Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.34 no.3 Bogotá July/Sept. 2019
https://doi.org/10.22516/25007440.292
Original articles
Effectiveness of vitamins C and E adjuvant to standard triple therapy for Helicobacter pylori in a cohort from the Peruvian Amazon
1Facultad de Medicina Humana, Universidad Nacional de San Martín, San Martín, Perú
2Servicio de Gastroenterología, Hospital Essalud II-Tarapoto, San Martín, Perú
3Área de Microbiología y Parasitología, Universidad Nacional de San Martín, San Martín, Perú
4Universidad Continental, Lima, Perú
Introduction and Objectives:
Adjuvant therapy with vitamins C and E has been proposed to increase standard triple therapy’s Helicobacter pylori eradication rate. This study tested this hypothesis in a cohort of patients from the Peruvian Amazon.
Material and Methods:
We retrospectively evaluated a cohort of 50 patients at Tarapoto Hospital who were treated for H. pylori infections from July to December 2016. Of these, 25 were randomly selected to receive standard triple therapy of 1 g amoxicillin, 500mg clarithromycin and 20mg omeprazole twice a day for 14 days plus adjuvant vitamins C and E. The other 25 only received standard triple therapy. The effectiveness of both treatments was estimated and compared using a general linear regression model using the Poisson family of distributions and log link with H. pylori eradication confirmed by histopathology as the outcome of interest.
Results:
A comparison of the two groups found no significant differences in their baseline symptoms, histopathological diagnoses, ages (38 ± 11 years vs. 36 ± 10 years) or genders (65% male vs. 63% male). A comparison of the effectiveness both treatments found a non-significant increase in eradication rates of 9.5% (91% vs. 82%, incidence rate ratio = 1.11; 95% confidence interval: 0.92 to 1.36).
Conclusions:
Adjuvant therapy with vitamins C and E may help increase the effectiveness of standard triple therapy for H. pylori in patients in the Peruvian Amazon, although this hypothesis needs to be confirmed in a clinical trial.
Keywords: Ascorbic acid; Vitamin E; drug therapy; Helicobacter pylori; Peru
Introducción y objetivos:
la terapia con vitaminas C y E ha sido propuesta como adyuvante a la terapia triple estándar (TTE) con el fin de incrementar la tasa de erradicación del Helicobacter pylori (H. pylori). En este estudio probamos esta hipótesis en una cohorte de pacientes de la Amazonía peruana.
Materiales y métodos:
retrospectivamente, evaluamos una cohorte de 50 pacientes infectados con H. pylori del Hospital de Tarapoto en el período comprendido entre julio-diciembre de 2016; de estos, 25 fueron tratados con TTE (amoxicilina 1 g, claritromicina 500 mg y omeprazol 20 mg, dos veces al día por 14 días) en adyuvancia con las vitaminas C y E, y 25 fueron tomados al azar (1:1), quienes solo recibieron TTE. Se estimó y comparó la efectividad de ambos tratamientos utilizando un modelo regresión general lineal con familia Poisson y link log, teniendo como desenlace de interés la erradicación del H. pylori confirmada por histopatología.
Resultados:
al comparar la cohorte de expuestos y con los no expuestos, no se encontraron diferencias significativas en sus características basales, incluyendo edad (38 ± 11 frente a 36 ± 10 años), género masculino (65 % frente a 63 %), síntomas y diagnóstico histopatológico. Al comparar la efectividad de ambos tratamientos, se encontró un incrementó no significativo en las tasas de erradicación del 9,5 % (91 % frente a 82 %, razón de tasas de incidencia = 1,11; intervalo de confianza [IC] 95 %: 0,92 a 1,36).
Conclusiones:
la terapia adyuvante con vitaminas C y E podría ayudar a incrementar la efectividad de la TTE para H. pylori en pacientes de la Amazonía peruana, aunque se requiere confirmar esta hipótesis en un ensayo clínico.
Palabras clave: Ácido ascórbico; vitamina E; Helicobacter pylori; Perú
Introduction
Worldwide, H. pylori infections are some of the most common bacterial infections in adults. 1 In developing countries, prevalence is estimated to be above 70%, while in developed countries it is close to 35%. 2 This bacterium colonizes the stomach and usually produces symptoms in 32% of cases. The most frequent are abdominal pain, regurgitation, heartburn, nausea and hyporexia. 3 Currently, the World Health Organization (WHO) lists H. pylori as a type 1 carcinogen due to its close relationship with gastric cancer. 4 For this reason, and because it is a highly pathogenic microorganism, initiation of eradication therapy is recommended at the time of diagnosis. 5
Currently, first-line H. pylori eradication treatment is standard triple therapy (STT) which consists of administration of two antibiotics, 2 g of amoxicillin twice daily and 500 mg of clarithromycin twice daily, and 20 mg of omeprazole, a proton pump inhibitor, twice daily for two weeks. 5 Until the last decade, the best H. pylori eradication rates achieved were in the range of 77% to 82%. 6,7 Since then, various modifications have been proposed for increasing these rates. Among them are quadruple therapy, sequential therapy, hybrid therapy and adjuvant therapy with vitamins C and E. 8
Adjuvant therapy with vitamins C and E has generated great expectations because it is relatively safe and accessible and because some reports have indicated that it can raise eradication rates to above 90%. 9 Vitamin C’s adjuvant effect has been shown to be due to its ability to inhibit the formation of N-nitrous compounds and reactive oxygen metabolites in the gastric mucosa. 10 Both of these are considered vital for growth and carcinogenicity of H. pylori. 11 The adjuvant effect of vitamin E is due to its ability to inhibit lipid peroxidation and oxidative stress in the microenvironment created by H. pylori in the gastric mucosa. 12 These two factors are also considered to be essential factors for growth and pathogenesis of H. pylori. 13
The first studies that evaluated the effects of adjuvant therapy with vitamins C and E yielded unfavorable results, 14 and the first metaanalysis published by Li in 2011 stated that there were insufficient significant findings evidence to recommend the therapy (relative risk = 0.93; 95% confidence interval (CI): 0.56 to 1.53) 15 However, newer studies show satisfactory and significant results with eradication rates for adjuvant therapy with vitamins C and E exceed those of standard triple therapy by 20%. 16 According to the latest published reports, the difference could be even larger for patients with low levels of antioxidant capacity and iron deficiency anemia. 17,18 This is very important for Peru where the prevalence of iron deficiency anemia exceeds 40%. 19 In the Peruvian jungle regions including the department of San Martín, prevalence of iron deficiency anemia is around 24%. 20 In most cases, this pathology is associated with malabsorption of iron related to low concentrations of vitamin C. 21 For this reason, our study attempts to determine the efficacy of the use of vitamins C and E, two antioxidants, as treatment adjuvant to STT for H. pylori in a cohort of patients from the Peruvian Amazon.
Materials and methods
Study Design
A retrospective cohort study was conducted in the district of Tarapoto in the province of San Martín (6 ° 29′00 ″ S, 76 ° 22′00 ″ W, population ~ 118,000) in the department of San Martín, in the northeastern region of Peru. The entire cohort consisted of patients with H. pylori infections diagnosed at the Social Security Hospital (EsSalud) of Tarapoto between July and December 2016. Patients who received STT plus adjuvant treatment with vitamins C and E were labeled the “exposed” cohort in this study. In order to estimate the effects attributable to adjuvant treatment with vitamins C and E, the exposed cohort was compared with a control cohort that was labelled “unexposed”. For this, a random sample (1:1) was taken from hospital records by simple random sampling from the total number of patients who were diagnosed with H. pylori infections and treated with STT during the same period of time. The control cohort was not exposed to adjuvant therapy with vitamins C and E. To compare the effectiveness of the treatment, both cohorts (exposed and unexposed) were followed retrospectively to measure and compare the eradication rates of H. pylori. In order to avoid information bias, eradication of H. pylori was defined a priori as a confirmed histopathological and upper endoscopy diagnosis.
Population and Sample
The exposed cohort and the unexposed cohort consisted of patients who were adults aged 18 to 60 years old, who had symptoms of abdominal pain, regurgitation, heartburn, nausea and/or hyporexia, and who had been diagnosed histopathologically with H. pylori. The exposed cohort consisted of those patients treated during the study period while the unexposed cohort was chosen randomly (sample 1: 1) from among all patients who had been diagnosed and treated previously and who met the criteria. Both cohorts received STT, but only the exposed cohort also received adjuvant treatment with vitamins C and E. Patients were excluded if they had had prior treatment for H. pylori, histories of gastric or duodenal ulcers, neoplasms of any kind, had been diagnosed as carriers of any metabolic disease, if they were pregnant or lactating women, if they had been treated with antibiotics in the six months prior to the study, if they were allergic to penicillins or other antibiotics, and if they had histories of previous gastric surgery. The sample size was estimated on the basis that a minimum of 50 patients (25 exposed and 25 unexposed) would be required to find differences in eradication rates over 25%, a 70% eradication rate was assumed in the unexposed, and an exploratory 90% CI and a study power of 80% were set. To maximize the power of study, from the beginning we planned to include all patients who met the selection criteria during the study period
Standard Triple Therapy for H. pylori
STT was provided for free to all patients by the Social Security hospital (EsSalud) of Tarapoto. STT, considered the first line H. pylori treatment, 8 consists of twice daily oral administration of 1 g of amoxicillin, 500 mg of clarithromycin, and 20 mg of omeprazole for 14 days.
Standard Triple Therapy Plus Adjuvant Treatment with Vitamins C and E
The use of STT plus adjuvant treatment with vitamins C and E is a potential new alternative therapy for H. pylori. It consists of twice daily administration of 500 mg of vitamin C and 200 IU of vitamin E until 30 days after the completion of STT. 9
Data Collection
The medical records from the Social Security hospital (EsSalud) in Tarapoto of all of these patients were used as the primary source of information. In the case of the exposed cohort, all data of interest were identified by the principal investigator (Wildor Samir Cubas, WSC) and taken from patients’ medical records. For the unexposed patients, a form was first elaborated with patients’ medical record numbers in chronological order. Next, the sample was taken, and then the data was collected. In both cases, age, gender, work environment, origin, main symptoms, disease duration and endoscopic and histopathological findings before and after therapy were recorded. All the variables of interest were measured in the standard way and collected retrospectively from the medical records of each study subject. To facilitate data collection, a checklist with ranges of values and pre-established categories was developed to ensure reliable data collection. Once this process was finished, the data were typed in duplicate and any discrepancies were resolved by another review of the medical records.
Data Analysis
Our descriptive analysis of baseline clinical-epidemiological characteristics of the study population included standard deviations (SD) of quantitative variables and absolute and relative frequencies of qualitative variables. Fisher’s exact test and the χ² test were used for comparison of proportions of the baseline characteristics of the exposed and unexposed. A multivariate Poisson regression analysis was performed to estimate the efficacy attributable to adjuvant therapy with vitamins C and E. The nested model method controlled by baseline characteristics was used to isolate the effect of adjuvant treatment. In this analysis, age, gender, adherence to treatment, disease time and number of symptoms were taken as potential confounding factors. In all cases, data analysis was performed using the STATA MP v13 statistical package considering a 95% CI.
Ethical Considerations
The ethics committee of the Hospital Nacional Docente Madre Niño “San Bartolomé” in Lima, Peru reviewed and approved the protocol of this study. The confidentiality of information was respected although informed consent was not necessary because the data was obtained retrospectively.
Results
Study Population Characteristics
Between July and December 2016, a total of 50 patients with H. pylori infections were evaluated and received eradication therapy. Of these, five patients (10%) were excluded from the analysis because they did not complete treatment. Of these five, two belonged to the exposed group and three belonged to the unexposed group. Among the 45 patients analyzed, no differences were found between exposed and unexposed patients (23 versus 22) in terms of male predominance (65% vs. 64%) and average age (38 ± 11 versus 36 ± 10 years ), which was 37 ± 11 years (range: 19-59 years). The majority of patients came from the city of Tarapoto (74% versus 64%), and the rest came from rural areas of the town’s periphery (26% versus 36%). The majority worked in the public sector (52% vs. 63%), and the rest were private sector workers (39% vs. 31%) or unemployed (9% vs. 5%). The main reason these patients came to the Gastroenterology Department of the Social Security Hospital (EsSalud) in Tarapoto was abdominal pain (43% versus 40%) which was followed by regurgitation (22% versus 27%), heartburn (17% vs. 18%), hyporexia (13% vs. 5%) and nausea (4% vs. 9%). Clinical manifestations had presented more than seven days prior to coming to the hospital in most cases (74% versus 77%). According to endoscopic findings prior to eradication therapy, the most frequent gastric lesions were antral gastritis (43% vs. 55%), pangastritis (43% vs. 27%) and mild intestinal metaplasia (13% versus 18%)
Efficacy of H. Pylori Eradication Therapies
The overall efficacy of H. pylori eradication therapies, the eradication rate, was estimated at 87% (95% CI: 76% to 97%). That for the exposed group was 91% (95% CI: 79-100%) while that for the unexposed group was 82% (95% CI: 64-99%). Based on these eradication rates, a ratio of incidence rates of 1.12 (95% CI 0.88 to 1.41) was calculated.
Discussion
Adjuvant therapy with vitamins C and E has been reported to be an effective alternative for increasing the eradication rate of H. pylori attributable to STT. Although this may be the case for H. pylori patients in the Peruvian Amazon, the results of our study do not allow us to make this conclusion even though our data indicate that adjuvant therapy increased the eradication rate by 9.5% (91% vs. 82%; incidence rate ratio = 1.11; 95% CI: 0.92 to 1.36). Nevertheless, this effect was statistically not significant (Figure 1). This could be because the effect does not exist or because it exists, but its magnitude is too small to have been detected with a power of study as small as ours.
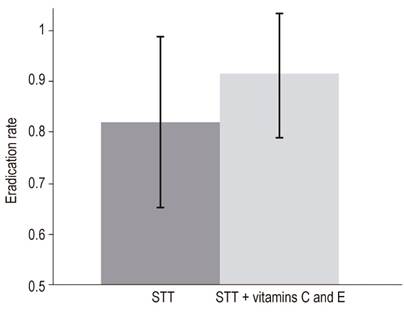
Figure 1 Effectiveness attributable to adjuvant use of vitamins C and E with standard triple therapy for patients infected with H. pylori in the Peruvian Amazon. Adjuvant treatment with vitamins C and E may increase eradication rates obtained with STT in patients infected with H. pylori (91% vs. 82%, incidence rate ratio = 1.11; 95% CI : 0.92 to 1.36).
According to several previous studies, the eradication rates attributable to STT plus adjuvant vitamins C and E can be as high as 94%. 9 This eradication rate is very similar to the 91% found in our study (Table 1). These rates may be explained by deficiencies of its antioxidant capacities of the populations studied. This was evidenced in an experimental study carried out in Asia where the patients in the sample infected with H. pylori also had low levels of antioxidant capacity. After administration of eradication therapy plus adjuvant vitamins C and E, eradication rates were higher than with standard triple therapy. 16 Subsequent studies have found that low levels of antioxidants are related to increased virulence and persistence of H. pylori, and that increasing antioxidant levels can affect survival of the bacteria. 22,23 Another factor involved in H. pylori pathogenesis is its direct relationship with iron deficiency anemia in infected individuals resulting from poor absorption of iron when concentrations of antioxidants are low. This is the case with vitamin C in patients with H. pylori infections. 17,21 This may add to the problem in South American countries like Peru where the latest reports of H. pylori prevalence and anemia exceed 60% and 40%, respectively. 19,24. In the Peruvian Amazon, including the department of San Martín, anemia’s prevalence is approximately 24%. 20
Table 1 Eradication rate of H. Pylori according to exposure to therapy with vitamin C and E
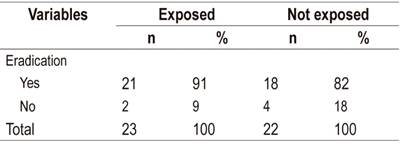
Source: clinical records of patients infected with H. pylori from the Gastroenterology Service of the Social Security Hospital (EsSalud) of Tarapoto between July and December 2016.
The findings of our work indicate that STT plus adjuvant vitamins C and E has therapeutic results that are superior to those of STT alone (91% vs. 82%). Given the close relationship between H. pylori infections, anemia, and low levels of antioxidants, we can infer that supplementing eradication therapies with vitamins C and E may have indirectly improved antioxidant levels in this study’s subjects which could have contributed to raising the H. pylori eradication rate above that of STT alone. Although some studies done in the past decade suggest that the evidence for recommending this adjuvant therapy is insufficient, 15 a number of other studies have demonstrated its therapeutic effectiveness. 25,26,27
As in other studies, H. pylori infections were observed most frequently in male adult patients 24,28,29,30,31 However, contrary to expectations, the majority of patients infected with H. pylori (68%) came from urban areas of Tarapoto while a minority came from rural areas (Table 2). This may be due to a design effect, since H. pylori infections are commonly reported to be associated with poor socioeconomic conditions and poor basic services with limited access to drinking water. However, in Peru potable water does not seem to prevent new H. pylori infections. In fact, according to a recent study carried out in Lima, where the majority of the population has access to drinking water, it is common to find remains of H. pylori genetic material in drinking water. 32 What is even more worrisome is that strains of H. pylori that are resistant to standard levels of sodium hypochlorite (chlorine) are not uncommon, either. 33
Table 2 Population characteristics
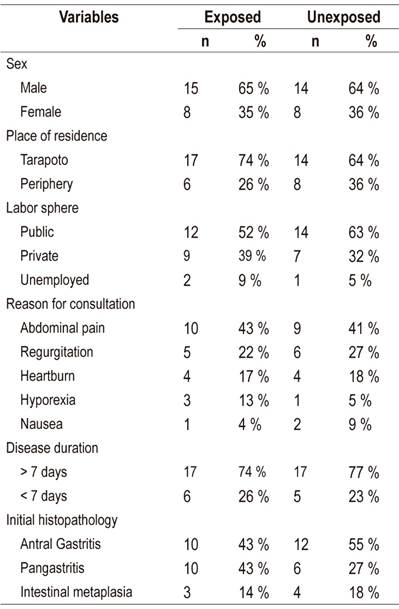
Source: clinical records of patients infected with H. pylori from the Gastroenterology Service of the Social Security Hospital (EsSalud) of Tarapoto between July and December 2016.
In conclusion, adjuvant treatment with vitamins C and E may increase the effectiveness of standard triple therapy for H. pylori in patients in the Peruvian Amazon. Nevertheless, to demonstrate this conclusively more experimental research is needed.
Referencias
1. Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017 Aug;153(2):420-429. https://doi.org/10.1053/j.gastro.2017.04.022 [ Links ]
2. Zamani M, Ebrahimtabar F, Zamani V, Miller WH, Alizadeh-Navaei R, Shokri-Shirvani J, et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther. 2018 Apr;47(7):868-876. https://doi.org/10.1111/apt.14561 [ Links ]
3. Suzuki H. Helicobacter pylori-Associated Upper Gastrointestinal Symptoms: FD or HpD? Dig Dis Sci. 2017 Jun;62(6):1391-1393. https://doi.org/10.1007/s10620-017-4556-4 [ Links ]
4. Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1-241. [ Links ]
5. Fallone CA, Chiba N, van Zanten SV, Fischbach L, Gisbert JP, Hunt RH, et al. The Toronto Consensus for the Treatment of Helicobacter pylori Infection in Adults. Gastroenterology . 2016 Jul;151(1):51-69.e14. https://doi.org/10.1053/j.gastro.2016.04.006 [ Links ]
6. Gisbert JP, Calvet X. Review article: the effectiveness of standard triple therapy for Helicobacter pylori has not changed over the last decade, but it is not good enough. Aliment Pharmacol Ther. 2011 Dec;34(11-12):1255-68. https://doi.org/10.1111/j.1365-2036.2011.04887.x [ Links ]
7. Novoa Reyes I, Caravedo Martínez M, Huerta-Mercado TJ, De los Ríos Senmache R, Pinto Valdivia J, Bussalleu Rivera A. Recurrencia de la infección gástrica con Helicobacter pylori en adultos peruanos con distrés postprandial dos años después de la erradicación exitosa. Rev Gastroenterol del Perú. 2014;34(1):15-21. [ Links ]
8. Malfertheiner P, Megraud F, O’Morain CA, Gisbert JP, Kuipers EJ, Axon AT, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017 Jan;66(1):6-30. https://doi.org/10.1136/gutjnl-2016-312288 [ Links ]
9. Sezikli M, Cetinkaya ZA, Sezikli H, Güzelbulut F, Tiftikçi A, Ince AT, et al. Oxidative stress in Helicobacter pylori infection: does supplementation with vitamins C and E increase the eradication rate? Helicobacter. 2009 Aug;14(4):280-5. https://doi.org/10.1111/j.1523-5378.2009.00686.x [ Links ]
10. Zhang ZW, Abdullahi M, Farthing MJ. Effect of physiological concentrations of vitamin C on gastric cancer cells and Helicobacter pylori. Gut. 2002 Feb;50(2):165-9. https://doi.org/10.1136/gut.50.2.165 [ Links ]
11. Zhang ZW, Farthing MJ. The roles of vitamin C in Helicobacter pylori associated gastric carcinogenesis. Chin J Dig Dis. 2005;6(2):53-8. https://doi.org/10.1111/j.1443-9573.2005.00194.x [ Links ]
12. Traber MG, Stevens JF. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic Biol Med. 2011 Sep 1;51(5):1000-13. https://doi.org/10.1016/j.freeradbiomed.2011.05.017 [ Links ]
13. Sugimoto N, Yoshida N, Nakamura Y, Ichikawa H, Naito Y, Okanoue T, et al. Influence of vitamin E on gastric mucosal injury induced by Helicobacter pylori infection. Biofactors. 2006;28(1):9-19. https://doi.org/10.1002/biof.5520280102 [ Links ]
14. Everett SM, Drake IM, White KL, Mapstone NP, Chalmers DM, Schorah CJ, et al. Antioxidant vitamin supplements do not reduce reactive oxygen species activity in Helicobacter pylori gastritis in the short term. Br J Nutr. 2002 Jan;87(1):3-11. https://doi.org/10.1079/BJN2001477 [ Links ]
15. Li G, Li L, Yu C, Chen L. Effect of vitamins C and E supplementation on Helicobacter pylori eradication: a meta-analysis. Br J Nutr. 2011 Dec;106(11):1632-7. https://doi.org/10.1017/S0007114511003813 [ Links ]
16. Sezikli M, Cetinkaya ZA, Güzelbulut F, Sezikli H, Özkara S, Coşgun S, et al. Efficacy of vitamins supplementation to therapy on Helicobacter pylori eradication in patients with low antioxidant capacity. Clin Res Hepatol Gastroenterol. 2011 Nov;35(11):745-9. https://doi.org/10.1016/j.clinre.2011.07.001 [ Links ]
17. Hudak L, Jaraisy A, Haj S, Muhsen K. An updated systematic review and meta-analysis on the association between Helicobacter pylori infection and iron deficiency anemia. Helicobacter . 2017 Feb;22(1). https://doi.org/10.1111/hel.12330 [ Links ]
18. Franceschi F, Annalisa T, Teresa DR, Giovanna D, Ianiro G, Franco S, et al. Role of Helicobacter pylori infection on nutrition and metabolism. World J Gastroenterol. 2014 Sep 28;20(36):12809-17. https://doi.org/10.3748/wjg.v20.i36.12809 [ Links ]
19. Alcázar L. Impacto económico de la anemia en el Perú. Lima: GRADE; Acción contra el Hambre; 2012. p. 19-24. [ Links ]
20. Tarqui-Mamani C, Sanchez-Abanto J, Alvarez-Dongo D, Espinoza-Oriundo P, Jordan-Lechuga T. Prevalencia de anemia y factores asociados en adultos mayores peruanos. Rev Peru Med Exp Salud Pública. 2015;32(4):687-92. https://doi.org/10.17843/rpmesp.2015.324.1759 [ Links ]
21. Lane DJ, Jansson PJ, Richardson DR. Bonnie and Clyde: Vitamin C and iron are partners in crime in iron deficiency anaemia and its potential role in the elderly. Aging (Albany NY). 2016 May;8(5):1150-2. https://doi.org/10.18632/aging.100966 [ Links ]
22. Sezikli M, Çetinkaya ZA, Güzelbulut F, Çimen B, Özcan Ö, Özkara S, et al. Effects of alpha tocopherol and ascorbic acid on Helicobacter pylori colonization and the severity of gastric inflammation. Helicobacter . 2012 Apr;17(2):127-32. https://doi.org/10.1111/j.1523-5378.2011.00925.x [ Links ]
23. Hagag AA, Amin SM, El-Fiky RB, El-Sayad ME. Study of Serum Levels of Some Oxidative Stress Markers in Children with Helicobacter pylori Infection. Infect Disord Drug Targets. 2018;18(1):52-59. https://doi.org/10.2174/1871526517666170102115116 [ Links ]
24. Pareja Cruz A, Navarrete Mejía PJ, Parodi García JF. Seroprevalencia de infección por Helicobacter pylori en población adulta de Lima, Perú 2017. Horizonte Médico. 2017;17(2):55-8. https://doi.org/10.24265/horizmed.2017.v17n2.08. [ Links ]
25. Tümgör G, Baran M, Çakır M, Yüksekkaya HA, Aydoğdu S. Comparison of standard and standard plus vitamin E therapy for Helicobacter pylori eradications in children. Turk J Gastroenterol. 2014 Dec;25 Suppl 1:99-103. https://doi.org/10.5152/tjg.2014.5592 [ Links ]
26. Demirci H, Uygun İlikhan S, Öztürk K, Üstündağ Y, Kurt Ö, Bilici M, et al. Influence of vitamin C and E supplementation on the eradication rates of triple and quadruple eradication regimens for Helicobacter pylori infection. Turk J Gastroenterol. 2015 Nov;26(6):456-60. https://doi.org/10.5152/tjg.2015.0233 [ Links ]
27. Sezikli M, Çetinkaya ZA, Güzelbulut F, Yeşil A, Coşgun S, Kurdaş OÖ. Supplementing vitamins C and E to standard triple therapy for the eradication of Helicobacter pylori. J Clin Pharm Ther. 2012 Jun;37(3):282-5. https://doi.org/10.1111/j.1365-2710.2011.01286.x [ Links ]
28. Bui D, Brown HE, Harris RB, Oren E. Serologic Evidence for Fecal-Oral Transmission of Helicobacter pylori. Am J Trop Med Hyg. 2016 Jan;94(1):82-8. https://doi.org/10.4269/ajtmh.15-0297 [ Links ]
29. Castillo Contreras O, Maguiña Quispe J, Benites Goñi H, Chacaltana Mendoza A, Guzmán Calderón E, Dávalos Moscol M, et al. Prevalencia de Helicobacter pylori en pacientes sintomáticos de consulta externa de la Red Rebagliati (EsSalud), Lima, Perú, en el período 2010-2013. Rev Gastroenterol Perú. 2016;36(1):49-55. [ Links ]
30. Wex T, Venerito M, Kreutzer J, Götze T, Kandulski A, Malfertheiner P. Serological prevalence of Helicobacter pylori infection in Saxony-Anhalt, Germany, in 2010. Clin Vaccine Immunol. 2011 Dec;18(12):2109-12. https://doi.org/10.1128/CVI.05308-11 [ Links ]
31. Zhang M, Zhou YZ, Li XY, Tang Z, Zhu HM, Yang Y, Chhetri JK. Seroepidemiology of Helicobacter pylori infection in elderly people in the Beijing region, China. World J Gastroenterol. 2014 Apr 7;20(13):3635-9. https://doi.org/10.3748/wjg.v20.i13.3635 [ Links ]
32. Boehnke KF, Brewster RK, Sánchez BN, Valdivieso M, Bussalleu A, Guevara M, et al. An assessment of drinking water contamination with Helicobacter pylori in Lima, Peru. Helicobacter . 2018 Apr;23(2):e12462. https://doi.org/10.1111/hel.12462 [ Links ]
33. Ramírez A, Chinga E, Mendoza D. Variación de la prevalencia del H. Pylori y su relación con los niveles de cloro en el agua de la Atarjea, Lima, Perú: Período 1985-2002. Rev Gastroenterol Perú. 2004;24(3):223-9. [ Links ]
Received: September 17, 2018; Accepted: December 18, 2018











 text in
text in 


