, Case JW, Putnam PE, Collins MH, Rothenberg ME. Quantity and distribution of eosinophils in the gastrointestinal tract of children. Pediatr Dev Pathol. 2006;9(3):210-8. doi: 10.2350/11-05-0130.1. [ Links ]
35. Collins MH. Histopathologic features of eosinophilic esophagitis and eosinophilic gastrointestinal diseases. Gastroenterol Clin North Am . 2014;43(2):257-68. doi: 10.1016/j.gtc.2014.02.007. [ Links ]
36. Aceves SS, Newbury RO, Dohil R, Bastian JF, Broide DH. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol . 2007;119(1):206-12. doi: 10.1016/j.jaci.2006.10.016. [ Links ]
37. Blaser MJ. Helicobacter pylori and esophageal disease: wake-up call? Gastroenterology . 2010;139(6):1819-22. doi: 10.1053/j.gastro.2010.10.037. [ Links ]
38. Dellon ES, Peery AF, Shaheen NJ, Morgan DR, Hurrell JM, Lash RH, et al. Inverse association of esophageal eosinophilia with Helicobacter pylori based on analysis of a US pathology database. Gastroenterology . 2011;141(5):1586-92. doi: 10.1053/j.gastro.2011.06.081. [ Links ]
39. Furuta K, Adachi K, Aimi M, Ishimura N, Sato S, Ishihara S, et al. Case-control study of association of eosinophilic gastrointestinal disorders with Helicobacter pylori infection in Japan. J Clin Biochem Nutr. 2013;53(1):60-2. doi: 10.3164/jcbn.13-15. [ Links ]
40. Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med. 2007;167(8):821-7. doi: 10.1001/archinte.167.8.821. [ Links ]
Received: June 26, 2018; Accepted: August 24, 2018











 text in
text in