Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.34 no.2 Bogotá Apr./June 2019
https://doi.org/10.22516/25007440.394
Review articles
Gastric cancer is a preventable disease: Strategies for intervention in its natural history
1Especialista en Cirugía General, Gastroenterología y Endoscopia Digestiva. Instituto Nacional de Cancerología. Bogotá D. C., Colombia
2Médico Cirujano Especialista en Psiquiatría, Magíster en Epidemiología Clínica y Especialista en Estadística. Universidad Nacional de Colombia e Instituto Nacional de Cancerología. Bogotá D. C., Colombia
Gastric cancer is a public health problem, but there are no usable mortality and survival statistics for Colombia. The country has no early diagnosis program or strategy, and gastric cancer is not prioritized as a health problem. Existing studies show that most patients are in advanced stages by the time they are diagnosed.
Ninety percent of gastric cancers are considered to be consequences of long inflammatory processes in the gastric mucosa. H Pylori infections are the most common etiology of gastritis which can progress to atrophy, metaplasia, dysplasia and cancer. Gastric atrophy establishes a cancerization field which is prone to molecular and phenotypic changes that end in cancerous growth. It is well understood that a disease’s natural history provides a rational pathological clinical understanding for primary and secondary prevention strategies. Well-established evidence shows that the combination of primary (H pylori eradication) and secondary strategies (diagnosis and endoscopic follow-up of pre-malignant lesions) can prevent or limit the progression of gastric carcinogenesis. The risk of gastric cancer associated with H pylori gastritis can be stratified according to the severity and extent of atrophy of the gastric mucosa. This approach has been adapted to many different countries according to specific incidences of gastric cancer, socio-economic conditions and cultural factors. This requires the complementary participation of gastroenterologists, surgeons, oncologists and pathologists.
In the face of this public health problem, there has been no action by health authorities or the medical association. For this reason, we have reviewed management strategies that allow intervening into the natural history of the disease to reduce its incidence and mortality rate.
The implementation and standardization of these management strategies in our environment may benefit patients who are at high risk for gastric cancer. These strategies can be implemented in a rational way, similar to what is being done with rectal cancer, in countries without screening programs all over the world.
Keywords: Gastric cancer; helicobacter pylori; prevention; natural history
El cáncer gástrico es un problema de salud pública. Las cifras de mortalidad y supervivencia son impresentables en nuestro país. En Colombia no existe ningún programa ni estrategias de diagnóstico temprano, ni tampoco es priorizado como un problema de salud. Los trabajos existentes demuestran que la mayoría de los pacientes cuando son diagnosticados presentan estadios avanzados.
Un 90 % de los canceres gástricos se consideran consecuencia de un largo proceso inflamatorio sobre la mucosa gástrica. La infección por Helicobacter pylori es la principal etiología de la gastritis, la cual puede progresar a atrofia, metaplasia, displasia y cáncer. La atrofia gástrica establece un campo de cancerización más propenso a cambios moleculares y fenotípicos que terminan en un cáncer en crecimiento. Es claro que la historia natural proporciona un racional entendimiento clínico patológico para estrategias de prevención primaria y secundaria. Una evidencia bien establecida demuestra que la combinación de las estrategias primarias (erradicación del H. pylori) y secundarias (diagnóstico y seguimiento endoscópico de lesiones premalignas) pueden prevenir o limitar la progresión de la carcinogénesis gástrica. El riesgo de cáncer gástrico asociado con la gastritis por H. pylori puede ser estratificado de acuerdo con la gravedad y extensión de la atrofia de la mucosa gástrica. Esta aproximación está adaptada a diferentes países, de acuerdo con su incidencia específica de cáncer gástrico, condición socioeconómica y factores culturales, que requiere de la participación complementaria de los gastroenterólogos, los cirujanos, los oncólogos y patólogos.
Frente a este problema de salud pública no hay ninguna acción por parte de las autoridades de salud ni del gremio médico. Por tanto, se revisan las estrategias de manejo que permitan intervenir la historia natural de la enfermedad con el objetivo de disminuir la incidencia y mortalidad.
La implementación y estandarización de estas estrategias de manejo en nuestro medio podrán beneficiar a los pacientes con riesgo incrementado para cáncer gástrico y pueden implementarse (en países sin programas de tamizaje) de una forma racional, similar a como se está haciendo con el cáncer colorrectal, en todo el mundo.
Palabras clave: Cáncer gástrico; Helicobacter pylori; prevención; historia natural
Introduction
All around the world, gastric cancer (GC) is a public health problem despite its decreasing incidence and mortality rate. 1 According to GLOBOCAN, 1,033,701 new cases of GC occurred and more than 782,685 deaths due to this disease occurred in 2018. 2 GC represents 5.7% of all new cancer cases and 8.2% of total cancer deaths in the world. 2,3 Japan and Korea have in the world’s highest incidences. High incidence areas are Asia, Eastern Europe, South America and Central America while low incidence areas are South Asia, North and East Africa, North America, Australia and New Zealand. 4 In Japan, where GC remains the most common type of cancer in both men and women, the incidence figures are 69.2/100,000 inhabitants and 28.6/100,000 inhabitants, respectively 4.
According to GLOBOCAN, 7,419 new cases of GC (7.3%) were detected in Colombia in 2018. Of these, 5,505 died. GC ranked third for the year, after breast and prostate cancer. It was followed by lung and colorectal cancer. For 2018, GC represented the first cause of cancer mortality (13.7%). 5
The risk of developing GC increases with age. It occurs most frequently between the ages of 50 and 80 and is uncommon in people under 30 years of age. 6
Despite Colombia’s significant case load, the country has no GC monitoring and prevention program nor has it prioritized GC as a public health problem. Existing research shows that the majority of GC patients are diagnosed in advanced stages which translates into very low survival figures. 7
GC is multifactorial with complex interactions of infectious agents such as helicobacter pylori and Epstein-Barr virus; environmental factors including high salt consumption, tobacco consumption and diets poor in fiber, fruit and vegetables; and a genetic component. The most important causative agent is H. pylori, a bacterial infection acquired in childhood. In the absence of adequate treatment, it may persist throughout life and induce a chronic inflammatory response that variably conditions development of atrophy, metaplasia, dysplasia and, finally, GC. 8
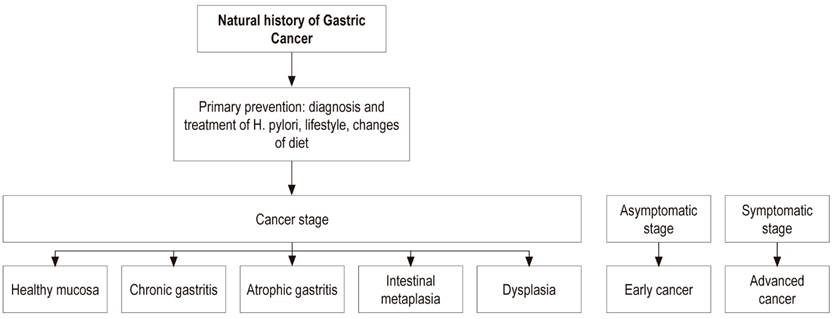
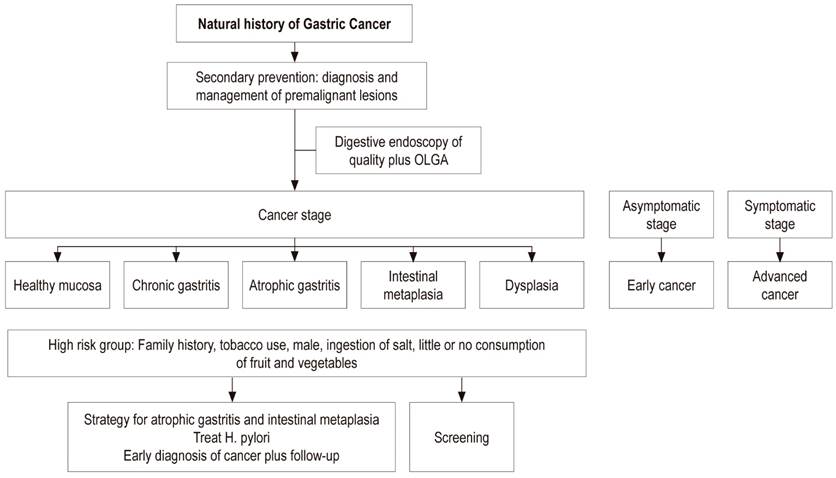
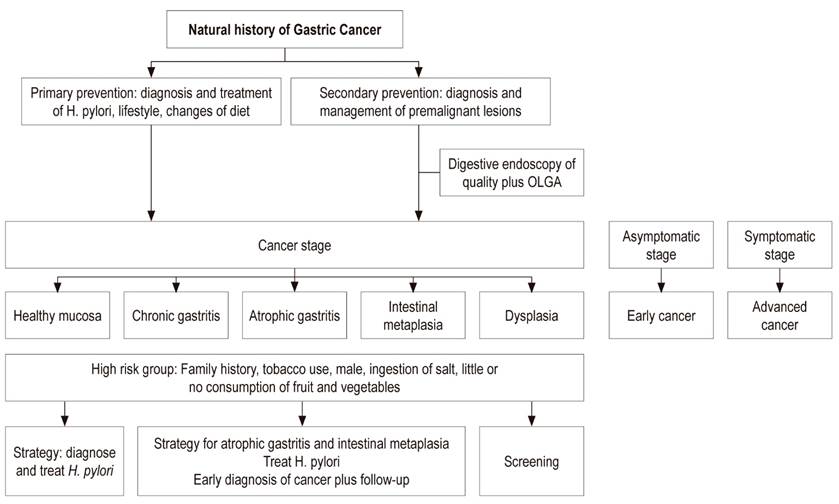
Primary prevention of GC aims at a diet rich in fiber with large amounts of fruit and vegetables plus diagnosis and treatment of H. pylori infection early in life. This strategy must be carried out before atrophy and intestinal metaplasia develop in the gastric mucosa. Secondary prevention aims at diagnosis and monitoring of preneoplastic lesions such as atrophy and intestinal metaplasia using the severity scales of histological staging known as the Operative Link on Gastritis Assessment (OLGA) and Operative Link on Gastric Intestinal Metaplasia assessment (OLGIM). 9
These recommendations are made because screening programs are not possible in countries such as in Colombia with low or intermediate economies where resources must be directed to immediate problems considered of greater urgency. 9
Unfortunately, in Colombia the diagnosis is made in advanced stages when there is no possibility of cure for this disease. In the face of this public health problem, there has been no action by the health authorities or the medical association. Therefore, proposing strategies to reduce incidence and mortality to the medical community of this country should be an important objective. 9
GC is a preventable disease. Within the literature there are strategies with adequate levels of evidence that allow action within the disease’s natural history to reduce incidence and mortality figures while improving survival through earlier diagnoses. The implementation of these cost-effective management strategies can be achieved in high-risk populations in a rational way similar to how colorectal cancer is being approached. 10
General objective
Colombia has no defined policies for controlling and preventing GC. Therefore, the objective of this study is to review intervention strategies aimed at primary and secondary prevention on the basis of our knowledge of GC’s natural history with the aim of reducing its incidence while improving mortality and early detection figures.
Natural history
Understanding the natural history of a type of cancer is crucial for designing effective intervention. (11)
In 1975 Pelayo Correa published “A model for the development of gastric cancer” in which he argued that the development of intestinal type GC, the most common subtype, originates in a 30 to 50-year-long process that begins with chronic atrophic gastritis and progresses to intestinal metaplasia, then to dysplasia and finally to cancer. That study postulated that the initial changes occurred in the first decade of life when colonization by H. pylori occurred. Correa initially postulated that the agents responsible for promoting this slow process from gastritis to cancer were related to the environment, based on studies of people migrating from high GC risk areas to low-risk areas. (12)
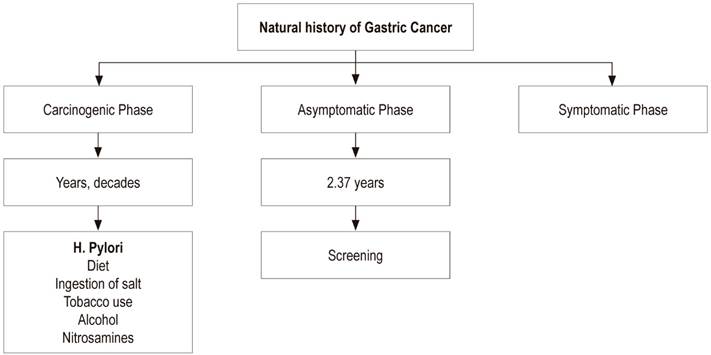
GC’s natural history has three phases: carcinogenic, asymptomatic and clinical or symptomatic (Table 1). (11)
Carcinogenic Phase (duration of years and decades)
H. pylori gastritis
The most widely accepted hypothesis is that H. pylori is the initial etiological factor of chronic gastritis leading to GC. This infection is acquired in childhood and slowly progresses toward gastric atrophy, intestinal metaplasia, dysplasia and invasive adenocarcinoma of the intestinal type. Its rate of progress varies and is modulated over many years by genetic, dietary and environmental factors which offer broad opportunities for intervention. 11,12
In 2002, Bedoya reported that 88% of children under 10 years of age showed some inflammatory changes of the gastric mucosa and changes that 5% presented chronic atrophic gastritis. 13
A review of gastric biopsies in a population aged one to 16 years by Archila et al. found H. pylori infections in 59% of these patients. There were small quantities of bacteria in 24.3%, moderate quantities in 20.1%, and abundant quantities in 14.6%. 13
H. pylori is the most important causative agent involved in the genesis of GC. The International Agency for Research on Cancer (IARC) has listed it as a Type I carcinogen since 1994. H. pylori is strongly associated with distal GC of the stomach although no relationship has been demonstrated with GC of the proximal and cardial regions. It has been estimated that more than 75% of gastric cancers worldwide are explained by H. pylori infections. There is also evidence that H. pylori infection is a necessary but not sufficient condition for gastric carcinogenesis. 15
Other etiological agents such as cigarettes, alcohol consumption and endogenous nitrosamine formation are recognized by the IARC as potential causal factors that in development of GC. Between 11% and 18% of cases may be associated with cigarettes. Diet and nutrition can also play a role in gastric oncogenesis. There is consistent evidence that consumption of fruit and vegetables is associated with decreased risks of GC (Figure 1). 15
GC’s association with family history (genetic component) has an odds ratio (OR) of 2.0 to 8.0, depending on the country. There are also studies that show higher prevalence of H. pylori infections and premalignant lesions in first-degree relatives of GC patients than in controls. 9,16
Genetic susceptibility caused by a mutation in E-cadherin, a crucial molecule in maintaining epithelial architecture, is assumed to be related to diffuse GC. 15
The first crucial event in gastric carcinogenesis is H. pylori infection. It activates the inflammatory response, and there is a high prevalence of H. pylori infections in GC patients. 15 Nevertheless, only a very small proportion of people who are infected patients with H. pylori ever develop GC: only one out of every 100 infected patients will develop GC. 17
This forces one to wonder why and how the disease develops in this minority of infected patients. One reason is variation in the pathogenicity of the bacteria. Research in this field has focused on genetic susceptibility due to polymorphisms in genes that govern gastric inflammation responses, the heterogeneity of H. pylori, and environmental influences such as salt from the diet or the presence of other species of Helicobacter within the gastrointestinal microbiota. 12
Considering GC to be the consequence of an infection has created enthusiasm for diagnosing and treating H. pylori in areas of high GC prevalence, 12 and it is clear that most GC is due to H. pylori infections rather than factors related to lifestyles. When it is suspected that cancer is caused by an infection, preventive measures are required in order to reduce both incidence and mortality. 12
HP infection is typically acquired in childhood, and mucosal transformation takes years and decades to pass through the chronic inflammatory process to states of atrophic gastritis and intestinal metaplasia. Consequently, eradication of the bacteria in young people could prevent this progression and reduce the risk of developing GC later in life. 11
Based on the arguments, the strategy of diagnosing and treating patients with gastritis associated with H. pylori was validated in Japan in 2009 and 2103 and gained support from the health care insurance system. This largest part of this population has non-atrophic gastritis following eradication treatment so endoscopic monitoring is not necessary. 18
The prevalence of H. pylori is high on the island of Matsú in Taiwan, and the incidence of GC is 50 per 100,000 inhabitants, 3 to 5 times higher than the overall incidence in Taiwan. The age of 30 was chosen as the cut-off for accelerating elimination of GC. A pilot screening study to diagnose and treat H. pylori infections was begun on the island in 2004. Initial results were very promising: incidence of GC decreased by 25% and the incidence of gastric atrophy feel by 77% compared to historical data. 19 It has been calculated that it is necessary to diagnose and treat 15 men in China and 245 women in the United States to avoid one case of GC. 20
Preventing and eradicating H. pylori infections before atrophic gastritis develops is the best means of reducing and/or eliminating GC. 21
In 2005, the Nobel Prize in Physiology was awarded to Marshall and Warren for the discovery of H. pylori and its role in gastritis and peptic ulcer. In addition, chronic inflammation is a common risk factor for carcinogenesis, and it has been suggested that primary prevention of GC could be achieved through a strategy of screening and treating H. pylori infection. 11
In 2013, an IARC working group reviewed the evidence accumulated in support of mass H. pylori eradication as a strategy for preventing GC. Based on the favorable results of controlled clinical studies and observational studies, a group of experts confirmed that this strategy is effective. 19 For this reason, the IARC recommended that health care agencies include this strategy in national cancer control programs.
In January 2014, a global consensus was reached in Kyoto, Japan for evaluation of the management of chronic gastritis associated with H. pylori. Its conclusions establish that H. pylori eradication could prevent GC and that all carriers of H. pylori should be treated in order to eradicate this pathogen. 21 Elimination of H. pylori from the population could eliminate approximately 75% of GC. 22
Our attention should be focused on how this strategy is carried out, for example, by identification of H. pylori patientss within the asymptomatic population and eradication before GC develops. However, the current strategy must depend on H. pylori infections and the incidence of GC within that population. 23
A metaanalysis of three studies (Forman, Parsonnet and Nomura) found that people infected with H. pylori had a great risk of developing GC than did uninfected people with an OR of 3.8. Uemura has shown that patients untreated H. pylori infections had a greater chance of progression to GC in the next 12 years than did uninfected patients. 12
Recent metaanalyses and studies with low statistical power indicate that the H. pylori eradication reduces the risk of GC developing by approximately 40% in primary prevention studies of asymptomatic individuals and by 54% as a tertiary prevention strategy against a second appearance of GC after endoscopic resection of early GC. It is not known if there is a cut-off time during the Correa cascade after which H. pylori eradication is no longer a deterrent to progression to GC. 12,24
A study by Lee et al. which included 24 publications (14 primary prevention studies and 10 tertiary prevention studies) with more than 48,000 individuals with follow-ups of 34,000 people/years has shown that the benefit of H. pylori eradication were more evident in areas where the incidence of GC is higher. However, risk reduction was evident in almost every individual evaluated in the study. Presumably, high-risk populations in low-risk countries, including immigrants who have been infected since childhood, benefit significantly from eradication. 12
In another study, 544 patients who had undergone early GC endoscopic surgery were randomized to receive H. pylori eradication treatment. Metachronous GC was detected in nine patients in the group that received treatment and in twenty-four of the patients in the group that did not receive treatment, with p <0.01. This indicates that the preventive effect of H. pylori eradication therapy in these patients significantly reduced the risk of metachronous GC. 25
One intervention strategy in the carcinogenic phase of GC’s natural history is the policy of diagnosing and treating H. pylori infections, especially before gastric atrophy and intestinal metaplasia occur. Nevertheless, patients with atrophy and metaplasia should also receive eradication therapy if bacterial infection is present even though there will be a time of no return after which therapy will have no justification because the mucosal damage will have already happened. 24
Some researchers are trying to pinpoint the moment at which H. pylori generates changes in a person’s deoxyribonucleic acid (DNA) and when that damage leads to irreversible development of cancer even if the infection is eradicated. 26 Determination of this point of no return will help define when eradicating the infection can guarantee the recovery of mucosal damage, stop the process and prevent the development of cancer.
A large number of medical professionals in Colombia do not have a clear, deep understandings of what gastritis implies in terms of risk, natural history, intervention and follow-up when faced with a pathology report of chronic atrophic gastritis with or without intestinal metaplasia. 14
The incidence of GC increases with age, and the impact of H. pylori eradication on the incidence of GC depends on the population studied. 23
Evidence suggests that all individuals with H. pylori gastritis should be treated. In countries with high-risk populations for GC, this strategy is recommended for young people under 20 years of age given that the infection is acquired in childhood. This knowledge may have clinical utility for stratifying individuals with H. pylori infection into those who are at high risk and those who are at low risk for GC in order to create personalized follow-up schemes. 12
The question of how to prevent GC is addressed in Figure 2. The results support a strategy of eradicating the bacteria in countries where H. pylori and GC are common. Then, the current strategy should be carried out depending on the prevalence of H. pylori and GC. 23 The participants in the 2014 Kyoto consensus unanimously recommended implementation of H. pylori eradication therapy before precancerous changes develop. 21 The reason is the risk of progression to gastric atrophy and to intestinal metaplasia would be reduced along with the later risk of GC.
This strategy of screening for and treating H. pylori infections seems to be the best approach for reducing cancer risk. However, the implementation of this strategy at the population level requires a systematic approach. The program must also be integrated into national health care priorities so that limited resources are effectively allocated and used. Implementation may require adoption of an appropriate strategy. Within the population there are subgroups that vary in risk, so that it is impossible for the approach to be the same for everyone. 11
Treating all patients with infections documented by histology or rapid urease test would not be justified because it would not be cost-effective. It is necessary to define a high-risk group within that population.
High Risk Group
High risk individuals might be defined as those from high-risk areas, especially in populations with an incidence greater than 20/100,000 inhabitants, who have a first degree family member with a history of GC, and who have histories of heavy smoking, and heavy salt and alcohol consumption (Figure 3). 24,27
Cost Analysis
The literature shows that diagnostic and treatment programs for H. pylori patients are more cost-effective in countries where the incidence of GC is higher than in low incidence countries. 30,31 Two studies have shown that the optimal screening age is between 20 and 30 years because screening in older cohorts was less cost-effective. 32
H. pylori screening is cost-effective because of the relatively low cost of H. pylori testing and treatment and the fact that screening is done only once. The estimated costs for detection and treatment of H. pylori are less than 1% of the costs of GC treatment in all studies. This means that the strategy of diagnosing and treating H. pylori entails considerable cost savings. GC consensus recommends blood test screening. In high prevalence populations, blood tests are used more frequently than the breath test while in low prevalence populations, the fecal antigen test is used more than the other two options. It should be taken into account that acceptability of the test is one of the requirements for introduction of this strategy in a study population. It has been found that blood tests and fecal antigen tests are more cost-effective than the breath test. Repeating screening and/or treatment and limiting treatment to those with CagA strains do not appear to be cost-effective policies. 32
A large-scale study for the prevention of colon cancer and gastric cancer through the detection of fecal occult blood and fecal antigen for H. pylori is being carried out in Taiwan. Patients infected with H. pylori receive treatment. The results of this study are not yet known. 11
Gastric Atrophy
Because GC develops over a long period of years to decades, the frequency of gastric atrophy is very low before the age of 40 (<5%). Only 5.9% of GC patients are younger than 40. 33,34
A study by Pelayo Correa in an area of high GC incidence reported that, in individuals over 40 years of age, the prevalence of chronic atrophic gastritis was 57%, the prevalence of intestinal metaplasia was 38%, and the prevalence of dysplasia was 10%. 35 This means that development of precancerous lesions and then identification of lesions can also take several years. Therefore, endoscopic follow-up of high risk patients can help identify malignant lesions early when they are still operable and there is a high probability of curing the patient. A 10-year follow-up study has reported that the figures for progression to GC for patients with atrophic gastritis, intestinal metaplasia, mild dysplasia and severe dysplasia are 0.8%, 1.8%, 4% and 33%, respectively. 36
Patients with atrophy or extensive intestinal metaplasia should be followed up with endoscopy every three years. Patients with moderate atrophy or intestinal metaplasia limited only to the antrum do not need follow-up. 37 Management should be individualized according to other factors such as family history of GC, geographic origin, smoking and salt consumption.
Dysplasia is a high risk indicator for GC and should be confirmed and classified by two pathologists due to interobserver variability.
European guidelines recommend that patients with extensive atrophic gastritis or extensive intestinal metaplasia should have endoscopic follow-ups every 3 years. The incidence of GC in 10 years of follow-up for patients with atrophic gastritis is 0.8%. For patients with intestinal metaplasia, it is 1.8%, so the endoscopic follow-up of these 2 groups should be different. 37
The operative link for gastritis assessment (OLGA) was established to evaluate the degree and location of atrophy. During diagnosis and follow-up of premalignant lesions, it is recommended that at least 5 samples be taken from patients undergoing endoscopy. Samples should include two from the antrum, two from the corpus, and one from the incisure. A biopsy sample from the incisure is needed because the prevalence of intestinal metaplasia is higher at this site than at any other location in the stomach. Intestinal metaplasia usually begins in the incisure and spreads to the antrum and the corpus. 37,38
Intestinal Metaplasia
Intestinal metaplasia is classified as either complete or incomplete. Whether intestinal metaplasia is reversible is a matter of controversy which is the reason a point of no return is under investigation. 39,40 Complete intestinal metaplasia is considered a short-term reactive process that usually regresses while incomplete intestinal metaplasia is related to chronic prolonged damage, so it is more likely to progress to dysplasia. 41
Patients with intestinal metaplasia may have up to 10 times more risk of GC than the general population. 42 There is controversy about the usefulness of classifying intestinal metaplasia in clinical practice. Incomplete intestinal metaplasia significantly increases the risk of GC beyond that of complete intestinal metaplasia. 2,42,37,39
Prevention and treatment of gastric atrophy and intestinal metaplasia decrease the prevalence of GC. H. pylori eradication is the fundamental management step while detection of GC in its early stages is the other strategy for these patients. 40
Correa proposed an algorithm for managing and monitoring preneoplastic lesions. For patients with intestinal metaplasia, the presence of H. pylori and the extent of intestinal metaplasia should be measured. If the infection is present, it should be treated. If intestinal metaplasia is extensive and incomplete, digestive endoscopy should be repeated every year and then every three years if the lesion persists. Otherwise monitoring is not required. 43 Patients with intestinal metaplasia who have at least one of these risk factors - incomplete intestinal metaplasia, family history, history of smoking, and salt consumption - may have a higher risk of developing GC and would probably benefit from more intense and frequent endoscopic monitoring. 33,37
Digestive endoscopy’s diagnostic performance has been poor in the West, so the diagnosis of gastric atrophy and intestinal metaplasia requires systematic biopsies of the corpus and the antrum. 44 The protocol for staging with the OLGA system includes 5 biopsies: two from the antrum, two from the corpus, and one from the incisure. A greater number of biopsies may increase sensitivity. 37,38,39
In a case-control study, the OLGA protocol identified 61.8% more cases of atrophy than did protocols with fewer biopsies. This could allow correction of the under diagnosis of gastric atrophy. 45
It would be justified to practice quality digestive endoscopy to search for premalignant lesions in the high-risk population from the age of 40. Their extent and the risk of GC according to the OLGA system would determine the frequency of endoscopic follow-up. 7,33,39
Intestinal metaplasia is a premalignant condition that can result from a process of adaption to an environmental stimulus such as H. pylori infection, smoking and/or high levels of salt consumption. 40 English studies that evaluated benefits of follow-ups for patients with intestinal metaplasia have found the incidence of GC to be 11%. Endoscopic follow-up was associated with earlier detection of GC and improved survival. 40
Cancer detection figures range from 33% to 85% in European studies of endoscopic follow-up of patients with intestinal metaplasia, dysplasia. 40
In the low-risk populations of the United States, the risk of progression is low and clinical follow-up is not indicated unless there are other risk factors for GC such as family history or Asian or Latin American country of origin. 42
A European consensus suggests that endoscopic follow-up should be performed with mapping and biopsies within one year of detection of low-grade dysplasia in a patient with intestinal metaplasia. The ideal frequency of endoscopic monitoring is not known. Follow-ups may be suspended when two consecutive endoscopies are negative for dysplasia. Unlike patients with low grade dysplasia, patients with high grade dysplasia should undergo surgical or endoscopic resection due to the high probability of coexisting invasive adenocarcinoma. Twenty-five percent of patients with high grade dysplasia can progress to adenocarcinoma within one year. If H. pylori infection is identified, it must be eradicated even though controversy remains as to whether empirical eradication should be performed when intestinal metaplasia is diagnosed. 42
The presence of incomplete intestinal metaplasia is a recognized predictor of increased risk for development of high grade dysplasia or GC in areas with high prevalence such as Japan. Several studies have concluded that incomplete intestinal metaplasia identifies patients at high risk of developing GC, and they require intensive follow-up (Figure 4). 46
Dysplasia
Gastric dysplasia is a precancerous lesion and is the penultimate stage in the cascade of gastric oncogenesis, as formulated by Correa. Therefore, identification, management and monitoring of this lesion is important for early detection and prevention of GC. Dysplasia is usually classified as low or high grade. 47
Patients with dysplasia are generally men and who are 10 years younger than their relatives with GC (61.35 years for dysplasia and 70 years for GC). 47
Dysplasia can be found anywhere in the stomach, but most often it is found in the antrum. Dysplasia is most often discovered incidentally during screening endoscopies. 47
The real risk of progression of dysplasia to carcinoma is unclear because it is difficult to establish the natural history of dysplasia. However, several studies have shown that high-grade dysplasia has a high risk of progressing to either carcinoma or synchronous carcinoma. Figures ranging from 60% to 85% have been reported in an interval of 4 to 48 months. It is also known that 25% of patients with high-grade dysplasia have progressed to carcinoma within one year of diagnosis. 39,47
High-grade lesions require endoscopic resection due to their potential for progression to carcinoma and coexistence with carcinoma. When lesions are not well defined endoscopically, it is recommended that they be followed up one year after diagnosis. Lesions with high grade dysplasia should be managed with endoscopic resection. 47
Sometimes endoscopic resection is indicated not only for diagnosis but for treatment of dysplasia.
Asymptomatic (Screening) Phase
The asymptomatic period is when cancer can be detected through screening tests before the typical symptoms needed for diagnosis appear. This phase is defined as the time from the onset of cancer to the onset of symptoms. It is the ideal time for screening programs. 11
This period is a theoretical concept that is currently impossible to measure in particular cases even though it is the statistically most important parameter for defining the screening interval in the general population. 11 This time has been defined for GC as 2.37 years on average. This is the reason the Koreans recommend screening every 2 years. 11 Nevertheless, this average changes with age: in 40 to 49 year old population, it is 1.25 years; from 50 to 59 years old, it is 3.18 years; and from 60 to 69 years old, it is 3.74 years. This may explain why endoscopic screening in high-risk groups should include follow-ups annually or every 2 years. 11
When cancer is diagnosed by screening, healing may be possible and patients may survive for long periods of time. However, this phase of GC is relatively short. Prostate cancer, which has a long asymptomatic phase, can be diagnosed early and asymptomatically through screening by testing for prostate specific antigen (PSA). 11
On the other hand, early GC progresses to advanced GC in 33 to 48 months, and it may be asymptomatic part of this time (Figure 5). 49
Symptomatic Phase
The initial stage of GC is practically asymptomatic, and symptoms appear when the disease is very advanced,. At that point, curative surgical treatment is often impossible. In this phase, only 10% of GC patients survive. 50
The risk of developing GC increases with age. GC occurs most frequently between 50 and 80 years of age. GC in people under 30 is rare. 6
Out of a total of 600 patients in the REGATA study, 5.9% were under 40 years old, 10.1% were between 40 and 49 years old, 18.9% were between 50 and 59 years and 65.1% were older than 60 years. GC is twice as frequent in men as in women. In this same study, 65% were men and 35% women. 34
In 2008, Adrada et al. published a series of GC patients in which 92.4% had advanced lesions. 51. Martínez et al. found that 97% of patients had advanced tumors. 52
Another important issue is the cost of handling patients with advanced lesions. Gaviria and Cubillos have established direct costs for diagnosis, staging, medical procedures and medical devices in caring for patients with advanced GC in Colombia. Costs are COP 12 million for stage II and COP 27 million for stage III. They established that the higher the stage, the higher the costs (Figure 6). 52
Conclusions
GC is an ideal candidate for preventive strategies. However, while primary prevention is facilitated by the recognized objective of H. pylori, effective secondary prevention strategies have obstacles such as high costs and the need for significant human and technical resources.
More than a decade ago a mathematical model showed that screening for H. pylori infections followed by eradication could be cost-effective in countries with high incidence of GC and high GC mortality rates. It was also shown that the benefit was only significant in a subgroup of patients without precancerous lesions. A metaanalysis of 7 studies conducted in areas of high incidence of GC demonstrated a reduction in the risk of GC among patients who underwent H. pylori eradication (relative risk [RR]: 0.65). This primary prevention strategy is cost-effective in countries with high incidences of GC.
Eighty-four percent of GC patients are above 50 years of age, and of this group 65.1% are from 60 to 70 years old. Generally, patients with dysplasia are men and are 10 years younger than their relatives with GC (61.35 years for dysplasia and 70 years for GC), so the strategy for the average population should be 10 years earlier than the age group with the greatest prevalence. In other words, the endoscopic surveillance and risk stratification for those over 50 year should be initiated.
The current incidence of GC in any population is dependent on a number of variables including the proportion infected by H. pylori, the severity of gastric atrophy and the speed of atrophy’s development.
It is necessary to change this disease’s landscape by creating sensitivity to this public health problem within the medical association and at the level of those responsible for health care policies. It is also necessary to develop clinical practice guidelines aimed at preventing GC.
Primary and secondary prevention strategies that impact the natural history of GC should be established.
Referencias
1. Den Hoed C, Kuipers E. Gastric Cancer: How can we reduce the incidence of this Disease? Curr Gastroenterol Rep. 2016;18(34):1-8. https://doi.org/10.1007/s11894-016-0506-0 [ Links ]
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cáncer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. https://doi.org/10.3322/caac.21492. [ Links ]
3. Park JY, von Karsa L, Herrero R. Prevention strategies for gastric cancer: a global perspective. Clin Endosc. 2014;47(6):478-89. https://doi.org/10.5946/ce.2014.47.6.478 [ Links ]
4. Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12(3):354-62. https://doi.org/10.3748/wjg.v12.i3.354. [ Links ]
5. World Health Organization. GLOBOCAN 2018: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2018. Colombia. WHO [internet] 2018 [acceso 12 de septiembre del 2018]. Disponible en: Disponible en: https://gco.iarc.fr/today/data/factsheets/populations/170-colombia-fact-sheets.pdf [ Links ]
6. Piazuelo M, Correa P. Gastric cancer: overview. Colomb Med. 2013;44(3):192-201. [ Links ]
7. Gómez M, Riveros J, Ruiz O, Concha A, Ángel D, Torres M, et al. Guía de práctica clínica para la prevención diagnóstico y tratamiento del cáncer gástrico temprano 2015. Rev Col Gastroenterol. 2015; 30 supl 1:34-42. [ Links ]
8. Correa P. Gastric cancer: overview. Gastroenterol Clin North Am. 2013;42(2):211-7. https://doi.org/10.1016/j.gtc.2013.01.002. [ Links ]
9. Choi IJ. Endoscopic gastric cancer screening and surveillance in high-risk groups. Clin Endosc. 2014;47(6):497-503. https://doi.org/10.5946/ce.2014.47.6.497 [ Links ]
10. Binefa G, Rodríguez-Moranta F, Teule A, Medina-Hayas M. Colorectal cancer: from prevention to personalized medicine. World J Gastroenterol. 2014;20(22):6786-808. https://doi.org/10.3748/wjg.v20.i22.6786 [ Links ]
11. Lee YC, Chiang TH, Liou JM, Chen HH, Wu MS, Graham DY. Mass Eradication of Helicobacter pylorito Prevent Gastric Cancer: Theoretical and Practical Considerations. Gut Liver. 2016;10(1):12-26. https://doi.org/10.5009/gnl15091 [ Links ]
12. Moss SF. The Clinical Evidence Linking Helicobacter pylori to Gastric Cancer. Cell Mol Gastroenterol Hepatol. 2016;3(2):183-91. https://doi.org/10.1016/j.jcmgh.2016.12.001 [ Links ]
13. Archila P, Tovar L, Ruiz M. Características histológicas de la gastritis crónica reportadas en las biopsias gástricas de niños de 1 a 16 años de edad en el Hospital Infantil de San José durante el periodo comprendido entre septiembre de 2008 a septiembre de 2010. Rev Col Gastroenterol. 2012;27(2):74-9. [ Links ]
14. Bedoya A, Sansón F, Yepes Y, Santacruz C, Cifuentes Y, Calvache D, et al. Prevalencia y severidad de las lesiones precursoras de malignidad en un área de alto riesgo de cáncer gástrico. Pasto 2012. Rev Col Gastroenterol. 2012;27(4):275-81. [ Links ]
15. González CA, Agudo A. Carcinogenesis, prevention and early detection of gastric cancer: where we are and where we should go. Int J Cancer. 2012;130(4):745-53. https://doi.org/10.1002/ijc.26430 [ Links ]
16. Yaghoobi M, McNabb-Baltar J, Bijarchi R, Hunt RH. What is the quantitative risk of gastric cancer in the first-degree relatives of patients? A meta-analysis. World J Gastroenterol. 2017;23(13):2435-42. https://doi.org/10.3748/wjg.v23.i13.2435 [ Links ]
17. Yoon H, Kim N. Diagnosis and management of high risk group for gastric cancer. Gut Liver. 2015;9(1):5-17. https://doi.org/10.5009/gnl14118 [ Links ]
18. Sugimoto M, Ban H, Ichikawa H, Sahara S, Otsuka T, Inatomi O, et al. Efficacy of the Kyoto Classification of Gastritis in Identifying Patients at High Risk for Gastric Cancer. Intern Med. 2017;56(6):579-86. https://doi.org/10.2169/internalmedicine.56.7775 [ Links ]
19. Leja M, You W, Camargo MC, Saito H. Implementation of gastric cancer screening - the global experience. Best Pract Res Clin Gastroenterol. 2014;28(6):1093-106. https://doi.org/10.1016/j.bpg.2014.09.005 [ Links ]
20. Ford AC, Forman D, Hunt RH, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348:g3174. https://doi.org/10.1136/bmj.g3174 [ Links ]
21. Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64(9):1353-67. https://doi.org/10.1136/gutjnl-2015-309252 [ Links ]
22. Wroblewski LE, Peek RM Jr. Helicobacter pylori in gastric carcinogenesis: mechanisms. Gastroenterol Clin North Am. 2013;42(2):285-98. https://doi.org/10.1016/j.gtc.2013.01.006 [ Links ]
23. Graham DY, Uemura N. Natural history of gastric cancer after Helicobacter pylori eradication in Japan: after endoscopic resection, after treatment of the general population, and naturally. Helicobacter. 2006;11(3):139-43. 10.1111/j.1523-5378.2006.00391.x [ Links ]
24. Rugge M. Gastric Cancer Risk in Patients with Helicobacter pylori Infection and Following Its Eradication. Gastroenterol Clin North Am. 2015;44(3):609-24. https://doi.org/10.1016/j.gtc.2015.05.009 [ Links ]
25. Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372(9636):392-7. https://doi.org/10.1016/S0140-6736(08)61159-9 [ Links ]
26. Grisales P. No hay enemigo pequeño: avances contra helicobacter pylori. Pesquisa. 2017;40:14-6. [ Links ]
27. Coelho LG, Maguinilk I, Zaterka S, Parente JM, do Carmo Friche Passos M, Moraes-Filho JP. 3rd Brazilian Consensus on Helicobacter pylori. Arq Gastroenterol. 2013 Apr;50(2). pii: S0004-28032013005000113. https://doi.org/10.1590/S0004-28032013005000001 [ Links ]
28. Rollán A, Cortés P, Calvo A, Araya R, Bufadel ME, González R, et al. Recommendations of the Chilean Association for Digestive Endoscopy for the management of gastric pre-malignant lesions. Rev Med Chil. 2014;142(9):1181-92. https://doi.org/10.4067/S0034-98872014000900013 [ Links ]
29. Lee YC, Chiang TH, Chou CK, Tu YK, Liao WC, Wu MS, et al. Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology. 2016;150(5):1113-24.e5. https://doi.org/10.1053/j.gastro.2016.01.028 [ Links ]
30. Asaka M. A new approach for elimination of gastric cancer deaths in Japan. Int J Cancer. 2013;132(6):1272-6. https://doi.org/10.1002/ijc.27965 [ Links ]
31. Teng AM, Kvizhinadze G, Nair N, McLeod M, Wilson N, Blakely T. A screening program to test and treat for Helicobacter pylori infection: Cost-utility analysis by age, sex and ethnicity. BMC Infect Dis. 2017;17(1):156. https://doi.org/10.1186/s12879-017-2259-2 [ Links ]
32. Lansdorp-Vogelaar I, Sharp L. Cost-effectiveness of screening and treating Helicobacter pylori for gastric cancer prevention. Best Pract Res Clin Gastroenterol. 2013;27(6):933-47. https://doi.org/10.1016/j.bpg.2013.09.005 [ Links ]
33. Rollan A, Ferreccio C, Gederlini A, Serrano C, Torres J, Harris P. Non-invasive diagnosis of gastric mucosal atrophy in an asymptomatic population with high prevalence of gastric cancer. World J Gastroenterol. 2006;12(44):7172-8. https://doi.org/10.3748/wjg.v12.i44.7172 [ Links ]
34. Oliveros R, Navarra LF. Diagnóstico, estadificación y tratamiento del cáncer gástrico en Colombia desde 2004 a 2008 (Regate- Colombia). Rev Col Gastroenterol. 2012;27(4):269-74. [ Links ]
35. Correa P, Haenszel W, Cuello C, Zabala D, Fontham E, Zarama G, et al. The gastric precursors in a high risk population: cross-sectional studies. Cancer Res. 1990;50:1731-6. [ Links ]
36. den Hoed CM, Holster IL, Capelle LG, de Vries AC, den Hartog B, Ter Borg F, et al. Follow-up of premalignant lesions in patients at risk for progression to gastric cancer. Endoscopy. 2013;45(4):249-56. https://doi.org/10.1055/s-0032-1326379 [ Links ]
37. Zullo A, Hassan C, Repici A, Annibale B. Intestinal metaplasia surveillance: searching for the road-map. World J Gastroenterol. 2013;19(10):1523-6. https://doi.org/10.3748/wjg.v19.i10.1523 [ Links ]
38. Rugge M, Pennelli G, Pilozzi E, Fassan M, Ingravallo G, Russo VM, et al. Gastritis: the histology report. Dig Liver Dis. 2011;43 Suppl 4:S373-84. https://doi.org/10.1016/S1590-8658(11)60593-8 [ Links ]
39. Dinis-Ribeiro M, Areia M, de Vries AC, Marcos-Pinto R, Monteiro-Soares M, O’Connor A, et al. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy. 2012;44(1):74-94. https://doi.org/10.1055/s-0031-1291491 [ Links ]
40. Park YH, Kim N. Review of atrophic gastritis and intestinal metaplasia as a premalignant lesion of gastric cancer. J Cancer Prev. 2015;20(1):25-40. https://doi.org/10.15430/JCP.2015.20.1.25 [ Links ]
41. Lage J, Uedo N, Dinis-Ribeiro M, Yao K. Surveillance of patients with gastric precancerous conditions. Best Pract Res Clin Gastroenterol. 2016;30(6):913-22. https://doi.org/10.1016/j.bpg.2016.09.004 [ Links ]
42. ASGE Standards of Practice Committee, Evans JA, Chandrasekhara V, Chathadi KV, Decker GA, Early DS, et al. The role of endoscopy in the management of premalignant and malignant conditions of the stomach. Gastrointest Endosc. 2015;82(1):1-8. https://doi.org/10.1016/j.gie.2015.03.1967 [ Links ]
43. Correa P, Piazuelo MB, Wilson KT. Pathology of gastric intestinal metaplasia: clinical implications. Am J Gastroenterol. 2010;105(3):493-8. https://doi.org/10.1038/ajg.2009.728 [ Links ]
44. Cañadas R. Metaplasia intestinal gástrica: ¿cómo la estamos abordando? Rev Col Gastroenterol. 2012;27(4):259-62. [ Links ]
45. Martinez D, Otero W, Ricaurte O. Impacto del sistema OLGA en la detección de gastritis crónica atrófica en Colombia: un estudio de casos y controles. Rev Col Gastroenterol. 2016;31(4)360-7. https://doi.org/10.22516/25007440.111 [ Links ]
46. Pittayanon R, Rerknimitr R, Klaikaew N, Sanpavat A, Chaithongrat S, Mahachai V, et al. The risk of gastric cancer in patients with gastric intestinal metaplasia in 5-year follow-up. Aliment Pharmacol Ther. 2017;46(1):40-45. https://doi.org/10.1111/apt.14082 [ Links ]
47. Sung JK. Diagnosis and management of gastric dysplasia. Korean J Intern Med. 2016;31(2):201-9. https://doi.org/10.3904/kjim.2016.021 [ Links ]
48. Bisschops R, Areia M, Coron E, Dobru D, Kaskas B, Kuvaev R, et al. Performance measures for upper gastrointestinal endoscopy: A European Society of Gastrointestinal Endoscopy quality improvement initiative. United European Gastroenterol J. 2016;4(5):629-656. https://doi.org/10.1177/2050640616664843 [ Links ]
49. Iwai T, Yoshida M, Ono H, Kakushima N, Takizawa K, Tanaka M, et al. Natural History of Early Gastric Cancer: a Case Report and Literature Review. J Gastric Cancer. 2017;17(1):88-92. https://doi.org/10.5230/jgc.2017.17.e9 [ Links ]
50. de Vries E, Uribe C, Pardo C, Lemmens V, Van de Poel E, Forman D. Gastric cancer survival and affiliation to health insurance in a middle-income setting. Cancer Epidemiol. 2015;39(1):91-6. https://doi.org/10.1016/j.canep.2014.10.012 [ Links ]
51. Adrada JC, Calambas F, Díaz JE, Delgado DO, Sierra CH. Características sociodemográficas y clínicas en una población con cáncer gástrico en el Cauca, Colombia. Rev Col Gastroenterol. 2008;23(4) 309-14. [ Links ]
52. Martínez J, Garzón M, Lizarazo J, Marulanda JC, Molano JC, Rey M, et al. Características de los pacientes con cáncer gástrico del departamento de Cundinamarca remitidos al Hospital universitario de la Samaritana entre los a-os 2004-2009. Rev Col Gastroenterol. 2010;25(4): 344-48. [ Links ]
53. Gaviria A, Cubillos L. Costos médicos directos en el tratamiento del cáncer gástrico en los estadios 0 a IIIB en pacientes adultos en Colombia. Colombia: UDCA; 2015. [ Links ]
Received: June 22, 2018; Accepted: November 19, 2018











 text in
text in