Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.34 no.1 Bogotá ene./mar. 2019
https://doi.org/10.22516/25007440.202
Case report
A Case Report of Crohn’s Disease and Primary Small Bowel Lymphoma
1Especialista en medicina interna, gastroenterología y endoscopia digestiva. Hospital Pablo Tobón Uribe, Medellín, Colombia
This is a case report of Crohn’s Disease with a primary small bowel lymphoma and a literature review of the topic. The 57 year-old patient had suffered from abdominal pain and distention for almost one year prior to diagnosis. Endoscopic studies had found inflamed ulcers in the distal ileum stenosis in the ileocecal region. Pathological and imaging findings were compatible with stenosing Crohn’s disease of the distal ileum. Inflammation markers including fecal calprotectin (more than 20 times the normal value) were elevated. Optimal medical treatment including steroids, an immunomodulator, and biological therapy with tumor necrosis factor antagonist (AntiTNF) was administered, and patient’s initial response was good. Nevertheless, endoscopic and imaging follow-ups found a lesion in the distal ileum that appeared to be a tumor. Surgery found a neoplastic hematolymphoid tumor in the distal ileum.
Keywords: Crohn’s disease; immune-proliferative disease of the small intestine; intestinal obstruction; magnetic resonance imaging
Paciente de 57 años, con cuadro clínico de casi 1 año de evolución de dolor abdominal, distensión, en estudios endoscópicos con úlceras en íleon distal de aspecto inflamatorio y estenosis en región ileocecal, con hallazgos patológicos e imagenológicos compatibles con enfermedad de Crohn estenosante de íleon distal y elevación de marcadores de inflamación incluyendo calprotectina fecal (más de 20 veces del valor normal) se dejó en tratamiento médico óptimo incluyendo esteroides, inmunomodulador y terapia biológica con antagonista de factor de necrosis tumoral (anti-TNF), experimentó buena respuesta clínica inicial, pero en el control endoscópico y de imágenes hubo evidencia de lesión de aspecto tumoral en el íleon distal, por lo que se planteó el manejo quirúrgico, con el que se confirmó una lesión neoplásica de origen hematolinfoide (linfoma) en el íleon distal. Se hace reporte de caso y revisión de la literatura.
Palabras clave: Enfermedad de Crohn; enfermedad inmunoproliferativa del intestino delgado; obstrucción intestinal; imagen por resonancia magnética
Introduction
Crohn’s disease (CD) is characterized by discontinuous lesions and transmural inflammation. It can affect any part of the gastrointestinal tract, but its most common locations are the ileocolonic region and the small intestine which account for almost 70% of cases. 1,2 Almost 80% of cases from other countries and regions are inflammatory with smaller numbers of stenosing and fistulizing presentations. 3 A study carried out at the Hospital Pablo Tobón Uribe has found that almost a third of CD patients there had the stenosing variety which may be related to late diagnosis. 4 There is no single diagnostic gold standard for CD, and diagnosis is confirmed by summation of clinical, endoscopic, imaging and histological findings, together with results of biochemical tests. 5,6 Performance of an ileocolonoscopy is essential when the distal ileum is compromised, since it is superior to other diagnostic strategies. 5
Patients with CD have a higher risk of developing neoplasms in the digestive tract, especially adenocarcinoma and lymphomas in the small intestine, than does the general population. The problem is that primary lymphomas in the small intestine are difficult to diagnose in the context of CD because the symptoms are often similar to, or superimposed on, inflammatory activity.
We present the case of a patient whose CD diagnosis had been established by clinical, imaging, endoscopic and histological findings, who developed a lymphoma in the distal ileum.
Clinical case
A 57-year-old cattle rancher was evaluated in primary care for what he considered to be back pain of mechanical origin in November 2015. He was treated with 15 mg of meloxicam per day for almost 2 months. In February 2016, he felt worse due to the appearance of abdominal pain and discomfort in the lower hemiabdomen. Magnetic resonance imaging (MRI) studies of the abdomen found lumbar osteochondrosis T12-L1 without other abnormalities. Total colonoscopy showed a normal colon without ileocecal valve compromise, but with deep, fibrin covered fibrin ulcers in the distal ileum. Biopsies revealed active chronic inflammation of the ulcers but no granulomas. Blood tests were negative for antibodies against neutrophil cytoplasm (ANCA) and positive for anti-Saccharomyces cerevisiae antibodies (ASCA) but did not show anemia. Ulcerated ileitis was considered likely in relation to non-steroidal anti-inflammatory drugs (NSAIDs). Suspension of NSAIDs was recommended and daily treatment with 3 g oral mesalazine for 1 month was begun. Abdominal distension and pain improved during this period.
Three months later the patient reported recurrence of his symptoms appearance with increased intensity. Now they were associated with 2 to 5 bouts of diarrhea with spots of blood per day. During the patient’s evaluation by gastroenterology he said he had not lost weight, did not suffer from night sweats, and had no extradigestive symptoms. Nitazoxanide, a broad spectrum antiparasitic was prescribed. The total colonoscopy was repeated two months later. Blood tests at that time showed markedly elevated fecal calprotectin of 2,100 mg/kg of stool (reference value up to 50), normal blood count, an erythrocyte sedimentation rate (ESR) of 28 and C-reactive protein (CRP) of 1.58 (reference value up to 0.8).
A follow-up colonoscopy performed on January 10, 2017 found a circumferential ulcer in the ileocecal valve with severe edema, erythema, and congestion (severe inflammatory stenosis) plus additional ulcers in the distal ileum. Multiple biopsies were taken (Figure 1), and the patient was hospitalized with high suspicion of stenosing CD. Biopsies showed ulcerated mucosa constituted by a stroma of vascularized fibro-connective tissue with marked inflammatory changes. Focal architectural was characterized by flattened villi, brush edges, and inflammatory and necrotic tissue debris. A dense lymphoplasmacytic infiltrate with lymphoid aggregates, atrophic crypts and loss of goblet cells was found without evidence of parasites, granulomas, or dysplastic changes.
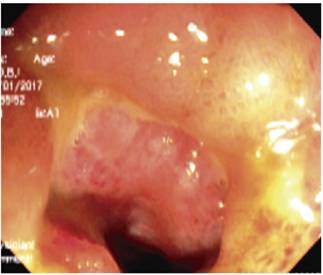
Figure 1 Severe inflammatory changes due to edema, erythema and a circumferential ulcer in the ileocecal valve and ulcers in the distal ileum.
Magnetic resonance (MR) enterography showed a marked concentric, asymmetric and irregular thickening of the submucosa of the distal ileal wall with extension to the ileocecal valve. The wall was estimated to be 1.4 cm thick with a longitudinal extension of 7 cm. Submucosal edema, restriction of diffusion and uniform post-contrast enhancement due to active inflammatory changes were found together with striation of mesenteric (brush sign) and non-necrotic adenopathies measuring less than 1 cm. No fibrosis was found (Figure 2). Tests for infectious diseases were negative.
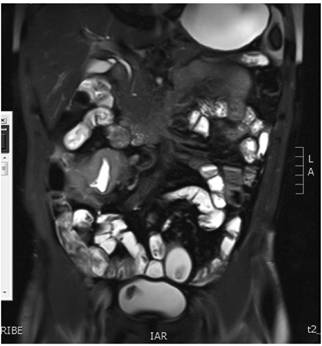
Figure 2 Magnetic resonance (MR) enterography showed marked thickening in the distal ileum with ulcerated areas and Crohn’s activity with inflammatory-fibrotic involvement.
Treatment with 50 mg of systemic hydrocortisone every 6 hours, azathioprine at 2 mg/kg/day, and anti-TNF (infliximab) at a dose of 5 mg/kg/dose (in induction schedule: weeks 0, 2 and 6) was begun. The patient’s clinical response was very good as shown by improvement of abdominal pain, distension and diarrhea. Acute phase reactants decreased, and the patient was discharged to be followed-up as an outpatient.
Four months later, the patient’s abdominal pain recurred, this time in association with bloating. Physical examination found a painful mass in the right iliac fossa. Total colonoscopy showed increased thickening of the distal ileum and a circumferential ulcer (Figure 3A), and a contrast abdominal CT scan showed a pseudotumor extending from the distal ileum to the ileocecal valve (Figure 3B). Ileocecal resection and a right hemicolectomy were performed with creation of an ileal transverse anastomosis. Almost 40 cm were resected (Figure 4). Pathology found an 8 cm diffuse large cell B lymphoma extending from mucosa to serosa. Resection margins were tumor free, and there was no vascular invasion, perineural invasion or lymph node involvement (Figure 5 A, B and C).
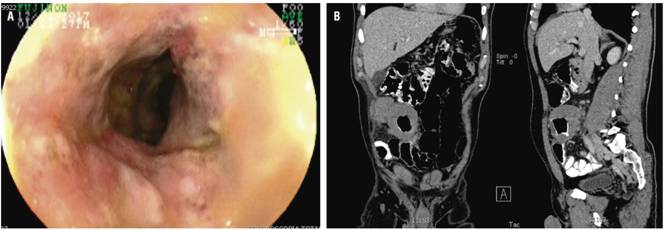
Figure 3 A. Increased thickening of wall in addition to severe inflammatory changes with circumferential ulceration in the ileocecal valve and distal ileum (4 months later). B. Contrast abdominal CT scan showing coronal section in which a tumor-like lesion is identified in the distal ileum.
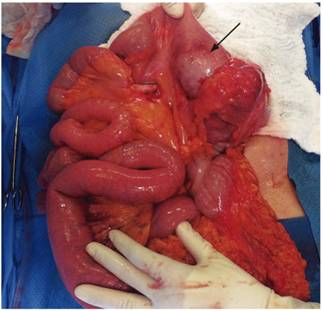
Figure 4 Photo during laparotomy in which a tumor-like lesion is identified. Ileocecal resection and a right hemicolectomy were performed with creation of an ileal transverse anastomosis.
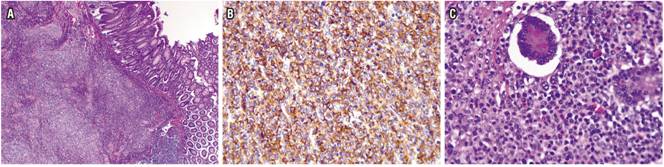
Figure 5 A. (4x) Diffuse neoplasm with focal ulceration that invades the entire thickness of the wall including the mucosa. B. Immunohistochemistry shows strong and diffuse positivity for CD20 in the membrane of the tumor lymphocytes. C. 40x photo shows multiple large neoplastic lymphocytes with vesicular nuclei in the mucosa. Lymphocytes are pleomorphic, have abundant mitotic figures, but there are no signs of compromised lymph node epithelium.
Outpatient blood tests and neck and chest CT scans ruled out lymph node involvement. Bone marrow aspiration also showed no evidence of compromise.
Discussion
The patient’s musculoskeletal symptoms were associated with abdominal pain and diarrhea and had serological, endoscopic and histological findings highly suggestive of stenosing CD with ileocecal involvement. This required treatment with biological anti-TNF and immunomodulatory therapy with azathioprine which led to notable clinical improvement. Nevertheless, endoscopic findings and images showed progressive worsening. After a follow-up months later found a palpable mass in the right iliac fossa, it was decided to perform laparoscopic surgical resection. A primary lymphoma of the small intestine was found in the surgical specimen.
Neoplasms of the small intestine are very rare, and their overall incidence is generally less than 1/100 000. 7 In the United States, small bowel cancer represents 0.4% of all neoplasms and accounts for only 0.2% of cancer deaths. 7,8 Primary small bowel lymphoma is even more unusual.
Patients with CD have a high risk of developing colorectal cancer (especially adenocarcinomas) and lymphomas in the small intestine (especially non-Hodgkin’s lymphoma in the terminal ileum), 9,10,11 but there is also data showing increasing incidence of small bowel adenocarcinoma among patients with CD. 12,13 Primary lymphoma in the small intestine is not easy to diagnose in patients with CD because the symptoms may be similar to, or superimposed on, inflammatory activity. Only the presence of a mass or signs of obstruction indicate this complication, as in the case presented.
As in ulcerative colitis, the risk of cancer in patients with CD correlates with the duration and extent of the disease, the presence of precancerous lesions (such as dysplasia) and age: it is more frequent when the diagnosis is at early ages. Population studies of patients with CD in the small intestine have estimated that the relative risk of carcinoma in the small intestine increases from 10 to 40 times. 13 The accumulated risk at 10 years has been estimated at 0.2% (95% confidence interval: 0.0 to 0.8) while the accumulated risk at 25 years has been estimated at 2.2% (95% CI: 0.7 to 6.4). 14
The most aggressive and most feared type of lymphoma during treatment with immunosuppressants in patients with IBD is hepatosplenic T-cell lymphoma which is usually fatal. Less than 30 cases have been reported worldwide. 15 Most of these were in men aged 12 to 58 years of age who were using treatment regimens combining anti-TNF and immunomodulators. Several other cases of young people on monotherapy with azathioprine have also been published. 15,16,17
In this case, the diagnosis of CD was based on clinical data, exams, images, and endoscopic and histological findings following recent recommendations. 5,6 Due to the evolution and findings in the first two colonoscopies showing no stenosis or mass, it was determined that the patient had developed the lymphoma in his small intestine as the result of the activity of CD. The striking feature of this case is the rapid progression to stenosis and the appearance of mass in such a short time.
A previous study had reported an average of 3.1 years of IBD at diagnosis of lymphoma and an average immunosuppressant treatment time prior to diagnosis of lymphoma of 20 months. 18) In that study, 782 patients with IBD, 238 of whom had immunosuppression, were followed up for eight years. Four cases with non-Hodgkin lymphoma were identified. All four had been treated with immunosuppressants: two with azathioprine, one with methotrexate and one with methotrexate and cyclosporine. The immunosuppression group had 59 times more risk of developing non-Hodgkin lymphoma than did the general population (p: 0.0001). 18 This clinical behavior indicates that the behavior of the lymphoma in our case was very aggressive. Many publications that have described how, in addition to the activity of the disease, the use of immunomodulators also increases the risk of lymphoma. 15,19-22
In this case, we are periodically conducting clinical follow-up by means MRI.
Calprotectin is a protein that binds to calcium and zinc, especially in the cytoplasm of cells that participate in the defense of pathogens such as neutrophils, monocytes and macrophages. Calprotectin shows bacteriostatic and fungistatic properties in vitro which underline its role in the attack of pathogens. Calprotectin accounts for up to 60% of the cytosolic protein in neutrophils, 23 but it has been observed to increase from 5 to 40 times under infectious and inflammatory conditions. Its levels are markedly elevated in the feces of patients with IBD. 24 It has an excellent negative predictive value in symptomatic patients and its positive predictive value is generally better than other markers of inflammation used. Its limitation is that it can also be elevated in other inflammatory conditions of the small intestine, 5 although there is no evidence that this marker rises significantly in intestinal lymphoma.
Conclusions
Neoplasms of the small intestine occur infrequently, and intestinal lymphoma is even rarer. Primary lymphoma of the small intestine may occur more frequently in the context of inflammatory diseases, and CD could significantly increase this risk. This is even more likely for stenosing CD that requires immunosuppressive therapies to control the disease.
Acknowledgements
We would like to thank Dr. Gabriel Varela Aguirre, a pathologist and oncologist at the Hospital Pablo Tobón Uribe in Medellin, Colombia, for his valuable collaboration and for the histopathology images from this case
REFERENCES
1. Garrido E, López A. Ileítis terminal: Diagnóstico diferencial. En: Marín-Jiménez I, Menchén-Viso L, Gomollón-García F (editores). Diagnóstico diferencial de la enfermedad inflamatoria intestinal. Elsevier; 2012. p. 23-32. [ Links ]
2. Hani A, Mosquera-Klinger G, Leguizamo AM. Presentación clínica de la enfermedad de Crohn. En: Aponte D, Gil F, Reyes G (editores). Diagnóstico en enfermedad inflamatoria intestinal. 1.a edición. Bogotá D. C.: Pulso ediciones S.L; 2014. p. 81-90. [ Links ]
3. Rieder F, Zimmermann EM, Remzi FH, Sandborn WJ. Crohn’s disease complicated by strictures: a systematic review. Gut. 2013;62(7):1072-84. doi: 10.1136/gutjnl-2012-304353. [ Links ]
4. Juliao F, Ruiz M, Flórez J, Donado J, Marín J, Arango C, et al. Fenotipo e historia natural de la enfermedad inflamatoria intestinal en un centro de referencia en Medellín-Colombia. Rev Colomb Gastroenterol. 2010;25(3):240-51. [ Links ]
5. Gomollón F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. J Crohns Colitis. 2017;11(1):3-25. doi: 10.1093/ecco-jcc/jjw168. [ Links ]
6. Yamamoto-Furusho JK, Bosques-Padilla F, de-Paula J, Galiano MT, Ibañez P, Juliao F, et al. Diagnosis and treatment of inflammatory bowel disease: First Latin American Consensus of the Pan American Crohn’s and Colitis Organisation. Rev Gastroenterol Mex. 2017;82(1):46-84. doi: 10.1016/j.rgmx.2016.07.003. [ Links ]
7. Schottenfeld D, Beebe-Dimmer JL, Vigneau FD. The epidemiology and pathogenesis of neoplasia in the small intestine. Ann Epidemiol. 2009;19(1):58-69. doi: 10.1016/j.annepidem.2008.10.004. [ Links ]
8. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71-96. doi: 10.3322/CA.2007.0010. [ Links ]
9. Hemminki K, Li X, Sundquist J, Sundquist K. Cancer risks in Crohn disease patients. Ann Oncol. 2009;20(3):574-80. doi: 10.1093/annonc/mdn595. [ Links ]
10. Holubar SD, Dozois EJ, Loftus EV Jr, Teh SH, Benavente LA, Harmsen WS, et al. Primary intestinal lymphoma in patients with inflammatory bowel disease: a descriptive series from the prebiologic therapy era. Inflamm Bowel Dis. 2011;17(7):1557-63. doi: 10.1002/ibd.21516. [ Links ]
11. Dawson MP, Cornes JS, Morson BC. Primary malignant lymphoid tumours of the intestinal tract. Report of 37 cases with a study of factors influencing prognosis. Br J Surg 1961;49:80-9. [ Links ]
12. Jess T, Loftus EV Jr, Velayos FS, Harmsen WS, Zinsmeister AR, Smyrk TC, et al. Risk of intestinal cancer in inflammatory bowel disease: a population-based study from olmsted county, Minnesota. Gastroenterology. 2006;130(4):1039-46. doi: 10.1053/j.gastro.2005.12.037. [ Links ]
13. Friedman S. Cancer in Crohn’s disease. Gastroenterol Clin North Am. 2006;35(3):621-39. doi: 10.1016/j.gtc.2006.07.008. [ Links ]
14. Jess T, Gamborg M, Matzen P, Munkholm P, Sørensen TI. Increased risk of intestinal cancer in Crohn’s disease: a meta-analysis of population-based cohort studies. Am J Gastroenterol. 2005;100(12):2724-9. doi: 10.1111/j.1572-0241.2005.00287.x. [ Links ]
15. Siegel C. Risk of lymphoma in inflammatory bowel disease. Gastroenterol Hepatol. 2009;5(11):784-90. [ Links ]
16. Mittal S, Milner BJ, Johnston PW, Culligan DJ. A case of hepatosplenic gamma-delta T-cell lymphoma with a transient response to fludarabine and alemtuzumab. Eur J Haematol. 2006;76(6):531-4. doi: 10.1111/j.1600-0609.2006.00646.x. [ Links ]
17. Navarro JT, Ribera JM, Mate JL, Granada I, Juncà J, Batlle M, et al. Hepatosplenic T-gammadelta lymphoma in a patient with Crohn’s disease treated with azathioprine. Leuk Lymphoma. 2003;44(3):531-3. Doi:10.1080/1042819021000035662. [ Links ]
18. Farrell RJ, Ang Y, Kileen P, O’Briain DS, Kelleher D, Keeling PW, et al. Increased incidence of non-Hodgkin’s lymphoma in inflammatory bowel disease patients on immunosuppressive therapy but overall risk is low. Gut . 2000;47(4):514-9. [ Links ]
19. Hecker R, Sheers R, Thomas D. Hodgkin’s disease as a complication of Crohn’s disease. Med J Aust. 1978;2(13):603. [ Links ]
20. Connell WR, Kamm MA, Dickson M, Balkwill AM, Ritchie JK, Lennard-Jones JE. Long-term neoplasia risk after azathioprine treatment in inflammatory bowel disease. Lancet. 1994;343(8908):1249-52. [ Links ]
21. Fraser AG, Orchard TR, Robinson EM, Jewell DP. Long-term risk of malignancy after treatment of inflammatory bowel disease with azathioprine. Aliment Pharmacol Ther. 2002;16(7):1225-32. [ Links ]
22. Kandiel A, Fraser AG, Korelitz BI, Brensinger C, Lewis JD. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut . 2005;54(8):1121-5. doi: 10.1136/gut.2004.049460. [ Links ]
23. Abraham BP, Kane S. Fecal markers: calprotectin and lactoferrin. Gastroenterol Clin North Am . 2012;41(2):483-95. doi: 10.1016/j.gtc.2012.01.007. [ Links ]
24. Konikoff MR, Denson LA. Role of fecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease. Inflamm Bowel Dis . 2006;12(6):524-34. [ Links ]
Received: January 29, 2018; Accepted: March 30, 2018











 texto en
texto en 


