Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.34 no.2 Bogotá abr./jun. 2019
https://doi.org/10.22516/25007440.398
Case report
Simultaneous appearance of early gastric cancer and GIST
1Especialista en Gastroenterología, Universidad Nacional de Colombia, Especialista en Medicina Interna, Universidad Nacional de Colombia, Hospital Universitario Nacional, Hospital Occidente de Kennedy, Unidad de gastroenterología y Ecoendoscopia (UGEC). Bogotá D. C., Colombia
2Especialista en Gastroenterología, Universidad Nacional de Colombia, Especialista en Medicina Interna, Pontificia Universidad Javeriana, Hospital Occidente de Kennedy, Hospital Universitario Nacional de Colombia. Bogotá D. C., Colombia
3Especialista en Gastroenterología, Universidad Nacional de Colombia, Especialista en Medicina Interna, Universidad San Martín, Hospital Occidente de Kennedy, Hospital Universitario Nacional de Colombia. Bogotá D. C., Colombia
We present the case of a 74-year-old male patient who was admitted with symptoms of upper digestive bleeding. Endoscopy of his upper digestive tract found an ulcerated lesion and a subepithelial lesion in his stomach. Complete studies including gastric endoscopic ultrasound showed a mucosal lesion infiltrating the submucosa which was suggestive of early gastric cancer as well as a subepithelial lesion on the muscle that was suggestive of a gastrointestinal stromal tumor (GIST). Staging showed no metastatic compromise, so surgery was performed, and histology subsequently confirmed the findings.
Keywords: Hematemesis; manes; gastric cancer; gastrointestinal stromal tumor; GIST; synchronous tumor; ki 67; surgery; endoscopic ultrasound; submucosal dissection; gastrectomy
Se presenta el caso clínico de un paciente masculino de 74 años quien ingresó con síntomas de sangrado digestivo alto, se realizó una endoscopia de vías digestivas altas y se encontraron dos lesiones en el estómago: una elevada de centro ulcerado y otra subepitelial, lo que llevó a completar los estudios con una ecoendoscopia gástrica que mostró una lesión mucosa que infiltraba la submucosa sugestiva de cáncer gástrico temprano y otra lesión subepitelial dependiente de la muscular, sugestivas de un tumor gastrointestinal estromal (GIST); los estudios de estatificación no evidenciaron compromiso metastásico, por lo que se llevó al manejo quirúrgico con la posterior confirmación histológica de los hallazgos.
Palabras clave: Hematemesis; melenas; cáncer gástrico; tumor gastrointestinal estromal; GIST; tumor sincrónico; ki 67; cirugía; ecoendoscopia; disección submucosa; gastrectomía
Introduction
Gastric cancer’s (GC) prevalence and mortality rate are high all around the worldwide. In 2008, there were 988,000 new cases of GC in the world, representing the fourth most frequent cancer after lung cancer, breast cancer and colon and rectal cancer: 738,000 deaths occurred making GC the second leading cause of cancer death. 1 In Colombia, it is the first cause of cancer death in men and the third cause of cancer death in women. 1 Digestive endoscopy is the diagnostic method of choice for GC, 2 but endoscopic ultrasonography (EUS) is a complementary method of choice for determining the depth of early GC. 3 It has the ability to visualize digestive tract strata with proven histological correlations. 3 Early GC is located in the mucosa and submucosa and may or may not involve lymph nodes. 4 Early GC is treated endoscopically by mucosectomy or endoscopic dissection of the submucosa, depending on the tumor’s size and morphological characteristics as determined by EUS. 5 Advanced GC invades beyond the submucosa and compromises regional and distant tissue. 5 Management includes surgery and chemotherapy and radiation therapy.
Gastrointestinal stromal tumors (GIST), with an incidence between 10 and 15 cases per million people, are the most common tumors of the gastrointestinal tract. 6 Although they are usually diagnosed incidentally from radiological or endoscopic studies, their most frequent clinical manifestation is gastrointestinal bleeding. 5 Their most frequent location is the stomach, 7 and histologically more than 95% of GIST are positive for the KIT protein (CD117). About 90% have a mutation either in the c-KIT gene or in the PDGFRA gene. 8 In endoscopy, it can be seen as a subepithelial lesion sometimes with a central ulceration. 9 In EUS it is a hypoechogenic lesion that is homogeneous and dependent on the muscular layer. EUS can be used in a complementary way to guide performance of a biopsy for use in histological diagnosis. 10 Computed axial tomography (CAT) is the imaging method of choice for characterizing an abdominal mass since it evaluates local extension and distance which is important because GIST can metastasize especially to the liver, omentum and peritoneal cavity. 11
Management of GC depends on its extension and size. The goal of surgical treatment is resection with free margins, but lymphadenectomy is not necessary in view of the fact that lymphatic involvement is rare. 11 Since forty to fifty percent of patients who undergo surgery may experience recurrences, 12 tyrosine kinase inhibitors appear to be an excellent alternative treatment. 13 Wedge resection is the surgical management of choice, 14 and laparoscopic techniques have fewer complications, shorter hospital stays and less bleeding than do open resection techniques. 15 The best way to treat lesions that are smaller than 2 cm is still not clear from the available evidence, so unless distance extension is documented, which is rare, management should be expectant. 16
This article presents the interesting case of one patient with simultaneous presentation of both of the pathologies discussed above.
Clinical case
The 74-year-old patient was admitted after three days of hematemesis and melena. Upper digestive endoscopy found an elevated, 20 mm in diameter lesion with an ulcerated center in the middle of the corpus towards the anterior wall as well as a 60 mm subepithelial lesion in the antrum. The initial endoscopic diagnosis a type 0-IIa elevated gastric lesion and a type 0-IIc subepithelial lesion (GIST?) (Figure 1). Multiple biopsies of the lesions were taken.
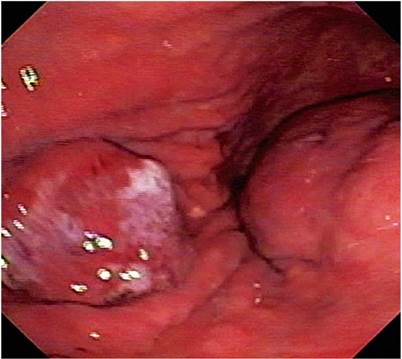
Figure 1 The image shows both lesions. The one on the left corresponds to early gastric cancer and the one on the right corresponds to GIST.
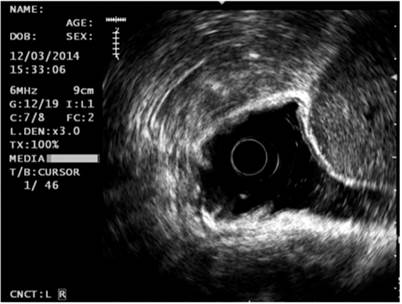
Based on the endoscopic findings, it was decided to extend the study through gastric endoscopic ultrasonography. It showed an elevated 20 mm hypoechoic lesion in the corpus that infiltrated into the mucosa and partially into the submucosa. In the antrum, a 60 mm in diameter subepithelial lesion with cystic spaces inside was found in the muscularis propria (Figure 2). No perilesional or celiac trunk adenopathy was found, and a diagnosis of early GC and GIST in the fourth layer was made. The biopsy taken from the lesion in the gastric corpus confirmed that it was a moderately differentiated gastric adenocarcinoma. A contrasted abdominal CT scan showed no metastasis from the GIST.
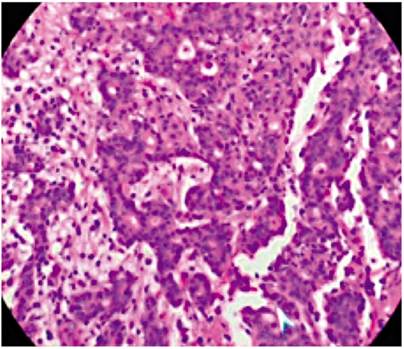
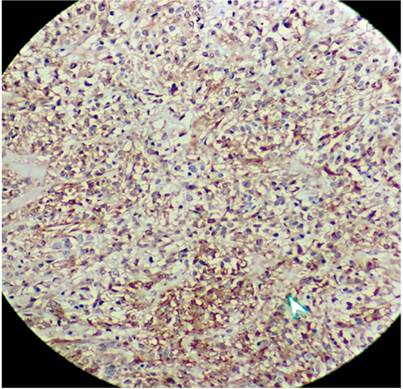
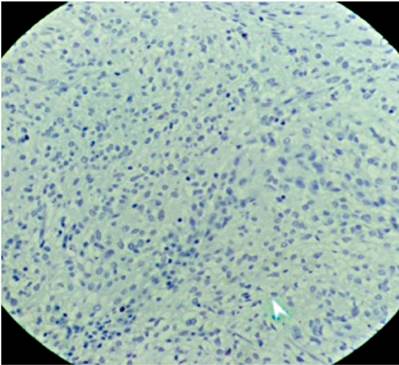
Submucosal dissection of the adenocarcinoma and surgical resection of the GIST were planned, but the patient developed acute bleeding due to ulceration of the GIST, so a subtotal gastrectomy with resection of the two lesions was performed. The pathology of the surgical specimen showed a moderately differentiated adenocarcinoma which only extended to the superficial submucosa (Figure 3). There was also a 7 x 7 cm antral lesion for which immunohistochemistry was positive for c-kit (Figure 4), positive for CD 34 positive, negative for S100. The mitotic index and ki 67 were both less than 2% (Figure 5). All nodes were negative for metastasis. The patient is asymptomatic, and evolution has been very satisfactory to date (1 year of follow-up). Since this was a case of early GC was early and the GIST was low risk, there was no need for complementary treatment.
Discussion
GIST does not occur frequently with other malignancies although there are a few case reports and case series. A series of cases published by Krame et al. has demonstrated a higher frequency of other types of tumors in patients who either had GIST at the time of the study or had suffered from GIST earlier. 17 The study covered 836 GIST patients and found that 31.9% had other types of neoplasms. Of these 43.5% were gastrointestinal, 34.1% were urogenital or breast cancer, 7.3% were hematological, and 7.3% were skin cancer. Nevertheless, most of these were found up to 5 years after the diagnosis of GIST, and no synchronous neoplasms were described. Another series of 101 patients by Goncalves were found that 13.8% had other types of tumors. Of these, 57.1% (8 cases) had GC, but none of them were synchronous. 18
Although a relationship between GIST and other neoplasms is already known, synchronous presentations have only been found in a very few case series. Wronski et al. published 28 cases of GIST with synchronous tumors and found that 57% of these were GC. 19 It is important to clarify that the type of studies that describe a relationship between GIST and GC cannot ascribe a causal association much less determine that one or the other pathology is a risk factor for the other.
Similarly, none of hypotheses about the occurrence of synchronous neoplasms with GIST have been proven yet. Larger follow-up studies and studies with larger sample sizes with comparisons with controls would be useful because they could establish whether there is a risk association between these pathologies. Nevertheless, reports like this illustrate possible association and thus refine the search for early GC in patients with GIST and vice versa.
In addition, the available evidence and its forcefulness require consideration of endoscopic management as the first-choice management for GC. The most effective type of endoscopic management is dissection of the submucosa. 5 On the other hand, laparoscopic wedge resection is the most appropriate choice for surgical management of gastric GISTs because it is high effective and has lower rate of adverse events. 16 In the case of this patient, the decision taken to perform subtotal gastrectomy was mostly guided by the development of severe bleeding.
Referencias
1. Ferlay J, Shin H, Bray F, Formar D, Mathers C, Parkin D. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893-917. https://doi.org/10.1002/ijc.25516 [ Links ]
2. Bowrey DJ, Griffin SM, Wayman J, Karat D, Hayes N, Raimes SA. Use of alarm symptoms to select dyspeptics for endoscopy causes patients with curable esophagogastric cancer to be overlooked. Surg Endosc. 2006;20(11):1725-8. https://doi.org/10.1007/s00464-005-0679-3 [ Links ]
3. Kwee R, Kwee T. Imaging in local staging of gastric cancer: a systematic review. J Clin Oncol. 2007;25(15):2107-16. https://doi.org/10.1200/JCO.2006.09.5224 [ Links ]
4. Tatsuta M, Iishi H, Okuda S, Oshima A, Taniguchi H. Prospective evaluation of diagnostic accuracy of gastrofiberscopic biopsy in diagnosis of gastric cancer. Cancer. 1989;63(7):1415-20. [ Links ]
5. Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition. Gastric cancer. 1998;1(1):10-24. https://doi.org/10.1007/PL00011681 [ Links ]
6. Sanchez-Hidalgo JM, Duran-Martinez M, Molero-Payan R, Rufian-Peña S, Arjona-Sanchez A, Casado-Adam A, et al. Gastrointestinal stromal tumors: A multidisciplinary challenge. World J Gastroenterol. 2018;24(18):1925-41. https://doi.org/10.3748/wjg.v24.i18.1925 [ Links ]
7. Harlan LC, Eisenstein J, Russell MC, Stevens JL, Cardona K. Gastrointestinal stromal tumors: treatment patterns of a populationbased sample. J Surg Oncol. 2015;111(6):702-7. https://doi.org/10.1002/jso.23879 [ Links ]
8. Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002; 33: 459-65. https://doi.org/10.1053/hupa.2002.123545 [ Links ]
9. Tio TL, Tytgat GN, den Hartog Jager FC. Endoscopic ultrasonography for the evaluation of smooth muscle tumors in the upper gastrointestinal tract: an experience with 42 cases. Gastrointest Endosc. 1990;36(4):342-50. [ Links ]
10. Ando N, Goto H, Niwa Y, Hirooka Y, Ohmiya N, Nagasaka T, et al. The diagnosis of GI stromal tumors with EUSguided fine needle aspiration with immunohistochemical analysis. Gastrointest Endosc. 2002;55(1):37-43. https://doi.org/10.1067/mge.2002.120323 [ Links ]
11. Everett M, Gutman H. Surgical management of gastrointestinal stromal tumors: analysis of outcome with respect to surgical margins and technique. J Surg Oncol 2008; 98: 588-593. https://doi.org/10.1002/jso.21030 [ Links ]
12. Dematteo RP, Gold JS, Saran L, Gönen M, Liau KH, Maki RG, et al. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer 2008;112(3):608-15. https://doi.org/10.1002/cncr.23199 [ Links ]
13. Gold JS, Dematteo RP. Combined surgical and molecular therapy: the gastrointestinal stromal tumor model. Ann Surg. 2006;244(2):176-84. https://doi.org/10.1097/01.sla.0000218080.94145.cf [ Links ]
14. Novitsky YW, Kercher KW, Sing RF, Heniford BT. Long-term outcomes of laparoscopic resection of gastric gastrointestinal estromal tumors. Ann Surg 2006;243(6):738-45. https://doi.org/10.1097/01.sla.0000219739.11758.27 [ Links ]
15. Bischof DA, Kim Y, Dodson R, Carolina Jimenez M, Behman R, Cocieru A, et al. Open versus minimally invasive resection of gastric GIST: a multiinstitutional analysis of short- and long-term outcomes. Ann Surg Oncol. 2014;21(9):2941-8. https://doi.org/10.1245/s10434-014-3733-3 [ Links ]
16. Balde AI, Chen T, Hu Y, Redondo N JD, Liu H, Gong W, et al. Safety analysis of laparoscopic endoscopic cooperative surgery versus endoscopic submucosal dissection for selected gastric gastrointestinal stromal tumors: a propensity scorematched study. Surg Endosc. 2017;31(2):843-51. https://doi.org/10.1007/s00464-016-5042-3 [ Links ]
17. Kramer K, Wolf S, Mayer B, Schmidt SA, Agaimy A, Henne-Bruns D, et al. Frequence, spectrum and prognostic impact of additional malignancies in patients with gastrointestinal stromal tumors. Neoplasia. 2015 Jan;17(1):134-40. https://doi.org/10.1016/j.neo.2014.12.001 [ Links ]
18. Gonçalves R, Linhares E, Albagli R, Valadao M, Vilhena B, Romano S, et al. Occurrence of other tumors in patients with GIST. Surg Oncol. 2010;19(4):140-3. https://doi.org/10.1016/j.suronc.2010.06.004 [ Links ]
19. Wronski M, Ziarkiewicz-Wroblewska B, Gornicka B, Cebulski W, Slodkowski M, Wasiutynski A, et al. Synchronous occurrence of gastrointes- tinal stromal tumors and other primary gastrointestinal neoplasms. World J Gastroenterol. 2006;12(33):5360. https://doi.org/10.3748/wjg.v12.i33.5360 [ Links ]
Received: January 30, 2018; Accepted: June 20, 2018











 texto en
texto en