Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista colombiana de Gastroenterología
versão impressa ISSN 0120-9957
Rev Col Gastroenterol vol.34 no.1 Bogotá jan./mar. 2019
https://doi.org/10.22516/25007440.242
Review articles
Diagnostic Approach to Cystic Pancreatic Neoplasms
1 Médico gastroenterólogo del Hospital Universitario de La Princesa. Madrid, España
2 Médico Gastroenterólogo del Hospital Universitario de La Princesa, Madrid, España.
3 Jefe del Servicio de Gastroenterología del Hospital Universitario de La Princesa, Madrid, España.
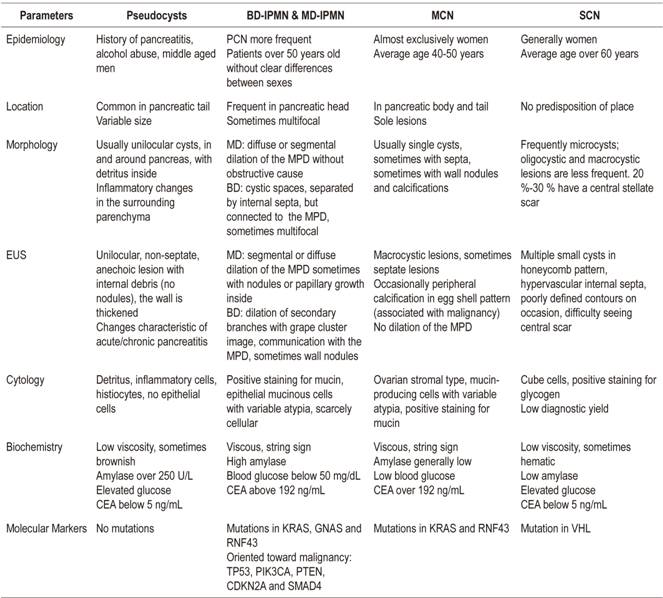
Cystic pancreatic lesions comprise a wide variety of lesions that are being increasingly diagnosed due to the more frequent use of imaging techniques and the aging of the population. Among these lesions, mucinous cystic neoplasms are especially relevant because of their malignant potential. Although abdominal ultrasound, computerized tomography and magnetic resonance imaging are usually the initial diagnostic imaging tests, morphological findings are often not enough for their differentiation. Endoscopic ultrasound has become the best test for their characterization because it allows morphological study and fluid analysis obtained by puncture of the lesion, although its diagnostic accuracy for the detection of mucinous and malignant cysts remains low. The importance of proper characterization is the early detection of preneoplastic as well as malignant lesions and to avoid unnecessary surgery. Clinical practice guidelines differ about the indications for endoscopic ultrasound, surgical treatment and follow-up of these lesions. Questions specially remains in the management of side-branch intraductal papillary neoplasm because of their lower risk of degeneration and their association with pancreatic cancer.
Keywords: Pancreatic cystic neoplasms; mucinous cystic neoplasm; molecular analysis; endoscopic ultrasound
Los quistes pancreáticos comprenden una amplia variedad de lesiones cada vez más frecuentemente diagnosticadas debido tanto al empleo creciente de técnicas de imagen como al envejecimiento de la población. Entre ellas, las neoplasias quísticas mucinosas son especialmente relevantes por su potencial de malignización. Aunque la ecografía abdominal, la tomografía axial computarizada y la resonancia magnética suelen ser las pruebas diagnósticas de imagen iniciales, muchas veces los hallazgos morfológicos no son suficientes para su diferenciación. La ecoendoscopia se ha convertido en la mejor prueba para su caracterización ya que permite realizar estudio morfológico y también del líquido obtenido mediante punción, aunque su precisión diagnóstica para la detección de quistes de estirpe mucinosa y de malignidad sigue siendo baja. La importancia de la adecuada caracterización radica tanto en la detección precoz de las lesiones preneoplásicas y malignas como en evitar cirugías innecesarias. La indicación de ecoendoscopia, de tratamiento quirúrgico y de seguimiento varía entre las distintas guías de práctica clínica estando actualmente en duda especialmente el tratamiento y seguimiento de las neoplasias quísticas mucinosas papilares intraductales de rama lateral por el menor riesgo de degeneración y su asociación con el cáncer de páncreas.
Palabras clave: Neoplasias quísticas pancreáticas; neoplasia quística mucinosa; análisis molecular; ecoendoscopia
Introduction
As a result of increasing use of magnetic resonance imaging (MRI) and abdominal computed tomography (CT) scans, the number of pancreatic cysts diagnosed has also increased. On average, these cysts are smaller in size than those found in the past. Pancreatic cysts have been identified in 3% of CAT scans and in up to 20% of MRIs performed for other reasons. 1,2 In addition, multiple studies have shown that the prevalence of these lesions increases with age which implies increasing numbers of diagnoses of pancreatic cysts given the ongoing aging of the population. 3,4 A recent prospective study of population cohorts after magnetic resonance cholangiopancreatography (MRC) has shown that pancreatic cysts were found in 49.1% of the study population. The study used a low cut-off size of 2 mm. 3 Most of these lesions are diagnosed incidentally, and they generally measure less than 10 mm. 3,5,6 This increase in the number of cysts diagnosed can generate great concern among patients and doctors due to their potential for becoming malignant. Since certain diagnoses are not always reached, performance of multiple costly tests, including invasive procedures and even unnecessary resection, can occur. This entails significant risks of morbidity and mortality.
Pancreatic cystic lesions include a wide variety of both neoplastic and non-neoplastic lesions (Table 1). Within the non-neoplastic group, the most frequent are pseudocysts which must be differentiated from neoplastic lesions such as true pancreatic cystic neoplasms (PCNs). Sometimes it is difficult to differentiate them exclusively by morphological criteria. In addition, only about 4% of asymptomatic cysts are pseudocysts, the majority are PCNs. 7
Table 1 Classification of pancreatic cystic lesions
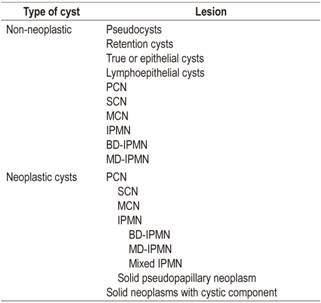
PCN: pancreatic cystic neoplasia; SCN: Serous cystic neoplasm; MCN: Mucinous cystic neoplasms; IPMN: intraductal papillary mucinous neoplasms; BD-IPMN: side branch intraductal papillary mucinous neoplasm; MD-IPMN: main branch intraductal papillary mucinous neoplasm.
The importance of correct identification of these cystic lesions lies in the potential of some of them for malignancy. These include solid-cystic pseudopapillary tumors of the pancreas (SCPTP), solid neoplasms with cystic degeneration, mucinous cysts, including the MCN category, and intraductal papillary mucinous neoplasms (IPMNs). IPMNs can be in either the main duct (MD-IPMN), in a branch duct (BD-IPMN) or mixed. Not all mucinous neoplasms have the same potential for degeneration. According to recent publications, MCNs’ potential malignancy ranges between 10% and 17%,8,9 MD-IPMNs potential malignancy ranges between 38% and 68%, 8,10,11 BD-IPMNs’ potential malignancy ranges between 12% and 47%8,10, solid pseudopapillary neoplasms potential malignancy ranges between 8% and 20%. 8 In contrast, serous cysts, simple cysts and lymphoepithelial cysts are considered to be benign. 8 It should be noted that there are anecdotal reports of serous cysts progressing to malignancy. 12
Pancreatic cancer, one of the most frequent causes of cancer-related death, has poor prognoses at the time of diagnosis. Taking all tumors into account, the 5-year survival rate is less than 10%. When only local lesions are considered the 5-year survival rate is 25%, and the annual mortality rate is almost equal to its morbidity rate. 13 Only 20% to 25% of pancreatic cancers are candidates for surgical treatment at diagnosis, and 80% of these will recur despite surgery. Consequently, early diagnosis and treatment are essential, and identification of precursor lesions of pancreatic adenocarcinoma, pancreatic intraepithelial neoplasms (PanIN) and PCN, is especially important. 14 It is estimated that precursor lesions require an average of 11.7 years to evolve into malignancy and take an additional 6.8 years to metastasize. 15 Theoretically, there is a diagnostic window in time sufficient for early detection of these lesions. However, in general terms, the risk of malignancy from PCN that is detected incidentally is low and represents 1% to 5% of total malignant pancreatic neoplasms. 16
Diagnosis
When a pancreatic cyst is found, the first step should be to differentiate between a pseudocyst and PCN through a combination of the patient’s clinical history, history of pancreatitis, imaging characteristics and cytological and biochemical analysis of fluid. It is important to bear in mind that patients with cystic neoplasms may have pancreatitis and that others without an apparent history of acute pancreatitis may present pseudocysts. 17 The next diagnostic objective should be to differentiate among types of PCNs to detect those with potential for malignancy (mucinous and solid pseudopapillary neoplasms). Finally, the physician should determine those suspected of being malignant.
The diagnostic approach to pancreatic cysts continuously evolving. Most are detected incidentally to CT scans or MRI performed for other reasons. MRI is the radiological test of choice for diagnosing and monitoring PCN because its greater spatial resolution offers better sensitivity for identification of solid small pancreatic lesions. The sequence of MR cholangiography allows detection of smaller cystic lesions which allows better definition of involvement of the major pancreatic duct (MPD) and better determination of whether or not nodules are present in the walls and septa. 18 In contrast, CT scans are the best choice when a malignancy or advanced disease is suspected because they can detect local invasions of structures and metastases. 17 Differentiation between the different types of cysts and determination of the risk of malignancy by only clinical and morphological criteria is suboptimal given that the different lesions may present similar characteristics. The diagnostic precision for determining the type of cyst of 40% to 95% for MR/MR cholangiography and 40% to 81% for CT scans. 18
Endoscopic ultrasound (EUS) allows assessment of morphological criteria and final needle aspiration for a fluid sample for later analysis. 19 Endosonographic characteristics that have been related to malignancy are ducts that are more than 3 cm in diameter, the presence of a solid component, wall thickening, main duct dilation, abrupt changes in the size of the MPD, distal atrophy of the pancreatic gland, and lymphadenopathies.1,10 Nevertheless, as with other imaging techniques, endosonographic characteristics are not sufficient for diagnosis of malignancy. Duct size over 3 cm has low sensitivity and specificity (74% and 49%, respectively) for diagnosis of advanced neoplasia, 14 and malignancy has been described in smaller lesions. 20 Endoscopic ultrasound elastography is a complementary technique that can be used for evaluation of solid pancreatic lesions although it has not proven useful for evaluation of pancreatic cystic lesions. 5
Endoscopic ultrasound guided fine-needle aspiration (EUS-FNA) is a safe technique that allows morphological study of the lesion and puncture-aspiration of its contents. A recent study by Kashab et al. concluded that the use of EUS-FNA together with a CAT scan or MRI increases accuracy of diagnosis of neoplasia in cystic lesions by 36% and 54%, respectively. 21 By making it possible to optimize diagnosis and therefore modify management of these patients, EUS-FNA has become the technique of choice for diagnosis of pancreatic lesions. 19
The liquid obtained can be used for cytological, biochemical and molecular study to define the type of cyst detect any malignancy that is present (Table 2). Even though cytology’s diagnostic yield and sensitivity are below 50% for mucinous cysts because samples frequently contain few or no cells for study, 22 it is very useful when it does provide a specific diagnosis. 1,23 Strategies to increase its performance include the use of contrast to visualize and locate any possible solid components from which a sample can be obtained while avoiding areas of mucus or detritus. 24
In general, complete emptying of liquid is recommended for biochemical analysis, determination of amylase and CEA levels and, according to recent evidence, measurement of glucose. 25,26 Amylase levels below 250 U/L exclude a diagnosis of pseudocyst with a specificity of 98%. 22 High levels orient towards IPMN or pseudocysts, although a finding of high amylase levels in PCN without communication with the MPD is not considered clinically relevant for differential diagnosis among PCN types. 27 CEA levels help differentiate mucinous lesions from non-mucinous lesions, but no optimal cut-off point has been established. The most commonly used cut-off point is 192 ng/mL. It has a sensitivity of 73%, a specificity of 84%, and diagnostic accuracy of 79%. 26 CEA levels have shown superiority with respect to other tumor markers such as CA 19.9, CA 72.4 and CA 15.3. Recently published studies describe mucinous cysts containing lower levels of glucose than non-mucinous cysts. 25 This determination is especially useful due to its low cost. Specifically, a study by Zikos et al. obtained a sensitivity of 95% and a specificity of 57% for detection of mucinous cysts in the laboratory. Using a glucometer with a cut-off point of 50 mg/dL, they obtained a sensitivity of 88% and a specificity of 78%. 25 Neither glucose nor CEA levels have been linked to malignancy. Finally, it should be noted that diagnoses that use morphological criteria and cytological and biochemical analysis can still be suboptimal. 28
Another important issue is selection of patients who should undergo EUS or EUS-FNA from among all patients diagnosed with pancreatic cysts. Several guidelines such as that of the 2012 International Association of Pancreatology (IAP) Guidelines (known as the Fukuoka guide), the 2013 European evidence-based guidelines on pancreatic cystic neoplasms and the 2015 American Gastroenterological Association (AGA) Guidelines in 2015 (Table 3) have tried to identify those PCN that would benefit from further investigation using echoendoscopy and FNA to obtain a sample for fluid analysis and make recommendations for follow-up and surgical treatment.1,6,10,23 For this purpose, the guidelines use parameters such as cyst size, the presence of a solid component and the involvement of the main pancreatic duct. Recently, after the 2016 Sendai meeting of the IAP decided to update their 2012 guidelines due to restrictions on monitoring PCN proposed by the AGA. The update was published in 2017. It pays special attention to the importance of wall nodules. It recommends surgery when nodules larger than 5 mm are found by EUS because this size of nodule is clearly related to malignancy. The update guidelines maintain the recommendation to continue following up on IPMNs. 29
Table 3 Recommendations for performance of diagnostic EUS-FNA
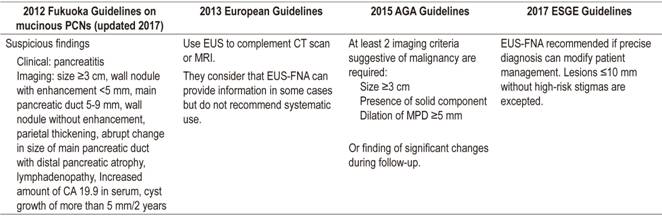
AGA: American Gastroenterological Association; ESGE: European Society of Gastrointestinal Endoscopy.
Results of the use of these guidelines are suboptimal and have been criticized by multiple authors. The recommendations of the AGA about interrupting follow-up have been especially criticized,19,30,31 and treatment and follow-up of BD-IPMN is especially unclear. 9,19,30,32,33. A retrospective study conducted by Lekkerkerker et al. evaluated the percentage of unnecessary surgeries and failure to detect malignancy if main clinical practice guidelines had been used for 115 patients who had undergone cyst resection. It concluded that the IAP and the European guidelines lead to the greatest numbers of unnecessary surgeries, twenty-eight percent of which would have been avoided by following the AGA recommendations instead. Nevertheless, 12% of the lesions with high-grade or invasive dysplasia would not have been detected, demonstrating lower sensitivity of AGA guidelines for detection of malignancy. 9
The progression of normal ductal pancreatic cells to tumor cells is characterized by accumulation of genetic mutations. Consequently, analysis of deoxyribonucleic acid (DNA) has been investigated as a means for differentiation between premalignant mucinous cysts and non-mucinous cysts and for detection of malignancy. 28 Several studies have shown that certain mutations are associated with certain types of cysts and could therefore help cyst characterization. 34,35 IPMNs are associated with mutations in KRAS, GNAS and RNF43; MCNs are associated with mutations in KRAS and RNF43, but not in GNAS; SCNs can present mutations in VHL; and solid pseudopapillary neoplasms are characterized by mutations in CTNNB1, but not in KRAS, GNAS, RNF43, or VHL. In addition, mutations in TP53, PIK3CA, PTEN, CDKN2A and SMAD4 are typical of advanced neoplasms in mucinous cysts, so molecular analysis could help both cyst characterization and identification of malignant cysts. 34,35
Currently, clinical practice guidelines recommend determination of KRAS and GNAS to identify mucinous lineage neoplasms in cases with doubtful diagnoses and in cases for which adequate characterization might modify treatment. 5,18 Use of other markers may not be necessary, although tests for specific markers may be useful in specific cases in the future.
Another currently available tool that does not increase adverse effects and which could improve diagnosis is contrast enhanced harmonic endoscopic ultrasound (CEH-EUS) which can detect vascularized structures (septa and nodules) even though cyst contents of mucus and detritus remains invisible. It is useful for differentiating wall nodules from mucin or debris accumulation and for differentiating cystic tumors from pseudocysts. All phases of pseudocysts except for the earliest do not absorb contrast and early phase pseudocysts can be enhanced. 36 Adequate detection of wall nodules is suboptimal with CT scans, MRI and EUS. 37 Several studies have shown that CEH-EUS detection is better than detection by these other methods, a point that is especially relevance for decisions about surgery for patients with IPMN or MCN since wall nodules are related to malignancy. 10,29,38 A recent study of 70 patients for whom surgical specimens were available found that the accuracy of preoperative EUS-CEH performed was superior to that of EUS mode B for determining whether a wall mural nodule was associated with malignancy. The sensitivity of both EUS-CEH and EUS was 97%, but the specificity of EUS-CEH was 75% and its accuracy was 84% while the specificity of EUS was only 40% and its accuracy was only 64%. 38 In addition, the study concluded that wall nodules detected with EUS-CEH that were more than 4 mm high were associated with malignancy (Odds ratio [OR]: 56) whereas, if measured with conventional EUS, the size should be greater (≥8 mm, OR: 15).
Treatment and follow up
While surgery is indicated in symptomatic cases, the difficulty lies in correctly detecting patients with malignant or high risk cysts. Pseudocysts and SCNs have little or no potential for malignancy, so treatment is reserved for symptomatic cases and for those in diagnostic doubt. 12 The Fukuoka, European and AGA guidelines recommend surgery for MCN, solid pseudopapillary neoplasms, MD-IPMN and mixed IPMN due to the risks of malignancy whenever the patient is a surgical candidate. 6,10,23 Nevertheless, there are differences in the thresholds of these recommendations in the case of BD-IPMN (Table 4). 1 For multifocal IPMNs, evaluation and treatment according to the individual characteristics of each lesion are recommended. 39
Table 4 Indications for surgical treatment according to the principal guidelines
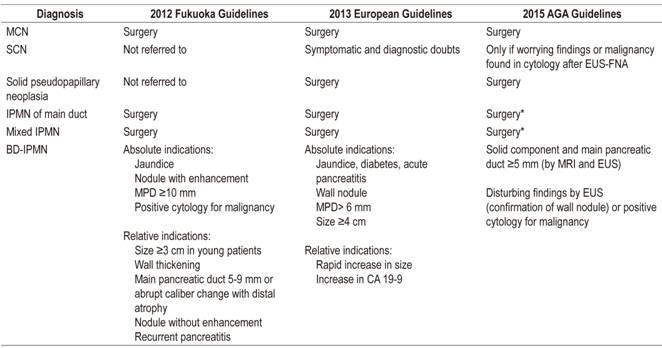
* Although it requires confirmation of solid nodule or positive cytology for malignancy in addition to dilating the MPD.
Whether or not follow-up is indicated depends on the type of cyst, an evaluation of its size and morphology, and MRI or EUS search for parameters suggesting early malignancy. Recommended follow-up intervals vary depending upon the guideline. The Fukuoka guide recommends follow-up with MRI or EUS with intervals depending on the size of the cysts. It recommends interrupting follow-up of resected MCNs and SCNs in the absence of invasive neoplasia. In contrast, the AGA recommends interrupting the follow-up of MCN, SCN and IPMN resected without high-grade dysplasia or invasive carcinoma as well as pancreatic cysts in the absence of changes after 5 years. 10,30
It is important to know that the simple presence of an IPMN implies a greater risk of pancreatic cancer than that of the general population. 7,13,31 Risks are up to 19.6 times higher. 2 According to various studies, patients treated for IPMNs risk recurrence and risk development of metachronous pancreatic adenocarcinoma in the remaining pancreas. 40 In addition, the risk of pancreatic adenocarcinoma continues more than 5 years of follow-up. 31 Consequently, long-term follow-up is recommended. 7 Therefore, multiple authors have criticized the guidelines of the AGA and propose an alternative approach that includes lowering the thresholds for performing EUS-FNA and for determination of molecular markers. 19,33
Conclusions
PCNs are frequently found by imaging tests but further characterization is important because of the potential malignancy of some PCNs. Initially, the diagnosis of pseudocyst should be excluded and cysts should be differentiated between mucinous neoplasms (IPMN and MCN) and serous neoplasms (SCN). In many cases, diagnosis by clinical and morphological criteria is not definitive, EUS-FNA followed by cytological and biochemical analysis becomes necessary. Molecular analysis in specialized centers is a tool that can, in the near future, help identify mucinous cysts in doubtful cases and can help detect malignancy. Once MD-IPMN, mixed IPMN or MCN has been diagnosed, surgery should be performed when possible. BD-IPMNs are managed according to the alarm criteria proposed by clinical practice guidelines. Except for symptomatic cases and cases with doubtful diagnoses, SCNs do not require treatment. It is currently recommended that follow-up of IPMNs not be interrupted even after surgical resection because of the risk of that a new IPMN or long-term pancreatic adenocarcinoma will develop.
Referencias
1. Chiang AL, Lee LS. Clinical approach to incidental pancreatic cysts. World J Gastroenterol. 2016;22(3):1236-45. doi: 10.3748/wjg.v22.i3.1236. [ Links ]
2. Munigala S, Gelrud A, Agarwal B. Risk of pancreatic cancer in patients with pancreatic cyst. Gastrointest Endosc. 2016;84(1):81-6. doi: 10.1016/j.gie.2015.10.030. [ Links ]
3. Kromrey ML, Bülow R, Hübner J, Paperlein C, Lerch MM, Ittermann T, et al. Prospective study on the incidence, prevalence and 5-year pancreatic-related mortality of pancreatic cysts in a population-based study. Gut. 2018;67(1):138-45. doi: 10.1136/gutjnl-2016-313127. [ Links ]
4. ASGE Standards of Practice Committee, Muthusamy VR, Chandrasekhara V, Acosta RD, Bruining DH, Chathadi KV, et al. The role of endoscopy in the diagnosis and treatment of cystic pancreatic neoplasms. Gastrointest Endosc . 2016;84(1):1-9. doi: 10.1016/j.gie.2016.04.014. [ Links ]
5. Dumonceau JM, Deprez PH, Jenssen C, Iglesias-Garcia J, Larghi A, Vanbiervliet G, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated January 2017. Endoscopy. 2017;49(7):695-714. doi: 10.1055/s-0043-109021. [ Links ]
6. Del Chiaro M, Verbeke C, Salvia R, Klöppel G, Werner J, McKay C, et al. European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis. 2013;45(9):703-11. doi: 10.1016/j.dld.2013.01.010. [ Links ]
7. Chernyak V, Flusberg M, Haramati LB, Rozenblit AM, Bellin E. Incidental pancreatic cystic lesions: is there a relationship with the development of pancreatic adenocarcinoma and all-cause mortality? Radiology. 2015;274(1):161-9. doi: 10.1148/radiol.14140796. [ Links ]
8. Basar O, Brugge WR. Pancreatic cyst guidelines: Which one to live by? Gastrointest Endosc . 2017;85(5):1032-1035. doi: 10.1016/j.gie.2016.11.003. [ Links ]
9. Lekkerkerker SJ, Besselink MG, Busch OR, Verheij J, Engelbrecht MR, Rauws EA, et al. Comparing 3 guidelines on the management of surgically removed pancreatic cysts with regard to pathological outcome. Gastrointest Endosc . 2017;85(5):1025-1031. doi: 10.1016/j.gie.2016.09.027. [ Links ]
10. Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12(3):183-97. doi: 10.1016/j.pan.2012.04.004. [ Links ]
11. Sugimoto M, Elliott IA, Nguyen AH, Kim S, Muthusamy VR, Watson R, et al. Assessment of a Revised Management Strategy for Patients With Intraductal Papillary Mucinous Neoplasms Involving the Main Pancreatic Duct. JAMA Surg. 2017;152(1):e163349. doi: 10.1001/jamasurg.2016.3349. [ Links ]
12. Jais B, Rebours V, Malleo G, Salvia R, Fontana M, Maggino L, et al. Serous cystic neoplasm of the pancreas: a multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas). Gut . 2016;65(2):305-12. doi: 10.1136/gutjnl-2015-309638. [ Links ]
13. Choi SH, Park SH, Kim KW, Lee JY, Lee SS. Progression of Unresected Intraductal Papillary Mucinous Neoplasms of the Pancreas to Cancer: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2017;15(10):1509-1520.e4. doi: 10.1016/j.cgh.2017.03.020. [ Links ]
14. Singh H, McGrath K, Singhi AD. Novel Biomarkers for Pancreatic Cysts. Dig Dis Sci. 2017;62(7):1796-1807. doi: 10.1007/s10620-017-4491-4. [ Links ]
15. Overbeek KA, Cahen DL, Canto MI, Bruno MJ. Surveillance for neoplasia in the pancreas. Best Pract Res Clin Gastroenterol. 2016;30(6):971-986. doi: 10.1016/j.bpg.2016.10.013. [ Links ]
16. Xu MM, Sethi A. Imaging of the Pancreas. Gastroenterol Clin North Am. 2016;45(1):101-16. doi: 10.1016/j.gtc.2015.10.010. [ Links ]
17. Brugge WR. Diagnosis and management of cystic lesions of the pancreas. J Gastrointest Oncol. 2015;6(4):375-88. doi: 10.3978/j.issn.2078-6891.2015.057. [ Links ]
18. European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut . 2018;67(5):789-804. doi: 10.1136/gutjnl-2018-316027. [ Links ]
19. Singhi AD, Zeh HJ, Brand RE, Nikiforova MN, Chennat JS, Fasanella KE, et al. American Gastroenterological Association guidelines are inaccurate in detecting pancreatic cysts with advanced neoplasia: a clinicopathologic study of 225 patients with supporting molecular data. Gastrointest Endosc . 2016;83(6):1107-1117.e2. doi: 10.1016/j.gie.2015.12.009. [ Links ]
20. Goh BK, Thng CH, Tan DM, Low AS, Wong JS, Cheow PC, et al. Evaluation of the Sendai and 2012 International Consensus Guidelines based on cross-sectional imaging findings performed for the initial triage of mucinous cystic lesions of the pancreas: a single institution experience with 114 surgically treated patients. Am J Surg. 2014;208(2):202-9. doi: 10.1016/j.amjsurg.2013.09.031. [ Links ]
21. Khashab MA, Kim K, Lennon AM, Shin EJ, Tignor AS, Amateau SK, et al. Should we do EUS/FNA on patients with pancreatic cysts? The incremental diagnostic yield of EUS over CT/MRI for prediction of cystic neoplasms. Pancreas. 2013;42(4):717-21. doi: 10.1097/MPA.0b013e3182883a91. [ Links ]
22. van der Waaij LA, van Dullemen HM, Porte RJ. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: a pooled analysis. Gastrointest Endosc . 2005;62(3):383-9. [ Links ]
23. Vege SS, Ziring B, Jain R, Moayyedi P; Clinical Guidelines Committee; American Gastroenterology Association. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148(4):819-22. doi: 10.1053/j.gastro.2015.01.015. [ Links ]
24. Fernández-del Castillo C, Adsay NV. Intraductal papillary mucinous neoplasms of the pancreas. Gastroenterology. 2010;139(3):708-713.e1-2. doi: 10.1053/j.gastro.2010.07.025. [ Links ]
25. Zikos T, Pham K, Bowen R, Chen AM, Banerjee S, Friedland S, et al. Cyst Fluid Glucose is Rapidly Feasible and Accurate in Diagnosing Mucinous Pancreatic Cysts. Am J Gastroenterol. 2015;110(6):909-14. doi: 10.1038/ajg.2015.148. [ Links ]
26. Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126(5):1330-6. [ Links ]
27. Nakai Y, Isayama H, Itoi T, Yamamoto N, Kogure H, Sasaki T, et al. Role of endoscopic ultrasonography in pancreatic cystic neoplasms: where do we stand and where will we go? Dig Endosc. 2014;26(2):135-43. doi: 10.1111/den.12202. [ Links ]
28. Springer S, Wang Y, Dal Molin M, Masica DL, Jiao Y, Kinde I, et al. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology. 2015;149(6):1501-10. doi: 10.1053/j.gastro.2015.07.041. [ Links ]
29. Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology . 2017;17(5):738-753. doi: 10.1016/j.pan.2017.07.007. [ Links ]
30. Lennon AM, Ahuja N, Wolfgang CL. AGA Guidelines for the Management of Pancreatic Cysts. Gastroenterology. 2015;149(3):825. doi: 10.1053/j.gastro.2015.05.062. [ Links ]
31. Date K, Ohtsuka T, Nakamura S, Mochidome N, Mori Y, Miyasaka Y, Oda Y, et al. Surveillance of patients with intraductal papillary mucinous neoplasm with and without pancreatectomy with special reference to the incidence of concomitant pancreatic ductal adenocarcinoma. Surgery. 2018;163(2):291-299. doi: 10.1016/j.surg.2017.09.040. [ Links ]
32. Basar O, Brugge WR. Which guidelines should be used for branch-duct intraductal papillary mucinous neoplasms? Gastrointest Endosc . 2016;84(3):446-9. doi: 10.1016/j.gie.2016.04.044. [ Links ]
33. Ridtitid W, DeWitt JM, Schmidt CM, Roch A, Stuart JS, Sherman S, et al. Management of branch-duct intraductal papillary mucinous neoplasms: a large single-center study to assess predictors of malignancy and long-term outcomes. Gastrointest Endosc . 2016;84(3):436-45. doi: 10.1016/j.gie.2016.02.008. [ Links ]
34. Jones M, Zheng Z, Wang J, Dudley J, Albanese E, Kadayifci A, et al. Impact of next-generation sequencing on the clinical diagnosis of pancreatic cysts. Gastrointest Endosc . 2016;83(1):140-8. doi: 10.1016/j.gie.2015.06.047. [ Links ]
35. Singhi AD, McGrath K, Brand RE, Khalid A, Zeh HJ, Chennat JS, et al. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut . 2018;67(12):2131-2141. doi: 10.1136/gutjnl-2016-313586. [ Links ]
36. Alvarez-Sánchez MV, Napoléon B. Contrast-enhanced harmonic endoscopic ultrasound imaging: basic principles, present situation and future perspectives. World J Gastroenterol. 2014;20(42):15549-63. doi: 10.3748/wjg.v20.i42.15549. [ Links ]
37. Farrell JJ, Fernández-del Castillo C. Pancreatic cystic neoplasms: management and unanswered questions. Gastroenterology. 2013;144(6):1303-15. doi: 10.1053/j.gastro.2013.01.073. [ Links ]
38. Kamata K, Kitano M, Omoto S, Kadosaka K, Miyata T, Yamao K, et al. Contrast-enhanced harmonic endoscopic ultrasonography for differential diagnosis of pancreatic cysts. Endoscopy. 2016;48(1):35-41. doi: 10.1055/s-0034-1393564. [ Links ]
39. Farrell JJ. Prevalence, Diagnosis and Management of Pancreatic Cystic Neoplasms: Current Status and Future Directions. Gut Liver. 2015;9(5):571-89. doi: 10.5009/gnl15063. [ Links ]
40. Miyasaka Y, Ohtsuka T, Tamura K, Mori Y, Shindo K, Yamada D, et al. Predictive Factors for the Metachronous Development of High-risk Lesions in the Remnant Pancreas After Partial Pancreatectomy for Intraductal Papillary Mucinous Neoplasm. Ann Surg. 2016;263(6):1180-7. doi: 10.1097/SLA.0000000000001368. [ Links ]
Received: April 04, 2018; Accepted: June 20, 2018











 texto em
texto em