Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.34 no.1 Bogotá Jan./Mar. 2019
https://doi.org/10.22516/25007440.354
Review articles
Endoscopic Intervention in Chronic Pancreatitis
1 Cirugía gastrointestinal y endoscopia. Jefe de Posgrado de Cirugía General, Universidad Pontificia Bolivariana. Profesor titular del grupo de gastrohepatología, Universidad de Antioquia. Cirugía y endoscopia del Instituto de Cancerología, Clínica las Américas. Medellín, Colombia
2 Profesor del Departamento de Cirugía de la Facultad de Medicina de la Universidad de Sao Paulo (FMUSP). Coordinador de la unidad de endoscopia biliopancreática, FMUSP. Vicepresidente de la Sociedad Interamericana de Endoscopia Digestiva (SIED; 2017-2018) Sao Paulo, Brasil
Chronic pancreatitis is an irreversible and progressive disorder of the pancreas characterized by inflammation, fibrosis and scarring. Exocrine and endocrine functions are lost often leading to chronic pain. Its etiology is multifactorial, although alcoholism is the most important risk factor in adults. If chronic pancreatitis is suspected, computed tomography with contrast is the best imaging modality. Although narcotics and antidepressants provide the greatest pain relief, more than half of all patients eventually require intervention by endoscopy or surgery.
Endoscopic retrograde cholangiopancreatography (ERCP) is an effective alternative for a variety of therapies for treating benign and malignant diseases of the pancreas. In the last 50 years, endoscopic treatment has evolved to become the first-line therapy for most acute and chronic inflammatory diseases of the pancreas. As this field progresses, it has become important for gastroenterologists to keep their knowledge of indications for this procedure up-to-date and to perform a sufficient volume of procedures to allow them to manage complex pancreatic endoscopic therapy. Keeping up-to-date should include an understanding of alternative approaches to pancreatic diseases including medical treatment, therapy guided by endoscopic ultrasound, management of symptomatic stenoses and stones, interventions on the celiac plexus, and drainage of pancreatic pseudocysts.
Keywords: Chronic pancreatitis; endoscopic cholangiography; pancreatic stones; pancreatic stenosis; pancreatic pseudocyst; celiac pleural block
La pancreatitis crónica es un trastorno irreversible y progresivo del páncreas caracterizado por inflamación, fibrosis y cicatrización. Las funciones exocrinas y endocrinas se pierden, lo que a menudo conduce al dolor crónico. La etiología es multifactorial, aunque el alcoholismo es el factor de riesgo más importante en los adultos. Si se sospecha pancreatitis crónica, la tomografía computarizada con contraste es la mejor modalidad de diagnóstico por imágenes. Aunque los narcóticos y los antidepresivos proporcionan el mayor alivio del dolor, más de la mitad de los pacientes eventualmente requiere una intervención por endoscopia o cirugía. La colangiopancreatografía retrógrada endoscópica es una alternativa eficaz para una variedad de terapias en el tratamiento de enfermedades benignas y malignas del páncreas. En los últimos 50 años, la endoterapia ha evolucionado hasta convertirse en la terapia de primera línea en la mayoría de las enfermedades inflamatorias agudas y crónicas del páncreas. A medida que avanza este campo, es importante que los gastroenterólogos mantengan un conocimiento adecuado de la indicación del procedimiento, mantengan el volumen de procedimiento suficiente para manejar la endoterapia pancreática compleja y comprendan enfoques alternativos a las enfermedades pancreáticas, incluidos el tratamiento médico, la terapia guiada por ecografía endoscópica, el manejo de las estenosis sintomáticas y cálculos, las intervenciones sobre el plexo celíaco y el drenaje de los pseudoquistes pancreáticos.
Palabras clave: Pancreatitis crónica; colangiografía endoscópica; cálculos pancreáticos; estenosis pancreática; pseudoquiste pancreático; bloqueo de plexo celíaco
Introduction
Chronic pancreatitis (CP) is diagnosed more and more frequently in medical practice. It is estimated that the incidence of CP varies between 5 and 14.4 cases per 100,000 inhabitants. 1,2 Intractable abdominal pain and associated morphological anomalies in the pancreatobiliary system are the main determinants of interventionist endoscopic treatment of CP. 3 There are multiple pain mechanisms in CP, and recent experimental evidence suggests that pancreatic ductal hypertension can activate pancreatic stellate cells which in turn can generate oxidative stress and subsequent inflammation. 4 One source of pain is the sum of chronic inflammation and oxidative stress which induces nociception, mechanical allodynia and inflammatory hyperalgesia, pancreatic neuropathy, peripheral neuroplasticity and central neuroplasticity. 5 In some patients, there are structural abnormalities within the duct or pancreatic parenchyma that may be responsible for, or that may perpetuate, this process. Such abnormalities are often the target for endoscopic or surgical intervention, but correction does not always translate into clinical improvement. This underscores the multimodal nature of the pain syndrome of these patients. Decompression can be performed using endoscopic and surgical approaches, but an endoscopic approach is currently recommended as the first line modality by the European Gastrointestinal Endoscopy Society (ESGE). 6
Before any endoscopic treatment is indicated, a meticulous morphological evaluation of the major pancreatic duct (MPD) (also known as the Wirsung duct) is mandatory for assessing the magnitude of the disease and local anatomical alterations that suggest neoplasia. Pancreatic cancer should be ruled out especially in Caucasian women patients over 50 years of age who have jaundice, manifest exocrine insufficiency, and who do not have any pancreatic calcifications. 7 The size and distribution of pancreatic ductal stones can be best assessed by computerized axial tomography (CT scan) although transabdominal ultrasound also provides a fairly good evaluation. Pancreatic ductal stenosis, biliary stenosis and anatomical variants such as pancreas divisum are best identified with magnetic resonance cholangiopancreatography (MRCP). MRCP also has advantages over endoscopic ultrasound (EUS) and CT scans for differentiating pancreatic pseudocysts (PP) from intrapancreatic necrosis (walled-off necrosis) in patients with CP who have had recent acute exacerbations. 8
CP is responsible for extensive health care use and is associated with disproportionately high socioeconomic costs. 9 Clinical management of this disease is often challenging because it frequently involves prescription of highly regulated opioids for pain control which are associated with numerous side effects and dependency problems. Current approaches to endoscopic CP treatment aim at removing stones that obstruct the pancreatic duct, pancreatic duct stenosis, PP drainage, celiac plexus block (CPB) and benign biliary stenoses. Each technique and its contribution to treatment of CP will be reviewed.
Indications and contraindications for endoscopic treatment of chronic pancreatitis
Intractable pain is the most common indication for endoscopic treatment of the pancreas in patients with CP, but the modality of choice depends on morphology:
Small stones can be extracted using endoscopic retrograde cholangiopancreatography (ERCP) with a pancreatic sphincterotomy using balloons and a Dormia basket. Calculi of more than 5 mm can be best fragmented by extracorporeal shock wave lithotripsy (ESWL) with or without placemtn of a pancreatic duct stent. 10
Currently, ESWL is the ESGE’s recommended first option. 6
Contraindications for ESWL include multiple calculi in the MPD, isolated calculi in the pancreatic tail, multiple MPD restrictions, moderate to massive ascites, pancreatic pseudocysts and masses in the pancreatic head. 11
Pancreatic duct stenosis can be treated by pancreatic sphincterotomy and stenting with or without dilation.
PP should be treated when there is an infection, symptomatic intracystic hemorrhaging, biliary obstruction, obstruction of the gastric outlet, early satiety, abdominal pain, weight loss and increased size of a pseudocyst. It has been suggested that prophylactic treatment of asymptomatic pseudocysts can be considered when the main vessels are compressed, when there is a pancreaticopleural fistula, when a pseudocyst that measures more than 5 cm does not become smaller after 6 weeks, and when a pseudocyst’s wall is more than 5 mm thick. 12
EUS can be used for drainage of the dilated MPD using rendezvous techniques when transpapillary access is not possible. 13
Treatment of pain refractory to standard intervention can be tried with EUS-guided CPB. 14
Endoscopic treatment of calculi in the pancreatic duct
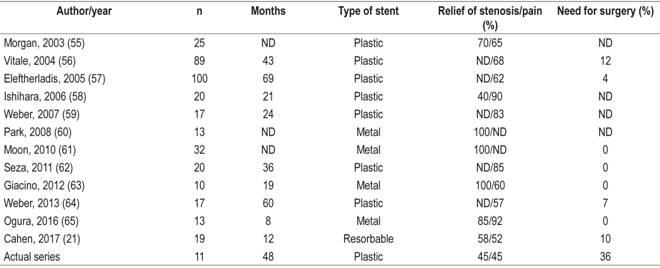
Pancreatic duct stones are biomineral concretions that can obstruct pancreatic ducts thereby increasing pancreatic duct pressure which can activate the inflammatory process that causes fibrosis. The objective of endoscopic treatment of calculi is to relieve obstruction by removal of stones (Figure 1). 15
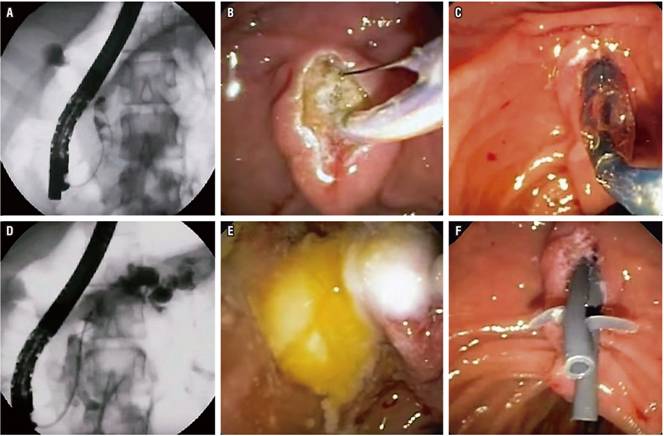
Figure 1 Management of calculi in the MPD in a patient with CP. A. Pancreatography shows several calculi in the pancreatic head. B. Pancreatic sphincterotomy. C. Dilation with sphincterotomy balloon. D. Extraction balloon passage. E. Extracted pancreatic stones. F. Ten cm passage of two 7 Fr pancreatic stents.
Techniques to fragment and eliminate pancreatic duct stones that have evolved over the years may require intraductal access through mechanical lithotripsy, electrohydraulic lithotripsy, laser-guided lithotripsy or may require an extracorporeal intervention such as ESWL. 16
Intraductal mechanical lithotripsy is performed with an endoscopic mechanical lithotripter. This technically challenging procedure has a high rate of complications and is rarely used today. Electrohydraulic lithotripsy is performed under direct pancreatoscopic visualization using a mother-child endoscopic system. It has the advantage of supplying energy to a focused area of the calculi. 17 Although technical modifications such as single operator use of the SpyGlass® Direct Visualization System and lithotripsy using a holmium laser have evolved over time, studies published in the literature are limited. 18 In addition, availability, high costs and operator expertise limit widespread use of these techniques.
ESWL is currently the first-line modality for treating painful obstructive pancreatic duct stones that measure more than 5 mm, particularly those located in the pancreatic head and body regions. 6 The objective is to reduce the calculi to fragments smaller than 3 mm which are then usually removed with ERCP and pancreatic sphincterotomy. A concomitant pancreatic ductal stent can be used to treat local stenosis.
For radiolucent calculi, a pancreatic sphincterotomy with placement of a naso-pancreatic drainage tube prior to ESWL can help accurately guide ESWL. 10 When there are multiple duct calculi, the calculi that is closest to the orifice of the MPD should be the main objective. Isolated intraductal stones located in the tail do not need to be treated with ESWL because these concretions are unlikely to result in enough ascending ductal pressure to cause pain. In addition, attempts to fragment duct stones in the tail could cause a collateral splenic lesion.
Derivative surgery is an alternative for managing pain and achieving decompression of ducts. It compares favorably with endoscopic treatment without ESWL. However, due to the morbidity associated with surgical procedures such as Frey’s surgery (Figure 2), this is generally reserved for patients for whom non-surgical treatments have failed. 19
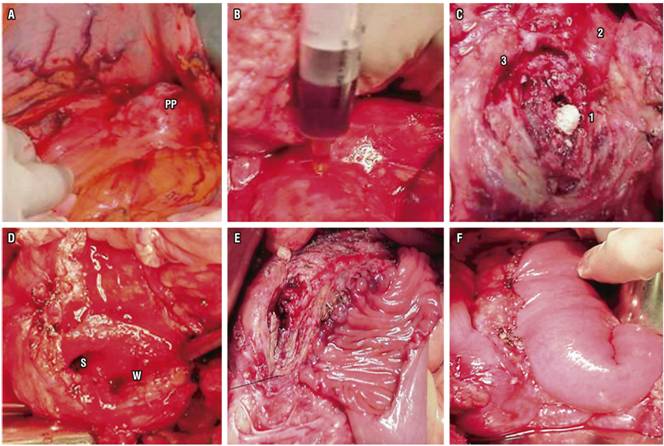
Figure 2 Frey’s surgery for CP. A. Transcavity approach to PP. B. Puncturing anterior pancreatic body to locate main pancreatic duct. C. Calculi (1) in the MPD (2), another calculus in the Santorini duct (3). D. Open MOD (W) and Santorini duct (S) in the pancreatic head. E. Partial anastomosis of the jejunal loop isolated in the anterior pancreas. F. Completed anastomosis in two planes.
Endoscopic treatment of stenosis of the major pancreatic duct
Stenosis is found in the MPD in up to 18% of patients with CP, and the priority is to rule out malignancy. Similar to the principle and problems created by obstructive stones, it is thought that these stenoses contribute to symptoms by causing pancreatic ductal hypertension. For this reason therapy aims at improving these narrow segments in order to decompress the ductal system. The approach usually involves pancreatic sphincterotomy followed by dilation of the stenosis and placement of a stent in the pancreatic duct (Figure 3). Immediate relief of pain with this treatment has been reported in 65% to 95% of cases, and sustained relief of pain has been reported in 32% to 68% of patients. 20
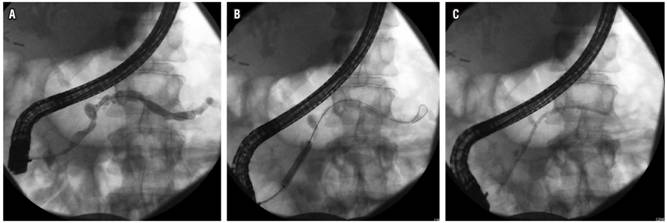
Figure 3 Management of benign stenosis with dilation and stent in a patient with CP. A. Stenosis in the head of the pancreas. B. Balloon dilation of stenosis. C. Passage of pancreatic stent.
Currently, the placement of a single 10 Fr polyethylene stent with successive changes yearly, even in the absence of symptoms, is the first-line treatment for dominant stenosis of the MPD. 6
Although straight plastic stents and pig tail stents have been widely used to treat pancreatic duct strictures, there is still no ideal stent. So far, several modifications have been made in pancreatic stent technology: S type stents, stents with lateral flaps, bumpy stents, and even biodegradable stents. 21 Various stents have been tested in animal models and in short-term clinical trials with small sample sizes. Data on the validation of these results and long-term data on efficacy and safety are needed before these stents can be used routinely.
One technique under study for treating main duct stenoses that persist for more than 12 months after treatment with a single plastic stent is the deployment of multiple plastic stents placed side by side simultaneously. 22
An international multicenter collaborative study has shown that minimally invasive pancreatic duct drainage by EUS in pancreatic stenosis after failed ERCP is safer than surgery and even more effective. 23
Endoscopic treatment of pancreatic pseudocysts
PPs are collections of encapsulated pancreatic juice with well-defined inflammatory walls. They develop in 20% to 40% of patients with CP. Unlike PP of acute origin, those of CP patients rarely remit spontaneously. Intervention is indicated when symptoms of abdominal pain, nausea, vomiting, early satiety, weight loss or gastric or biliary obstructions or infections persist. Some treatments have been proposed for large, persistent asymptomatic collections, for those that have thick walls (recognizing it is not common to be asymptomatic with cysts> 6 cm), for those associated with fistulas, for changes of the main pancreatic duct, and for calculi of the pancreatic duct. Endoscopic drainage of pseudocysts can be performed by transmural and transpapillary approaches. 24
It is important to evaluate abnormal ductal anatomy, including ductal leaks and whether or not there is communication with the pseudocyst, with an MRCP or ERCP prior to treatment. An increase in the size of a pseudocyst in images suggests ductal communication with the pseudocyst. Ductal obstruction should be managed prior to endoscopic treatment of pseudocysts in order to achieve a higher success rate and avoid recurrence. Similarly, if arterial pseudoaneurysms are detected, they should be embolized before endoscopic treatment because of the high mortality rate from hemorrhaging of pseudoaneurysms close to pseudocysts. 25
Transpapillary drainage during ERCP is most useful for small, solitary, communicating pseudocysts located in or near the head and body of the pancreas. This type of drainage is also feasible and useful for treating large and multiple PPs although the results are no better or worse than those of transmural drainage. 26
Transmural drainage can be done by creating communication between the pseudocyst and the stomach (cystogastrostomy) or the pseudocyst and the duodenum (cystoduodenostomy). After puncture, at least two double pig tail plastic stents should be placed through the puncture to keep the aperture between the pseudocyst and the stomach or duodenum open. The stent must not be removed for at least two months following insertion, and a cross-sectional image must be obtained in order to assess whether the cyst has resolved before the stent can be removed. 27
If there images show a sectioned MPD, the stent should be kept in situ indefinitely to achieve the best results. An attempt to pass the stent through the rupture in the duct is also associated with long-term success. 28
Before the use of EUS to guide pseudocyst drainage, compression of the gastric or duodenal wall was essential because puncture and drainage was performed through the wall. Under linear echoendoscopic guidance, drainage can be performed with superior results even for pseudocysts that do not bulge and even beyond the gastric or duodenal lumen. 29 EUS can also help delimit an avascular area for puncture which is especially helpful for patients with extensive collateral vascularization secondary to portal hypertension. EUS also helps distinguish pseudocysts from cystic neoplasms. 30
Endoscopic treatment of biliary stenosis in patients with CP
Benign biliary stenosis is found in many patients with CP. The clinical estimates of prevalence vary a great deal, ranging from 3% to 46%. 31 In general, benign biliary stenoses present as fibrous calcified circumferential strictures that usually develop within the pancreatic portion of the common bile duct. This makes it possible for biliary obstruction to develop from extrinsic compression related to pancreatic edema and fluid collection. When a stenosis is identified, it is important to exclude malignancy. Patients may experience pain, nausea, weight loss, jaundice, pruritus, and 10% may progress to development of cholangitis or biliary cirrhosis. 32
Cholangitis is a clear indication that endoscopic intervention should be used to treat a patient with CP, but it is less clear whether it should be used as a preventive procedure. There are numerous observations of patients with cholestasis due to benign biliary stenosis related to CP who were successfully managed without biliary drainage. Nevertheless, it is unclear how to predict who will progress or develop other complications or whether liver fibrosis will return after successful biliary drainage. The main indications for endoscopic intervention have been adopted from surgery. In addition to the presence of symptoms, indications include secondary biliary cirrhosis, stones in the common bile duct, progression of biliary stenosis based on increased proximal dilation of the biliary ductal, jaundice that persists for more than one month, and alkaline phosphatase greater than two to three times the upper limit of normal. 6
EUS guided procedures after ERCP fails
The development of linear EUS has allowed new approaches for drainage of the pancreatobiliary system when conventional ERCP fails.
After the initial description by Harada 33, several other studies have evaluated the feasibility and efficacy of pancreatobiliary drainage directed by EUS. The fundamental principle is to use EUS to guide a large gauge needle to perforate the MPD through the gastric or duodenal wall. Once successful access to the MPD is achieved, the duct can be drained using rendezvous techniques or the transmural route. 34 In view of the technical challenge posed by EUS guided rendezvous procedures and the high frequency of complications, this procedure is currently recommended only for selected patients at tertiary care centers which have the appropriate infrastructure and experienced specialists. 35
EUS guided CPB
CPB is a treatment option aimed at disruption of the afferent pathway of pain transmission from the pancreas. 36 It typically involves injecting a mixture of local anesthetic and a corticosteroid into the celiac plexus. This is different from celiac plexus neurolysis with an ethanol injection, which should not be used to treat benign pancreatic disease. 37 Steroids are used as a substitute for ethanol to prolong the effect of the treatment. The combination of bupivacaine and triamcinolone was also the regimen selected in the largest prospective study on EUS guided CPB. 38
CPB can be performed percutaneously, but EUS guided CPB has better results and lower risks of complications such as paraplegia which is associated with the percutaneous technique. 14 In view of its dubious efficacy (short-term pain relief, if any) and frequent complications, CPB should be seen as rescue or bridge therapy for patients who do not respond to conventional medical and endoscopic treatment and who are not ideal candidates for surgery. 39 Although EUS guided celiac ganglia neurolysis with absolute alcohol is justified in cases of pancreatic cancer, it should be avoided in cases of CP since the fibrosis resulting from the alcohol injection could hinder subsequent surgery.
Comparative randomized trials have shown that EUS-guided CPB is superior to both fluoroscope-guided CPB and CT-guided CPB in terms of both pain relief and patient preference. Improvement of pain can be expected in 50% to 60% of patients treated with EUS-guided CPBs, but improvement does not usually persist, so many the procedure’s benefits disappear after several months. Some patients may respond to sequential CPB, but this management strategy has not been not proven, and risk accumulates with each intervention. 12
Complications
Although mechanical and electrohydraulic intraductal lithotripsy procedures are associated with increased risk of complications, ESWL is a relatively safe procedure. The usual complications of ESWL include acute pancreatitis, splenic lesions, petechiae, hemorrhaging, cobble stone patterns and perforations. Acute pancreatitis is the most important and occurs most frequently. A recent study of 1,470 procedures found that ESWL had a general complication rate of 6.7%. The study documented an odds ratio (OR) of 1.28 for the development of post-ESWL complications in the presence of pancreatic divisum and the interval between PC diagnosis, respectively. Male gender emerged as a possible independent protective factor against moderate to severe complications, with an OR of 0.19. 40
Common problems encountered with pancreatic stents include migration and obstruction. Pancreatic stent patency is usually between 6 and 12 months. 41
Complications of endoscopic drainage of pseudocysts include hemorrhaging, infection, and retroperitoneal leakage. Complications occur in approximately 4% of patients, although mortality is usually low (0.5%). (42) Complications such as hemorrhaging and ruptures are minor with the transpapillary method, but the risk of infection is greater.
Complications of CPB commonly include transient diarrhea, exacerbation of pain, hypotension, and occasional infections. Death is rare. 43
Results
Extracorporeal Shock Wave Lithotripsy (ESWL)
The effectiveness of ESWL is usually measured in terms of complete stone fragmentation, stone removal and pain relief. Recently, it has been shown that solitary and low density calculi are independent predictors of complete clearance by ESWL. 44 Table 1 lists studies that have reported complete removal of calculi from the duct and pain relief in response to ESWL with or without ERCP.
Table 1 21st century studies showing the results of ESWL with or without ERCP to relieve pain and clear calculi from the major pancreatic duct in cases of CP
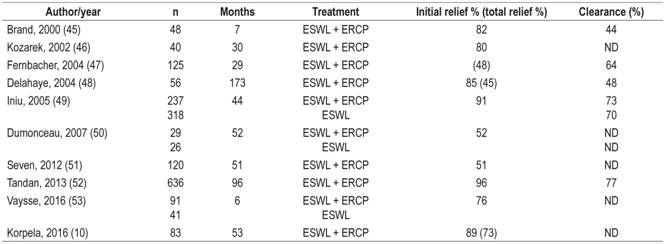
ND: no data.
A study of cohort of 636 patients by Tandan has recently demonstrated complete pain relief in 68.7% of patients followed up for two to five years and 60.3% of patients followed up for more than five years after ESWL. 52 Complete clearance of the ducts was observed in 77.5% and 76% of patients in the intermediate and long-term follow-up groups, respectively. Although stones recurred 14.1% of patients in the intermediate follow-up group and 22.8% in the long-term group, none of the patients in the long-term follow-up group required repetition of ESWL, and only 3.8% of the patients in the intermediate follow-up group did. This study suggests that when ESWL is performed when stones are first diagnosed, pain relief is likely to persist for a longer time.
A study of 120 patients by Seven et al. demonstrated pain relief in 85% of patients after a mean follow-up time of 4.3 years. 51 Complete pain relief was seen in 50% of the patients, and there were significant improvements in quality of life scores (visual analogue scale 7.3 [2.7] versus 3.7 [2.4], p <0.001). The proportion of patients without pain after four years of follow-up was significantly higher than for those who underwent surgery (61% vs. 21%, p = 0.009). The longest follow-up period in this study was more than 7 years.
A study by Choi et al. has shown that the use of secretin before ESWL results in greater elimination of stones. 54 That study found that intravenous administration of 16 μg of secretin before ESWL resulted in 63% stone clearance compared to 46% when secretin was not used. Multiple logistic regression suggested that the use of secretin and pancreatic stenting prior to ESWL are independent predictors of complete or near total clearance of the MPD.
Stenting the Pancreatic Duct to Treat Stenosis
Table 2 lists the most important 21st century studies that have evaluated the role of the pancreatic duct stents for treating stenosis of the MPD.
Table 2 21st century studies with management by endoscopic cholangiography of pancreatic stenoses with stents in patients with CP
ND: no data.
Long-term clinical success after stent placement in MPD stenosis is measured by the absence of pain one year after stent removal. An additional stent is not necessary if the contrast has been eliminated one or two minutes prior to the anastomosis and if a 6 Fr catheter can easily pass through the stenosis. 11
Pancreatic stenting is technically successful in 85% to 98% of cases and is associated with immediate pain relief in 65% to 95% of patients. Pain relief was maintained for 32% to 68% of patients in 14 to 58 months follow-ups. 66 A recent study of 17 patients who underwent endoscopic pancreatic stenting found that 57% remained completely free of pain (without relapse) after 5 years. 64
It has been shown that the placement of multiple plastic stents to treat pancreatic stenoses relieved pain in 84.2% of cases and had technical and functional success rates of 100% and 94.7%, respectively. Stent migration and reoperation were observed in 10.5% and 15.8% of cases, respectively. 67
The use of metal stents was associated with 100% technical and functional success. Although 85.2% of patients did not present pain in the short-term follow-up, complications were observed in 26.8%. These included stent migration in 8.2% and reoperation in 9.8% of patients. 66 Currently, the use of metal stents is recommended only in clinical trials with planned exchanges within one year because metal stent permeability lasts only one year in the MPD.
Drainage of Pancreatic Pseudocysts
Although endoscopic and long-term surgical drainage of pseudocysts have similar morbidity and recurrence, the mortality rate of endoscopic drainage is only 0.2% while that of surgical drainage is 2.5%. Endoscopic treatment has also been found to be significantly better than surgery in terms of cost, hospital stay and patient quality of life up to 3 months after drainage. 68
Transpapillary and transmural drainage of PP have been found to have a similar long-term success rates, but the former has a lower morbidity rate (1.8% versus 15.4%, p <0.008). Although transmural drainage of pseudocysts can be performed by conventional drainage or EUS-guided, the success rate is greater with drainage guided by EUS because it does not require intraluminal compression. Results are most favorable for pseudocysts located in the head of the pancreas (Figure 4). 11
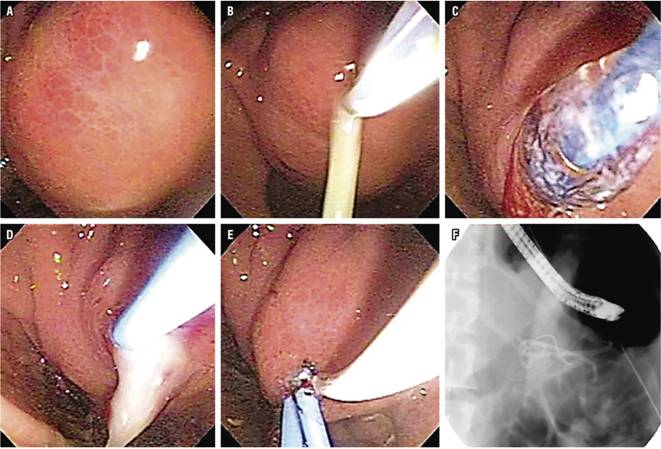
Figure 4 Endoscopic drainage of PP in CP. A. PP bulging in the gastric antrum. B. Puncture with precut needle with pus output. C. Transmural dilation with balloon up to 10 mm. D. Passage of first 7 Fr. pig tail catheter E. Passage of second pig tail catheter. F. Fluoroscopic image of double drainage.
A retrospective study has found that therapeutic failure of endoscopic drainage of the PP is independently associated with the placement of a single stent and with stent durations of less than 6 weeks. A recent randomized controlled trial has shown that recurrence of pseudocysts is associated with early removal of stents. 69
Most studies have evaluated the role of endoscopic pseudocyst drainage for treating both acute and chronic pancreatitis. Since the revision of the Atlanta classification, 70 it is increasingly recognized that PP are uncommon in cases of acute pancreatitis. 71 It is likely that many cases diagnosed as pseudocysts in patients with acute pancreatitis were actually instances of walled necrosis. Consequently, results obtained from studies with pseudocyst drainage are currently better when only CP patients with true PP are considered.
Biliary Stenosis due to CP
Endoscopic management of benign biliary stenosis due to CP usually involves placement of a biliary stent through ERCP. Balloon-only dilation performs poorly because these stenoses are not easily resolved and because clinical relapse with restenosis is not uncommon after stent removal. The current options are placement of one or several plastic stents or placement of a self-expanding metal stent (SEMS). One review found that the average long-term success, usually defined as resolution of the stenosis, with the placement of a plastic endoscopic biliary stent may be around 37% at 32 months. 72 The same review found that the permeability range of SEMS was 37% to 100% over a 45-month follow-up average. A broad systematic review that compared these two approaches found the greatest success with uncovered SEMS (80%). The success rate for use of a single plastic stent was 36%, but the selected studies did not present sufficient data about the use of multiple stents. (73) Other experiences have shown high success rate with multiple plastic stents. Expert opinion favors this approach but does not support the routine use of biliary SEMS for this indication. 6. Obstruction and infection are some of the biggest problems with biliary stent placement. (Figure 5).
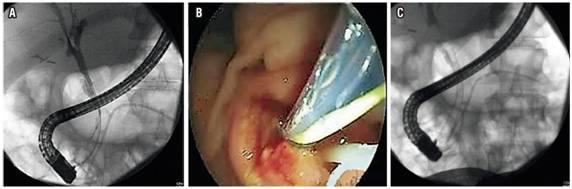
Figure 5 Endoscopic management of biliary stenosis by CP. A. Cholangiogram showing distal biliary stenosis. B. Endoscopic passage of metal biliary stent. C. Expanded metal biliary stent and plastic stent in the pancreas.
How frequently stents need to be replaced is also a common question, and patient adherence to recommended replacement also varies. Patients are often asked to return at intervals of three to four months, but the low rate of occlusion with multiple plastic stents may prolong the replacement interval. 74 There is also no consensus on whether and under what conditions endoscopic treatment should be used before considering surgery. Answering this question requires multidisciplinary discussion.
Finally, benign biliary stenoses can be found in many patients with CP, but an important early step is exclusion of malignancy. These stenoses have the potential to cause numerous symptoms including jaundice, cholangitis and secondary biliary cirrhosis. A decision to treat is usually based on development of symptoms or to prevent infection and cirrhosis.
Both endoscopic and surgical options are available, but direct comparisons are needed. Most stenoses recur and may require several endoscopic treatments with balloon dilation and stent replacement every one to two years before a more durable outcome is achieved. Optimal stent selection continues to be evaluated, but the results with the use of multiple plastic stents are equivalent to those of SEMS. Replacement of stents should also be done every few months. Poor patient adherence to scheduled stent replacement increases the risks of adverse events. 75
EUS Guided Drainage after failed ERCP
A recent study by Shah et al. 76 has reported a success rate of up to 75% for the pancreatic rendezvous procedure guided by EUS. Another Spanish multicenter study of 125 patients showed an overall technical success rate of 67.2% and overall clinical success rate of 63.2% for biliopancreatic access guided by EUS. 77 Another study by Ergun et al. 78 observed significant reductions in the pain score and in the diameter of the MPD during long-term follow-up (median: 38 months [ range: 3-120]) of patients whose EUS-guided drainage was successful.
EUS Guided CPB
The roles of percutaneous CPB and EUS-guided CPB to relieve pain in CP patients have been controversial. 14 Even when beneficial, the effects are short-lived. Fifty-five percent of patients improve for the first four to eight weeks, but this proportion falls to 26% after twelve weeks and further to 10% after 24 weeks. 38 The results are even poorer for patients under 45 years of age who had previously undergone pancreatic surgery. 37
Conclusions
CP is a challenging disease whose primary symptom is pain. The most commonly described reasons for endoscopic intervention are stones that obstruct the pancreatic duct, pancreatic duct stenosis, PP, celiac nerve plexus blockage, and treatment of benign biliary strictures. Endoscopic Treatment has a role in each of these disorders, and its capacity has been expanded or refined with the development of new technologies such as EUS which allows extra-anatomic approaches.
In summary:
The primary symptom of CP is pain.
Pancreatic duct stones should be removed endoscopically if possible even though pain does not always respond to removal of stones.
Stenosis of the main pancreatic duct can be managed with a stent, but malignancy must always be excluded.
CPB is rarely effective for long-term pain management in cases of CP.
Pseudocysts should be operated on only if they are thought to cause pain or intestinal obstruction.
Compression of the common bile duct is treated with long-term stents.
Referencias
1. Domínguez Muñoz JE, Lucendo Villarín AJ, Carballo Álvarez LF, Tenías JM, Iglesias García J. Spanish multicenter study to estimate the incidence of chronic pancreatitis. Rev Esp Enferm Dig. 2016;108(7):411-6. doi: 10.17235/reed.2016.4056/2015. [ Links ]
2. Lizarazo-Rodríguez JI. Fisiopatología de la pancreatitis crónica. Rev Col Gastroenterol. 2008;23:290-8. [ Links ]
3. Majumder S, Chari ST. Chronic pancreatitis. Lancet. 2016;387(10031):1957-66. doi: 10.1016/S0140-6736(16)00097-0. [ Links ]
4. Yang D, Forsmark CE. Chronic pancreatitis. Curr Opin Gastroenterol. 2017;33(5):396-403. doi: 10.1097/MOG.0000000000000377. [ Links ]
5. Talukdar R, Reddy DN. Pain in chronic pancreatitis: managing beyond the pancreatic duct. World J Gastroenterol. 2013;19(38):6319-28. doi: 10.3748/wjg.v19.i38.6319. [ Links ]
6. Dumonceau JM, Delhaye M, Tringali A, Dominguez-Munoz JE, Poley JW, Arvanitaki M, et al. Endoscopic treatment of chronic pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2012;44(8):784-800. doi: 10.1055/s-0032-1309840. [ Links ]
7. Kirkegård J, Mortensen FV, Cronin-Fenton D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Am J Gastroenterol. 2017;112(9):1366-1372. doi: 10.1038/ajg.2017.218. [ Links ]
8. Issa Y, Kempeneers MA, van Santvoort HC, Bollen TL, Bipat S, Boermeester MA. Diagnostic performance of imaging modalities in chronic pancreatitis: a systematic review and meta-analysis. Eur Radiol. 2017;27(9):3820-3844. doi: 10.1007/s00330-016-4720-9. [ Links ]
9. Hall TC, Garcea G, Webb MA, Al-Leswas D, Metcalfe MS, Dennison AR. The socio-economic impact of chronic pancreatitis: a systematic review. J Eval Clin Pract. 2014;20(3):203-7. doi: 10.1111/jep.12117. [ Links ]
10. Korpela T, Udd M, Tenca A, Lindström O, Halttunen J, Myrskysalo S, et al. Long-term results of combined ESWL and ERCP treatment of chronic calcific pancreatitis. Scand J Gastroenterol. 2016;51(7):866-71. doi: 10.3109/00365521.2016.1150502. [ Links ]
11. Talukdar R, Reddy DN. Pancreatic Endotherapy for Chronic Pancreatitis. Gastrointest Endosc Clin N Am. 2015;25(4):765-77. doi: 10.1016/j.giec.2015.06.010. [ Links ]
12. Adler JM, Gardner TB. Endoscopic Therapies for Chronic Pancreatitis. Dig Dis Sci. 2017;62(7):1729-1737. doi: 10.1007/s10620-017-4502-5. [ Links ]
13. Okuno N, Hara K, Mizuno N, Hijioka S, Kuwahara T, Fujita A, et al. Advanced technique for the treatment of chronic calculous pancreatitis using endoscopic ultrasound-guided pancreatic duct drainage. Endoscopy. 2017;49(8):E197-E199. doi: 10.1055/s-0043-110666. [ Links ]
14. Moura RN, De Moura EG, Bernardo WM, Otoch JP, Bustamante FA, Albers DV, et al. Endoscopic-ultrasound versus percutaneous-guided celiac plexus block for chronic pancreatitis pain. A systematic review and meta-analysis. Rev Gastroenterol Peru. 2015;35(4):333-41. [ Links ]
15. Kim YH, Jang SI, Rhee K, Lee DK. Endoscopic treatment of pancreatic calculi. Clin Endosc. 2014;47(3):227-35. doi: 10.5946/ce.2014.47.3.227. [ Links ]
16. Tandan M, Talukdar R, Reddy DN. Management of Pancreatic Calculi: An Update. Gut Liver. 2016;10(6):873-880. doi: 10.5009/gnl15555. [ Links ]
17. Ang TL. Chronic pancreatitis with pancreatic duct stricture and calculi treated by fully covered self-expandable metal stent placement and intraductal pancreatoscopy-guided laser lithotripsy. Endoscopy. 2017;49(6):E145-E146. doi: 10.1055/s-0043-105570. [ Links ]
18. Bekkali NL, Murray S, Johnson GJ, Bandula S, Amin Z, Chapman MH, et al. Pancreatoscopy-Directed Electrohydraulic Lithotripsy for Pancreatic Ductal Stones in Painful Chronic Pancreatitis Using SpyGlass. Pancreas. 2017;46(4):528-530. doi: 10.1097/MPA.0000000000000790. [ Links ]
19. Castaño R, Puerta JD, Ruiz M, Hoyos S. Cirugía de Frey para la pancreatitis crónica. Rev Col Cirugía. 2003;18:139-47. [ Links ]
20. Talukdar R, Nageshwar Reddy D. Endoscopic therapy for chronic pancreatitis. Curr Opin Gastroenterol. 2014;30(5):484-9. doi: 10.1097/MOG.0000000000000091. [ Links ]
21. Cahen DL, van der Merwe SW, Laleman W, Poley JW, Bruno MJ. A biodegradable non-covered self-expandable stent to treat pancreatic duct strictures in chronic pancreatitis: a proof of principle. Gastrointest Endosc. 2018;87(2):486-491. doi: 10.1016/j.gie.2017.08.018. [ Links ]
22. Ohyama H, Mikata R, Ishihara T, Sakai Y, Sugiyama H, Yasui S, et al. Efficacy of multiple biliary stenting for refractory benign biliary strictures due to chronic calcifying pancreatitis. World J Gastrointest Endosc. 2017;9(1):12-18. doi: 10.4253/wjge.v9.i1.12. [ Links ]
23. Tyberg A, Sharaiha RZ, Kedia P, Kumta N, Gaidhane M, Artifon E, et al. EUS-guided pancreatic drainage for pancreatic strictures after failed ERCP: a multicenter international collaborative study. Gastrointest Endosc. 2017;85(1):164-169. doi: 10.1016/j.gie.2016.07.030. [ Links ]
24. Hao L, Pan J, Wang D, Bi YW, Ji JT, Xin L et al. Risk factors and nomogram for pancreatic pseudocysts in chronic pancreatitis: A cohort of 1998 patients. J Gastroenterol Hepatol. 2017;32(7):1403-1411. doi: 10.1111/jgh.13748. [ Links ]
25. Pang TC, Maher R, Gananadha S, Hugh TJ, Samra JS. Peripancreatic pseudoaneurysms: a management-based classification system. Surg Endosc. 2014;28(7):2027-38. doi: 10.1007/s00464-014-3434-9. [ Links ]
26. Amin S, Yang DJ, Lucas AL, Gonzalez S, DiMaio CJ. There Is No Advantage to Transpapillary Pancreatic Duct Stenting for the Transmural Endoscopic Drainage of Pancreatic Fluid Collections: A Meta-Analysis. Clin Endosc. 2017;50(4):388-394. doi: 10.5946/ce.2016.091. [ Links ]
27. Ge PS, Weizmann M, Watson RR. Pancreatic Pseudocysts: Advances in Endoscopic Management. Gastroenterol Clin North Am. 2016;45(1):9-27. doi: 10.1016/j.gtc.2015.10.003. [ Links ]
28. Larsen M, Kozarek RA. Management of Disconnected Pancreatic Duct Syndrome. Curr Treat Options Gastroenterol. 2016;14(3):348-59. doi: 10.1007/s11938-016-0098-7. [ Links ]
29. Guo J, Saftoiu A, Vilmann P, Fusaroli P, Giovannini M, Mishra G, et al. A multi-institutional consensus on how to perform endoscopic ultrasound-guided peri-pancreatic fluid collection drainage and endoscopic necrosectomy. Endosc Ultrasound. 2017;6(5):285-291. doi: 10.4103/eus.eus_85_17. [ Links ]
30. Alali A, Mosko J, May G, Teshima C. Endoscopic Ultrasound-Guided Management of Pancreatic Fluid Collections: Update and Review of the Literature. Clin Endosc. 2017;50(2):117-125. doi: 10.5946/ce.2017.045. [ Links ]
31. Oza VM, Kahaleh M. Endoscopic management of chronic pancreatitis. World J Gastrointest Endosc. 2013;5(1):19-28. doi: 10.4253/wjge.v5.i1.19. [ Links ]
32. Saluja SS, Kalayarasan R, Mishra PK, Srivastava S, Chandrasekar S, Godhi S. Chronic pancreatitis with benign biliary obstruction: management issues. World J Surg. 2014;38(9):2455-9. doi: 10.1007/s00268-014-2581-4. [ Links ]
33. Harada N, Kouzu T, Arima M, Asano T, Kikuchi T, Isono K. Endoscopic ultrasound-guided pancreatography: a case report. Endoscopy. 1995;27(8):612-5. doi: 10.1055/s-2007-1005769. [ Links ]
34. Chapman CG, Waxman I, Siddiqui UD. Endoscopic Ultrasound (EUS)-Guided Pancreatic Duct Drainage: The Basics of When and How to Perform EUS-Guided Pancreatic Duct Interventions. Clin Endosc. 2016;49(2):161-7. doi: 10.5946/ce.2016.011. [ Links ]
35. Shimamura Y, Mosko J, Teshima C, May GR. Endoscopic Ultrasound-Guided Pancreatic Duct Intervention. Clin Endosc. 2017;50(2):112-116. doi: 10.5946/ce.2017.046. [ Links ]
36. Sabbagh LC, Aponte D, Cañadas R, Torres M, Álvarez E, Prieto R, et al. Guía de práctica clínica para el uso de ultrasonido endoscópico en pancreatitis crónica, lesiones quísticas y sólidas del páncreas en adultos. Rev Col Gastroenterol 2015;30(Supl 1):97-104. [ Links ]
37. Sey MS, Schmaltz L, Al-Haddad MA, DeWitt JM, Calley CS, Juan M, et al. Effectiveness and safety of serial endoscopic ultrasound-guided celiac plexus block for chronic pancreatitis. Endosc Int Open. 2015;3(1):E56-9. doi: 10.1055/s-0034-1377919. [ Links ]
38. Gress F, Schmitt C, Sherman S, Ciaccia D, Ikenberry S, Lehman G. Endoscopic ultrasound-guided celiac plexus block for managing abdominal pain associated with chronic pancreatitis: a prospective single center experience. Am J Gastroenterol. 2001;96(2):409-16. doi: 10.1111/j.1572-0241.2001.03551.x. [ Links ]
39. Fusaroli P, Caletti G. Is there a role for celiac plexus block for chronic pancreatitis? Endosc Int Open. 2015;3(1):E60-2. doi: 10.1055/s-0034-1391392. [ Links ]
40. Li BR, Liao Z, Du TT, Ye B, Zou WB, Chen H, et al. Risk factors for complications of pancreatic extracorporeal shock wave lithotripsy. Endoscopy. 2014;46(12):1092-100. doi: 10.1055/s-0034-1377753. [ Links ]
41. Samuelson A, Zeligman B, Russ P, Austin GL, Yen R, Shah RJ. Pancreatic Duct Changes in Patients With Chronic Pancreatitis Treated With Polyethylene and Sof-Flex Material Stents: A Blinded Comparison. Pancreas. 2016;45(2):281-5. doi: 10.1097/MPA.0000000000000471. [ Links ]
42. Zerem E, Hauser G, Loga-Zec S, Kunosić S, Jovanović P, Crnkić D. Minimally invasive treatment of pancreatic pseudocysts. World J Gastroenterol. 2015;21(22):6850-60. doi: 10.3748/wjg.v21.i22.6850. [ Links ]
43. Gimeno-García AZ, Elwassief A, Paquin SC, Sahai AV. Fatal complication after endoscopic ultrasound-guided celiac plexus neurolysis. Endoscopy. 2012;44 Suppl 2 UCTN:E267. doi: 10.1055/s-0032-1309709. [ Links ]
44. Ohyama H, Mikata R, Ishihara T, Tsuyuguchi T, Sakai Y, Sugiyama H, et al. Efficacy of stone density on noncontrast computed tomography in predicting the outcome of extracorporeal shock wave lithotripsy for patients with pancreatic stones. Pancreas. 2015;44(3):422-8. doi: 10.1097/MPA.0000000000000277. [ Links ]
45. Brand B, Kahl M, Sidhu S, Nam VC, Sriram PV, Jaeckle S, et al. Prospective evaluation of morphology, function, and quality of life after extracorporeal shockwave lithotripsy and endoscopic treatment of chronic calcific pancreatitis. Am J Gastroenterol. 2000;95(12):3428-38. doi: 10.1111/j.1572-0241.2000.03190.x. [ Links ]
46. Kozarek RA, Brandabur JJ, Ball TJ, Gluck M, Patterson DJ, Attia F, et al. Clinical outcomes in patients who undergo extracorporeal shock wave lithotripsy for chronic calcific pancreatitis. Gastrointest Endosc. 2002;56(4):496-500. doi: 10.1067/mge.2002.128105. [ Links ]
47. Farnbacher MJ, Schoen C, Rabenstein T, Benninger J, Hahn EG, Schneider HT. Pancreatic duct stones in chronic pancreatitis: criteria for treatment intensity and success. Gastrointest Endosc. 2002;56(4):501-6. doi: 10.1067/mge.2002.128162. [ Links ]
48. Delhaye M, Arvanitakis M, Verset G, Cremer M, Devière J. Long-term clinical outcome after endoscopic pancreatic ductal drainage for patients with painful chronic pancreatitis. Clin Gastroenterol Hepatol. 2004;2(12):1096-106. [ Links ]
49. Inui K, Tazuma S, Yamaguchi T, Ohara H, Tsuji T, Miyagawa H, et al. Treatment of pancreatic stones with extracorporeal shock wave lithotripsy: results of a multicenter survey. Pancreas. 2005;30(1):26-30. [ Links ]
50. Dumonceau JM, Costamagna G, Tringali A, Vahedi K, Delhaye M, Hittelet A, et al. Treatment for painful calcified chronic pancreatitis: extracorporeal shock wave lithotripsy versus endoscopic treatment: a randomised controlled trial. Gut. 2007;56(4):545-52. doi: 10.1136/gut.2006.096883. [ Links ]
51. Seven G, Schreiner MA, Ross AS, Lin OS, Gluck M, Gan SI, et al. Long-term outcomes associated with pancreatic extracorporeal shock wave lithotripsy for chronic calcific pancreatitis. Gastrointest Endosc. 2012;75(5):997-1004.e1. doi: 10.1016/j.gie.2012.01.014. [ Links ]
52. Tandan M, Reddy DN, Talukdar R, Vinod K, Santosh D, Lakhtakia S, et al. Long-term clinical outcomes of extracorporeal shockwave lithotripsy in painful chronic calcific pancreatitis. Gastrointest Endosc. 2013;78(5):726-33. doi: 10.1016/j.gie.2013.05.012. [ Links ]
53. Vaysse T, Boytchev I, Antoni G, Croix DS, Choury AD, Laurent V, et al. Efficacy and safety of extracorporeal shock wave lithotripsy for chronic pancreatitis. Scand J Gastroenterol. 2016;51(11):1380-5. doi: 10.1080/00365521.2016.1209688. [ Links ]
54. Choi EK, McHenry L, Watkins JL, Sherman S, Fogel EL, Coté GA, et al. Use of intravenous secretin during extracorporeal shock wave lithotripsy to facilitate endoscopic clearance of pancreatic duct stones. Pancreatology. 2012;12(3):272-5. doi: 10.1016/j.pan.2012.02.012. [ Links ]
55. Morgan DE, Smith JK, Hawkins K, Wilcox CM. Endoscopic stent therapy in advanced chronic pancreatitis: relationships between ductal changes, clinical response, and stent patency. Am J Gastroenterol. 2003;98(4):821-6. doi: 10.1111/j.1572-0241.2003.07381.x. [ Links ]
56. Vitale GC, Cothron K, Vitale EA, Rangnekar N, Zavaleta CM, Larson GM, et al. Role of pancreatic duct stenting in the treatment of chronic pancreatitis. Surg Endosc. 2004;18(10):1431-4. doi: 10.1007/s00464-003-8933-z. [ Links ]
57. Eleftherladis N, Dinu F, Delhaye M, Le Moine O, Baize M, Vandermeeren A, et al. Long-term outcome after pancreatic stenting in severe chronic pancreatitis. Endoscopy. 2005;37(3):223-30. [ Links ]
58. Ishihara T, Yamaguchi T, Seza K, Tadenuma H, Saisho H. Efficacy of s-type stents for the treatment of the main pancreatic duct stricture in patients with chronic pancreatitis. Scand J Gastroenterol. 2006;41(6):744-50. doi: 10.1080/00365520500383597. [ Links ]
59. Weber A, Schneider J, Neu B, Meining A, Born P, Schmid RM, et al. Endoscopic stent therapy for patients with chronic pancreatitis: results from a prospective follow-up study. Pancreas. 2007;34(3):287-94. doi: 10.1097/mpa.0b013e3180325ba6. [ Links ]
60. Park DH, Kim MH, Moon SH, Lee SS, Seo DW, Lee SK. Feasibility and safety of placement of a newly designed, fully covered self-expandable metal stent for refractory benign pancreatic ductal strictures: a pilot study (with video). Gastrointest Endosc. 2008;68(6):1182-9. doi: 10.1016/j.gie.2008.07.027. [ Links ]
61. Moon SH, Kim MH, Park DH, Song TJ, Eum J, Lee SS, et al. Modified fully covered self-expandable metal stents with antimigration features for benign pancreatic-duct strictures in advanced chronic pancreatitis, with a focus on the safety profile and reducing migration. Gastrointest Endosc. 2010;72(1):86-91. doi: 10.1016/j.gie.2010.01.063. [ Links ]
62. Seza K, Yamaguchi T, Ishihara T, Tadenema H, Tawada K, Saisho H, et al. A long-term controlled trial of endoscopic pancreatic stenting for treatment of main pancreatic duct stricture in chronic pancreatitis. Hepatogastroenterology. 2011;58(112):2128-31. doi: 10.5754/hge09346. [ Links ]
63. Giacino C, Grandval P, Laugier R. Fully covered self-expanding metal stents for refractory pancreatic duct strictures in chronic pancreatitis. Endoscopy. 2012;44(9):874-7. doi: 10.1055/s-0032-1309774. [ Links ]
64. Weber A, Schneider J, Neu B, Meining A, Born P, von Delius S, et al. Endoscopic stent therapy in patients with chronic pancreatitis: a 5-year follow-up study. World J Gastroenterol. 2013;19(5):715-20. doi: 10.3748/wjg.v19.i5.715. [ Links ]
65. Ogura T, Onda S, Takagi W, Kitano M, Sano T, Okuda A, et al. Placement of a 6 mm, fully covered metal stent for main pancreatic head duct stricture due to chronic pancreatitis: a pilot study (with video). Therap Adv Gastroenterol. 2016;9(5):722-8. doi: 10.1177/1756283X16651855. [ Links ]
66. Shen Y, Liu M, Chen M, Li Y, Lu Y, Zou X. Covered metal stent or multiple plastic stents for refractory pancreatic ductal strictures in chronic pancreatitis: a systematic review. Pancreatology. 2014;14(2):87-90. doi: 10.1016/j.pan.2013.12.005. [ Links ]
67. Costamagna G, Bulajic M, Tringali A, Pandolfi M, Gabbrielli A, Spada C, et al. Multiple stenting of refractory pancreatic duct strictures in severe chronic pancreatitis: long-term results. Endoscopy. 2006;38(3):254-9. doi: 10.1055/s-2005-921069. [ Links ]
68. Varadarajulu S, Bang JY, Sutton BS, Trevino JM, Christein JD, Wilcox CM. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology. 2013;145(3):583-90.e1. doi: 10.1053/j.gastro.2013.05.046. [ Links ]
69. Arvanitakis M, Delhaye M, Bali MA, Matos C, De Maertelaer V, Le Moine O, et al. Pancreatic-fluid collections: a randomized controlled trial regarding stent removal after endoscopic transmural drainage. Gastrointest Endosc. 2007;65(4):609-19. doi: 10.1016/j.gie.2006.06.083. [ Links ]
70. Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102-11. doi: 10.1136/gutjnl-2012-302779. [ Links ]
71. Acevedo-Piedra NG, Moya-Hoyo N, Rey-Riveiro M, Gil S, Sempere L, Martínez J, et al. Validation of the determinant-based classification and revision of the Atlanta classification systems for acute pancreatitis. Clin Gastroenterol Hepatol. 2014;12(2):311-6. doi: 10.1016/j.cgh.2013.07.042. [ Links ]
72. Arslanlar S, Jain R. Benign biliary strictures related to chronic pancreatitis: balloons, stents, or surgery. Curr Treat Options Gastroenterol. 2007;10(5):369-75. [ Links ]
73. van Boeckel PG, Vleggaar FP, Siersema PD. Plastic or metal stents for benign extrahepatic biliary strictures: a systematic review. BMC Gastroenterol. 2009;9:96. doi: 10.1186/1471-230X-9-96. [ Links ]
74. Lawrence C, Romagnuolo J, Payne KM, Hawes RH, Cotton PB. Low symptomatic premature stent occlusion of multiple plastic stents for benign biliary strictures: comparing standard and prolonged stent change intervals. Gastrointest Endosc. 2010;72(3):558-63. doi: 10.1016/j.gie.2010.05.029. [ Links ]
75. Khan MA, Baron TH, Kamal F, Ali B, Nollan R, Ismail MK, et al. Efficacy of self-expandable metal stents in management of benign biliary strictures and comparison with multiple plastic stents: a meta-analysis. Endoscopy. 2017;49(7):682-694. doi: 10.1055/s-0043-109865. [ Links ]
76. Shah JN, Marson F, Weilert F, Bhat YM, Nguyen-Tang T, et al. Single-operator, single-session EUS-guided anterograde cholangiopancreatography in failed ERCP or inaccessible papilla. Gastrointest Endosc. 2012;75(1):56-64. doi: 10.1016/j.gie.2011.08.032. [ Links ]
77. Vila JJ, Pérez-Miranda M, Vazquez-Sequeiros E, Abadia MA, Pérez-Millán A, González-Huix F, et al. Initial experience with EUS-guided cholangiopancreatography for biliary and pancreatic duct drainage: a Spanish national survey. Gastrointest Endosc. 2012;76(6):1133-41. doi: 10.1016/j.gie.2012.08.001. [ Links ]
78. Ergun M, Aouattah T, Gillain C, Gigot JF, Hubert C, Deprez PH. Endoscopic ultrasound-guided transluminal drainage of pancreatic duct obstruction: long-term outcome. Endoscopy. 2011;43(6):518-25. doi: 10.1055/s-0030-1256333. [ Links ]
Received: April 02, 2018; Accepted: July 30, 2018











 text in
text in 


