Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.34 no.2 Bogotá Apr./June 2019
https://doi.org/10.22516/25007440.393
Original articles
A randomized controlled clinical trial of the efficacy and safety of colonoscopy preparation using a single four liter dose of polyethylene glycol (PEG) vs. two 2 liter doses of PEG vs. two low volume (1L + 1L) doses of PEG
1Servicio de Gastroenterología y Endoscopia Digestiva, Clínica Universitaria Colombia, Fundación Universitaria Sanitas. Bogotá, Colombia
2Grupo de Epidemiología y Evaluación en Salud Pública, Universidad Nacional de Colombia. Bogotá, Colombia
3Instituto de investigación Sanitas. Bogotá, Colombia
4Jefe de Gastroenterología, Clínica Colsanitas. Bogotá, Colombia
5Pediatra, Organización Sanitas internacional. Bogotá, Colombia
Introduction:
Colonoscopy is the gold standard for evaluation of the colonic mucosa. Colon cleansing in preparation for colonoscopy depends on finding of polyps which can be adenomatous with malignant potential and the possibility of degenerating into colon cancer.
Objective:
This study’s objective was to compare the efficacy and safety of three types of preparations for colon cleansing: a single four liter dose of polyethylene glycol (PEG) vs. two 2 liter doses of PEG vs. two low volume (1L + 1L) doses of PEG.
Methods:
This is a randomized controlled clinical trial of patients who underwent elective colonoscopy at a University clinic. It was blinded for the doctor who evaluated colon cleansing. Seventy four patients 74 patients were randomized into each group. The main parameter of effectiveness was integral preparation of adequate quality measured on the Boston scale. Secondary parameters were the percentage of adverse events, tolerability and detection rate of polyps.
Results:
Complete preparation of the entire colon was achieved significantly more often with 4 liters divided into two 2 liter doses followed by the other divided alternative (1 L + 1 L). It was achieved least frequently with in the single dose: 79.7%, 75.7% and 63.5%, respectively, p = 0.019. Differences were also found in the detection of polyps (13.5%, 24.3% and 9.5%, respectively, p = 0.037). ) There were no differences in presentation of at least one adverse event (p = 0.254) or in tolerability (p = 0.640).
Conclusions:
The two divided dose preparations had higher colon cleansing and polyp detection efficacies than did the single 4L dose while there were no differences in occurrence of adverse events and tolerability. The divided PEG 2L dose could be a very good option for elective colonoscopy preparation.
Keywords: Efficacy; safety; preparation; colon; polyethylene glycol; low volume
Introducción:
la colonoscopia es el examen estándar de oro para evaluar la mucosa del colon. De la limpieza del colon en la preparación intestinal para colonoscopia depende el hallazgo de pólipos, que pueden ser adenomatosos con potencial maligno y con la posibilidad de degenerarse en cáncer de colon.
Objetivo:
comparar la eficacia y la seguridad de tres tipos de preparaciones para la limpieza del colon: dosis única de polietilenglicol (PEG) 4 litros (4 L) y dosis divididas: PEG 4 L dividido (2 L + 2 L) y PEG 2 L dividido (1 L + 1 L) de volumen bajo.
Métodos:
en pacientes con una colonoscopia electiva de una clínica universitaria, se realizó un ensayo clínico controlado aleatorizado y ciego (para el médico que evaluó la limpieza del colon). Se asignaron 74 pacientes para cada grupo. El parámetro principal de eficacia fue la preparación integral de calidad adecuada medida con la escala de Boston, y los parámetros secundarios fueron el porcentaje de eventos adversos, la tolerabilidad y la tasa de detección de pólipos.
Resultados:
la preparación completa de todo el colon fue significativamente de mayor la calidad en la alternativa de 4 L divididos (2 L + 2 L), seguida de la otra alternativa dividida (1 L + 1 L) y menor en la dosis única (79,7 %, 75,7 % y 63,5 %, respectivamente, p = 0,019); también se encontraron diferencias en la detección de pólipos (13,5 %, 24,3 % y 9,5 %, p = 0,037) y sin diferencias en la presentación de al menos un evento adverso (p = 0,254) ni en la tolerabilidad (p = 0,640).
Conclusiones:
las dos preparaciones de dosis dividida tienen una mayor eficacia en la limpieza del colon en comparación con la dosis única de 4 L y en la detección de pólipos, mientras que no se evidencian diferencias en las preparaciones para la ocurrencia de eventos adversos y la tolerabilidad. La dosis de PEG 2 L dividido puede ser una muy buena opción para las preparaciones de colonoscopia electiva.
Palabras clave: Eficacia; seguridad; preparación; colon; polietilenglicol; bajo volumen
Introduction
Colonoscopy, the gold standard for evaluating the mucosa of the colon,1,2 is especially important for finding polyps which decreases the incidence and mortality from colon cancer since adenomatous polyps are potentially malignant.3,4. Colon cancer is the second leading cancer cause of death in women and the third in men. 5
Effective preparation for colonoscopy is important because it allows a gastroenterologist to efficiently detect more polyps and other pathologies of the colon.2,6 The degree of cleanliness of the colon determines the success of colonoscopy. 7 All colonoscopy examinations should state the quality of the colon preparation. The quality criterion should be to achieve good or very good preparation as measured by the Boston Bowel Preparation Scale (BBPS) in more than 95% of the explorations. 8-14
Intestinal preparations are evaluated on the bases of three criteria: efficacy, safety and tolerability. The efficacy of different bowel preparation regimens has been assessed and quantified in several studies, systematic reviews and meta-analyzes. Differences of regimens, dosages, dietary restrictions, patient characteristics, adjuvant agents and assessment methods among the various the studies have led controversy regarding their results. In 2014, the guidelines of the Multi-Society Task Force on Colorectal Cancer (MSTF) in the United States stated that a divided 4 liter dose of PEG with electrolytes provides high quality preparation. The guidelines also indicate that low-volume two liter formulations of PEG achieve intestinal cleansing in healthy patients without constipation and that the results are not inferior to the 4 L formulation. 10 This was supported by a recent metaanalysis of 47 randomized controlled clinical trials with 13,487 patients. It compared a single dose of PEG taken the day before colonoscopy with a divided dose of PEG (Odds ratio [OR]: 2.51; 95% confidence interval [CI]: 1.86-3.39) 15. Tolerability was better with 2 L PEG than with 4 L PEG (OR: 2.23; 95% CI 1.67-2.98). 2,10,15,16 The metaanalysis concluded that more uniform definitions should be developed through studies with parameters such as adverse effects, polyps, detection of adenomas and return to daily activities. 15
In Colombia, there are only a few studies of colon preparation for colonoscopy. A randomized, double-blind, cost-effectiveness study compared PEG and mannitol in a fourth-level hospital in Bogotá and concluded that both intestinal preparations for diagnostic colonoscopy provide similar colonic cleansing results. They are both safe, reliable and well-tolerated treatments, but Mannitol costs significantly less. 17 Efficacy was not compared.
At the Clínica Universitaria Colombia, PEG is the drug of choice for the gastroenterology service because it has good cleaning efficacy and is very safe for patients with fecal occult blood, digestive bleeding, chronic diarrhea, abdominal pain, and irritable bowel symptoms as well as being sage for colon cancer screening. 18,19
Because of the need to optimize quality of preparation, a randomized blinded clinical trial was designed to evaluate the efficacy and safety of colon cleansing with three different PEG preparations including the low volume 2 L divided PEG dose (Two one liter doses each with two envelopes of PEG 3350).
Materials and methods
Study Design
This is a randomized, blind, parallel, controlled clinical trial that evaluates the efficacy and safety of three preparations: 4 L PEG in a single dose, 4 L PEG divided (2 L + 2 L) and divided 2 L PEG (1 L + 1 L). Participants were equally allocated among the 3 groups (74:74:74). The doctor who evaluated colon cleansing using the BBPS did not know which preparation had been used.
Patients
Patients who were 18 to 75 years whose attending physician prescribed colonoscopy due to occult fecal blood, digestive bleeding, diarrhea, abdominal pain, and/or irritable bowel symptoms or for screening and who signed an informed consent form were included. Patients were excluded because of pregnancy, lactation, nausea, chronic vomiting, intestinal obstruction, neurological hypomotility syndrome, severe constipation (less than one deposition per week), colon resection> 50%, known allergy at PEG, major psychiatric disease, history of gastroparesis diagnosed by scintigraphy, and chronic renal failure under treatment by hemodialysis. Patients were selected from the gastroenterology department of the Clínica Universitaria Colombia, a fourth level hospital.
Result Variables
Primary Parameters of the Study
The primary parameters of this study were total scores on the BBPS by segments and integrally (the sum of the three segments) were used. Scores of six or higher were defined as adequate preparation while those under six were defined as inadequate preparation.
Secondary Study Parameters
Secondary study parameters were the percentage of adverse events, the rate of detection of adenomas (polyps) and the percentage of tolerability for the preparation of colon cleansing reported by the patient.
Sample Size
Sample size estimation for evaluation of differences among the three types of preparations was determined at a difference between the minimum preparation or equal preparations of 20% with a reliability of 95% and a power of 90%. The minimum size in the three groups was calculated at 74 (74:74:74) and with a loss adjustment of 10% (82:82:82).
Randomization
The biostatistical epidemiological method of permuted block randomization of patients was used. One was added to an evenly distributed random number between 0 and 1 that had been multiplied by six. Then it was rounded off to the lowest whole number. The possible permutations of the 3 study groups (1. ABC, 2. ACB, 3. BAC, 4. BCA, 5. CAB, 6. CBA) were taken into account. Then, a sequence of 74 random numbers between 1 and 6 were generated in Excel 2013 to obtain 74 random triples.
Three strategies were used to identify patients: 1) outpatients for whom a gastroenterologist indicated a need for colonoscopy; 2) telephone calls to patients scheduled for colonoscopy; and 3) email to patients scheduled for colonoscopy with subsequent telephone explanation. Patients who entered the gastroenterology service consecutively and met the selection criteria were randomly assigned the permutations chosen. Once the patient met the selection criteria including signed informed consent, the investigator gave her or him a sealed and numbered envelope with the previously randomized preparation (which the doctor who assessed the degree of colon cleansing did not know). Subsequently, s/he was given the data collection form which had been evaluated and approved by the Ethics and Research Committee. Forms were filled out and delivered by the patient on the day colonoscopy was performed.
Interventions: All preparations evaluated used either Nulytely® or Klean-Prep® PEG 3350.
Group 1: 4 L PEG divided (2 L + 2 L)
Patients were instructed to dissolve one envelope of PEG in 1 L of water and another envelope of PEG in another liter of water and take them at 8:00 pm the night before the examination. Then the instructions called for patients to repeat this procedure at 3:00 am if colonoscopy was scheduled in the morning. If the colonoscopy was scheduled in the afternoon, the patients were instructed to repeat the procedure at or after 8:00 am.
Group 2: 2 L PEG divided (low volume) (1 L + 1 L)
Patients were instructed to dissolve 2 envelopes of PEG in 1 L of water and take them at 8:00 pm the night before the examination. at 8:00 pm the night before the examination. Then the instructions called for patients to repeat this procedure at 3:00 am if colonoscopy was scheduled in the morning. If the colonoscopy was scheduled in the afternoon, the patients were instructed to repeat the procedure at 10:00 am.
Group 3: 4 L PEG in a single dose
Patients were instructed to individually dissolve 4 envelopes of PEG in 4 L of water. In other words, each envelope of PEG was to be dissolved in one L of water separately from the other three. Then the instructions called for patients to drink all four liters of water with PEG at 8:00 pm the night before the examination, if it had been scheduled for the morning. If the colonoscopy was scheduled in the afternoon, the patients were instructed to drink all four liters of water at 6:00 am on the day of the procedure.
As recommended by the guidelines of the American Society of Anesthesiologists (ASA), patients were asked not to consume any food orally for at least 4 hours prior to the procedure to avoid the risk of aspiration associated with sedation. 2
Once the colonoscopy had been performed, the doctor who performed the procedure evaluated the cleanliness of the colon according to the BBPS. Scores from 0 to 3 were assigned with 0 indicating inadequate, 1 indicating bad, 2 indicating good and 3 indicating excellent. Cohen’s kappa coefficient, a measure of intra-observer reliability, was 0.77. 3,12,13.
Data Collection Instrument
The data collection form included information on sex, age, comorbidities, abdominal surgery, type of preparation, evaluation of colon cleansing in three segments according to the BBPS, type of doctor who performed the colonoscopy (gastroenterology fellow or, gastroenterologist). It also included a subjective questionnaire about adverse events including abdominal distension, abdominal pain, vomiting, sleep disturbance and work or school absenteeism, a subjective rating of preparation tolerability of good, tolerable, bad or very bad, and questions about constipation. These questions asked about frequency of bowel movements (defining constipation as a bowel movement once every three or more days), hard feces, excessive effort, and need for digital manipulation to facilitate evacuation. Finally, the form included body mass index (BMI) and whether or not polyps were found during colonoscopy
Ethical Considerations
The clinical trial protocol was approved by the Ethics and Research Committee of the Fundación Universitaria Sanitas and the Organización Sanitas Internacional (CEIFUS 2748-16 of February 19, 2016). Written informed consent was obtained from all patients who participated in the study.
Statistical Analysis
For qualitative variables, simple frequencies and percentages were used to describe clinical and demographic characteristics. For quantitative variables, measures of central tendency (averages and medians) and measures of dispersion (standard deviation and range) were used. Normality of the distributions of numerical variables was evaluated with Kolmogórov-Smirnov tests and the Shapiro-Wilk test. Homogeneity of variances was assessed with Levene’s test. Kruskal-Wallis non-parametric analysis of variance (ANOVA) and multiple KW comparisons were also used. Pearson’s χ² test was used to measure differences in proportions of qualitative variables among the three preparations, and exact likelihood tests were used to measure expected values less than five. The information was systematized in an Excel 2016 database and debugged and processed with SPSS version 23 (IBM) and Stata 14.0.
Results
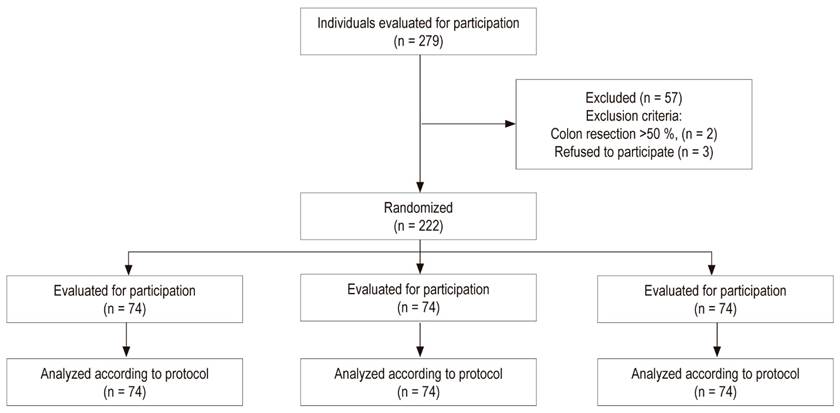
A total of 279 patients were evaluated. The effective sample size was 222 patients randomized into three groups (Figure 1).
Demographic and Clinical Characteristics
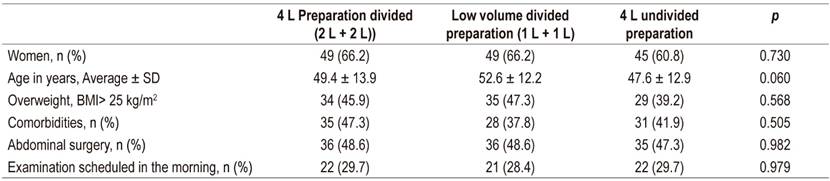
In the total study population, 60.8% of the study participants were women, and the average age of participants was 49.9 ± 13.1 years. Table 1 shows that there are no significant differences among the groups in terms of demographic and clinical characteristics of the patients included in the study.
Efficacy
Preparation Quality
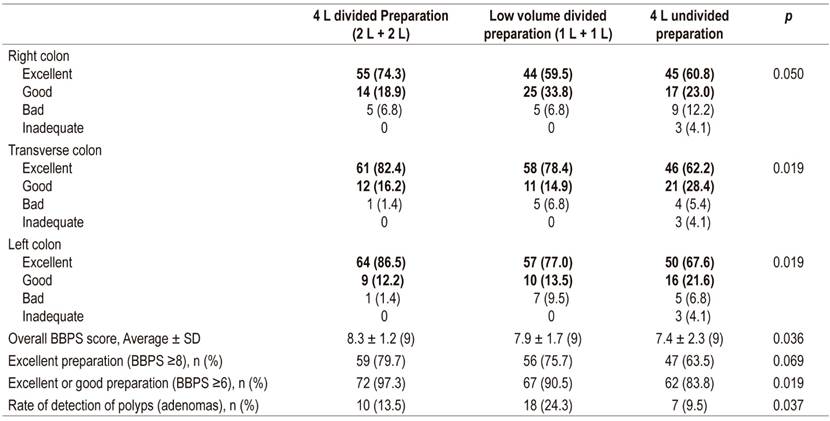
Cleaning quality was significantly better in the transverse and left colon with the two divided dose preparation schemes than in the single dose scheme. No statistically significant differences were found between the divided dose alternatives. In the right colon, the cleaning quality was better in the 2 L + 2 L divided dose group than in the other two schemes.
Statistically significant differences were found among the three schemes in the overall BBPS (sum of three segments). The highest score was in group 2 L + 2 L and the biggest difference was between that group and the 4 L group. There were no statistically significant differences between the two divided dose groups (2 L + 2 L vs. 1 L + 1 L).
The percentage of excellent or good results for the overall BBPS (≥6) was significantly higher in the 2 L + 2 L alternative, followed by the other divided alternative (1 L + 1 L), and lowest in the single dose (4 L) alternative (Table 2). Statistically significant differences were found in the polyp detection rate. Alternative 1 L + 1 L had the highest detection rate (Table 2).
Safety
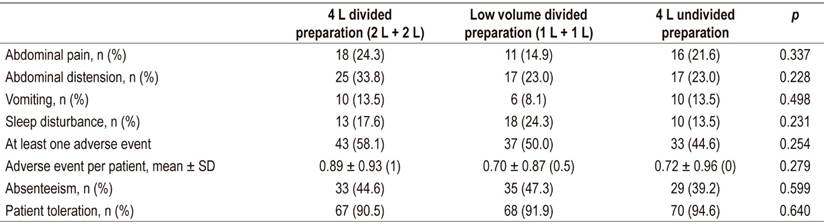
At least one adverse event was reported in 113 patients (50.9%). Descriptions of the various adverse events and their frequencies are presented in Table 3. School or work absenteeism was reported for 97 patients (43.7%), and abdominal distention and pain were the most frequently reported events. No statistically significant differences were observed in the study preparation schemes and no differences were found per individual event (when at least one adverse event or an average of adverse events was reported) (Table 3).
Table 3 Reported adverse effects and patient tolerability of colon preparation
SD: standard deviation
The overall tolerability scores of the scale subjectively measured in the data collection questionnaire as good, tolerable, bad or very bad were high, and there were no significant differences among the three schemes.
Discussion
This study was a randomized controlled clinical trial in which the BBPS was used for assessment. The overall sample included 222 patients and had a power of 90%, 95% reliability, and high quality information. Noninferiority of divided dose regimens with PEG for elective colonoscopy was evidenced. Four liter (2 L + 2 L) and low volume (1 L + 1 L) have divided doses high efficacy and safety profiles and have greater efficacy than do single doses. 15 For the transverse and left colon, the scores of the BBPS are better for split-dose regimens than for the single dose 4 L regimen. This is an important result, because divided doses could become the recommended system for colon cleansing before elective colonoscopy, as shown by the studies by Martel et al. 15 (OR 2.51; 95% CI 1.86- 3.39), Téllez-Ávila et al. 20 (p = 0.045) and Kilgore et al. 19 (OR 3.70; 95% CI 2.79-4.91) and as already shown in literature. 18,21-24
The efficacy of the low volume regimen (1 L + 1 L) is comparable with that of the normal divided volume (2 L + 2 L) for the preparation of the colon in elective colonoscopy. It is important to note that our Spanish and English literature search found no studies on low volume divided PEG regimens, which makes this study novel.
No statistically significant differences were found in colon cleansing between the two divided dose regimens for any of colon segments, nor were statistically significant differences found for frequency of adverse effects among the three regimens that were compared. This result would indicate that low volume alternatives can be recommended equally. To our knowledge, this is the first study that evaluates a divided low volume dose of 2 L. It shows no inferiority with respect to the alternatives evaluated. In the literature, only single low volume doses have been evaluated. They were found to be more effective and to have less adverse effects in the article published by Téllez-Ávila et al. in Mexico. They concluded that divided-dose and low-volume preparations were better than a single 4 L dose the day before the examination. 20
Our study found significant differences in the rates of detecting adenomas (p = 0.037) between the 2 L + 2 L and the 4 L single dose schemes. The detection of adenomas was higher in divided doses, especially in the 1 L + 1 L scheme. This finding should be subjected to additional analyses because the differences between the divided preparations cannot be attributable to the differences in the quality of the preparation as they were similar in the two divided dose groups.
Limitations
This analysis has some limitations. First, it was not possible to blind the patients to the alternative that they used even though the endoscopist was blinded at the time of the evaluation of the scale. Second, the patients took the preparation at home without direct control by researchers. Nevertheless, this mitigated by strict control and telephone and email follow-ups to remind, advise and guarantee compliance with instructions. Third, tolerability was assessed with a scale that had not been validated, so its results should be interpreted with caution. However, the main outcome, colon cleansing, was assessed with a validated instrument, the BBPS.
Conclusions
The two divided dose schemes, 4 L (2 L + 2 L) and low volume 2 L (1 L + 1 L), were most effective for colon cleansing according to the overall BBPS scores. No differences in safety were found between divided dose and single dose preparations. Both divided dose preparations were better than the single 4L dose given the day before the colonoscopy was performed. Polyps detection was greatest with the divided 2 L + 2 L dose.
REFERENCES
1. Wexner SD, Beck DE, Baron TH, Fanelli RD, Hyman N, Shen B, et al. A consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Gastrointest Endosc. 2006 Jun;63(7):894-909. https://doi.org/10.1016/j.gie.2006.03.918 [ Links ]
2. ASGE Standards of PracticeCommittee, Saltzman JR, Cash BD, Pasha SF, Early DS, Muthusamy VR, et al. Bowel preparation before colonoscopy. Gastrointest Endosc. 2015 Apr;81(4):781-94. https://doi.org/10.1016/j.gie.2014.09.048 [ Links ]
3. Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013 Sep 19;369(12):1095-105. https://doi.org/10.1056/NEJMoa1301969 [ Links ]
4. Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, et al. Colonoscopic Polypectomy and Long-Term Prevention of Colorectal-Cancer Deaths. N Engl J Med 2012; 366:687-696. https://doi.org/10.1056/NEJMoa1100370 [ Links ]
5. International Agency for Research on Cancer, World Health Organization. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Globocan [Internet]. 2012;1-6. Disponible en: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx? [ Links ]
6. Ávila Á, Parada JL, Benítez S. Preparación intestinal colónica con polietilenglicol y manitol: efectividad según la escala de Boston. Gen. Sociedad Venezolana de Gastroentereología. 2013;67(2):76-81. [ Links ]
7. Lichtenstein G. Bowel preparations for colonoscopy: a review. Am J Health Syst Pharm. 2009 Jan 1;66(1):27-37. https://doi.org/10.2146/ajhp080084 [ Links ]
8. Calderwood AH, Jacobson BC. Comprehensive validation of the Boston Bowel Preparation Scale. Gastrointest Endosc. 2010 Oct;72(4):686-92. https://doi.org/10.1016/j.gie.2010.06.068 [ Links ]
9. Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009 Mar;69(3 Pt 2):620-5. https://doi.org/10.1016/j.gie.2008.05.057 [ Links ]
10. Cohen LB. Advances in bowel preparation for colonoscopy. Gastrointest Endosc Clin N Am. 2015 Apr;25(2):183-97. https://doi.org/10.1016/j.giec.2014.11.003 [ Links ]
11. Lorenzo-Zúñiga V, Moreno-de-Vega V, Boix J. Preparation for colonoscopy: types of scales and cleaning products. Rev Esp Enferm Dig. 2012 Aug;104(8):426-31. https://doi.org/10.4321/S1130-01082012000800006 [ Links ]
12. González-Huix Lladó F, Figa Francesch M, Huertas Nadal C. Essential quality criteria in the indication and performance of colonoscopy. Gastroenterol Hepatol. 2010 Jan;33(1):33-42. https://doi.org/10.1016/j.gastrohep.2009.02.014 [ Links ]
13. Morán Sánchez S, Torrella E, Esteban Delgado P, Baños Madrid R, García A, Ono A, et al. Colonoscopy quality assessment. Rev Esp Enferm Dig. 2009 Feb;101(2):107-12, 112-6. [ Links ]
14. Jover R, Herráiz M, Alarcón O, Brullet E, Bujanda L, Bustamante M, et al. Clinical practice guidelines: quality of colonoscopy in colorectal cancer screening. Endoscopy. 2012 Apr;44(4):444-51. https://doi.org/10.1055/s-0032-1306690 [ Links ]
15. Martel M, Barkun AN, Menard C, Restellini S, Kherad O, Vanasse A. Split-Dose Preparations Are Superior to Day-Before Bowel Cleansing Regimens: A Meta-analysis. Gastroenterology. 2015 Jul;149(1):79-88. https://doi.org/10.1053/j.gastro.2015.04.004 [ Links ]
16. Xie Q, Chen L, Zhao F, Zhou X, Huang P, Zhang L, et al. A meta-analysis of randomized controlled trials of low-volume polyethylene glycol plus ascorbic acid versus standard-volume polyethylene glycol solution as bowel preparations for colonoscopy. PLoS One. 2014 Jun 5;9(6):e99092. https://doi.org/10.1371/journal.pone.0099092 [ Links ]
17. Forero E, Cardona H, Reyes G, Abello H, Rosas M, Sánchez C. Preparación intestinal para colonoscopia; comparación entre polietilenglicol y manitol: Estudio de costo efectividad, doble ciego aleatorizado. Rev Col Gastroenterol. 2005 Dec;20(4):60-71. [ Links ]
18. Sharara AI, Abou Mrad RR. The modern bowel preparation in colonoscopy. Gastroenterol Clin North Am. 2013 Sep;42(3):577-98. https://doi.org/10.1016/j.gtc.2013.05.010 [ Links ]
19. Kilgore TW, Abdinoor AA, Szary NM, Schowengerdt SW, Yust JB, Choudhary A, et al. Bowel preparation with split-dose polyethylene glycol before colonoscopy: a meta-analysis of randomized controlled trials. Gastrointest Endosc. 2011 Jun;73(6):1240-5. https://doi.org/10.1016/j.gie.2011.02.007 [ Links ]
20. Téllez-Ávila FI, Murcio-Pérez E, Saúl A, Herrera-Gómez S, Valdovinos-Andraca F, Acosta-Nava V, et al. Efficacy and tolerability of low-volume (2 L) versus single- (4 L) versus split-dose (2 L + 2 L) polyethylene glycol bowel preparation for colonoscopy: randomized clinical trial. Dig Endosc. 2014 Nov;26(6):731-6. https://doi.org/10.1111/den.12265 [ Links ]
21. El Sayed AM, Kanafani ZA, Mourad FH, Soweid AM, Barada KA, Adorian CS, et al. A randomized single-blind trial of whole versus split-dose polyethylene glycol-electrolyte solution for colonoscopy preparation. Gastrointest Endosc. 2003 Jul;58(1):36-40. https://doi.org/10.1067/mge.2003.318 [ Links ]
22. Aoun E, Abdul-Baki H, Azar C, Mourad F, Barada K, Berro Z, et al. A randomized single-blind trial of split-dose PEG-electrolyte solution without dietary restriction compared with whole dose PEG-electrolyte solution with dietary restriction for colonoscopy preparation. Gastrointest Endosc. 2005 Aug;62(2):213-8. https://doi.org/10.1016/S0016-5107(05)00371-8 [ Links ]
23. Adams WJ, Meagher AP, Lubowski DZ, King DW. Bisacodyl reduces the volume of polyethylene glycol solution required for bowel preparation. Dis Colon Rectum. 1994 Mar;37(3):229-33; discussion 233-4. https://doi.org/10.1007/BF02048160 [ Links ]
24. Sharma VK, Chockalingham SK, Ugheoke EA, Kapur A, Ling PH, Vasudeva R, et al. Prospective, randomized, controlled comparison of the use of polyethylene glycol electrolyte lavage solution in four-liter versus two-liter volumes and pretreatment with either magnesium citrate or bisacodyl for colonoscopy preparation. Gastrointest Endosc. 1998 Feb;47(2):167-71. https://doi.org/10.1016/S0016-5107(98)70351-7 [ Links ]
Received: September 13, 2018; Accepted: March 12, 2019











 text in
text in