Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.34 no.3 Bogotá July/Sept. 2019
https://doi.org/10.22516/25007440.328
Original articles
Characterization of patients with chronic hepatitis C treated in a high complexity hospital in Medellín
1Grupo Promoción y Prevención Farmacéutica. Universidad de Antioquia, Medellín, Colombia
2Unidad de Hepatología y Trasplante Hepático, Hospital Pablo Tobón Uribe; Grupo Gastrohepatología, Universidad de Antioquia, Medellín, Colombia
3Facultad de Medicina, Universidad de Antioquia, Medellín, Colombia
Introduction:
Throughout the world hepatitis C (HepC) is a public health problem. Estimates for its prevalence in Colombia range from 0.5% to 1% but 2.1 % for patients over 50 years of age. The Hepatology Unit at the Hospital Pablo Tobón Uribe (HPTU) has been a benchmark for management of HepC in Medellín and Colombia for years.
Objective:
To describe sociodemographic and clinical characteristics together with health outcomes of patients with chronic HepC who were treated at the HPTU between 2013 and 2018.
Materials and methods:
This is an observational, descriptive and retrospective study of patients with chronic HepC, treated between January 1, 2013 and March 31, 2018.
Results:
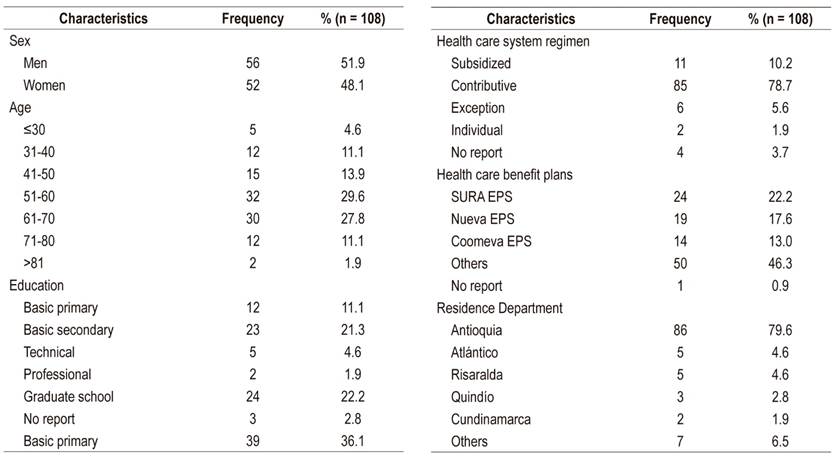
One hundred and eight patients were analyzed. The average age was 55.8 years (SD 13.7), 51.9% were men, and 78.7% belonged to the contributory health care scheme. Most frequently, the disease was transmitted by blood, and genotype 1 predominated in the group of patients analyzed. The effectiveness of interferon schemes was 46.9% while that of Direct-Acting Antivirals (DAA) was 94.6%. Adverse drug reactions were found in 68.2% of patients treated with interferon/ribavirin schemes but in only 25.9% of the patients treated with DAA.
Conclusions:
In this group of patients treated at HPTU, DAA were safer and more effective than interferon/ribavirin schemes.
Keywords: Hepatitis C; Colombia; antivirals; interferons; direct action antivirals
Introducción:
la hepatitis C (HepC) representa un problema de salud pública a nivel mundial. Se estima que en Colombia la prevalencia de virus de la hepatitis C (VHC) está entre el 0,5-1 %, y asciende al 2,1 % en pacientes mayores de 50 años. La Unidad de Hepatología del Hospital Pablo Tobón Uribe (HPTU) ha sido un referente en el manejo de la HepC en Medellín y Colombia durante años.
Objetivo:
describir las características sociodemográficas/clínicas y los resultados en salud de los pacientes con HepC crónica atendidos en el HPTU entre 2013 y 2018.
Materiales y métodos:
estudio observacional, descriptivo, retrospectivo de pacientes con HepC crónica atendidos entre el 1 de enero de 2013 y el 31 de marzo de 2018.
Resultados:
se analizaron 108 pacientes. La edad promedio fue de 55,8 años (desviación estándar [DE] 13,7), 51,9 % eran hombres, y 78,7 % pertenecían al régimen contributivo. El mecanismo de transmisión más frecuente fue la hemotransfusión; el genotipo 1 predominó en el grupo de pacientes analizados. La efectividad de los esquemas con interferón fue del 46,9 % y de los antivirales de acción directa (AAD) del 94,6 %. La presencia de reacciones adversas a medicamentos (RAM) fue del 68,2 % en pacientes con esquemas con interferón/ribavirina y del 25,9 % en pacientes con AAD.
Conclusiones:
se realiza la caracterización de los pacientes atendidos en el HPTU, en quienes los AAD han mostrado mayor efectividad y seguridad en comparación con esquemas con interferón/ribavirina.
Palabras clave: Hepatitis C; Colombia; antivirales; interferones; antivirales de acción directa
Introduction
Hepatitis C (HepC) is a global public health problem whose prevalence is between 2% and 3%. It progresses to chronic diseases, and 70% to 90% of patients develop chronic liver diseases including cirrhosis and hepatocellular carcinoma (HCC). 1,2 Especially vulnerable populations including injectable drug users and people with inadequate healthcare. In Colombia, it is estimated that the prevalence of HepC in the overall population is between 0.5% but that it is 2.1% in people over 50 years of age. 3
The goal of treatment is to reduce adverse health consequences such as terminal liver disease and HCC and reduce mortality from any cause by achieving sustained virological response (SVR). 4 This is defined as an unmeasurably small viral load at 12 weeks after the end of interferon-free therapies or at or 24 weeks interferon-based therapies. 5
Treatment of HepC has evolved considerably. Treatment with pegylated interferon (peg-IFN) and ribavirin (RBV) are not tolerated well by many patients and which only achieve SVR in 6% to 56% of patients. 2,6 Consequently, they are being replaced by direct action antivirals (DAAs) which achieve SVR in more than 90% of patients, have shorter treatment times and reduce the number of adverse events. 5,7,8 In the United States, second generation DAAs were approved in 2013. In Colombian they were considered to be vital drugs that were not available until simeprevir (SMV), daclatasvir (DCV) and asunaprevir went on the market in 2015. These were followed by the treatment regimen of paritaprevir/ombitasvir/ritonavir/dasabuvir (PrOD) in 2016 and by sofosbuvir (SOF) and ledipasvir (LDV) in 2017. 9
Hospital Pablo Tobón Uribe (HPTU) is a referral center for hepatology patients which is responsible for providing care for chronic HepC patients from various parts of Colombia. Nevertheless, systematic information on the characteristics of the diseases and patient population had not been published before now. Therefore, the objective of this work was to describe the sociodemographic, clinical characteristics and health outcomes of patients with HepC treated at the HPTU between 2013 and 2018.
Material and methods
Study Population
Patients with chronic HepC whose diagnoses were confirmed by detection of hepatitis C virus RNA and who were treated in the HPTU between January 1, 2013 and March 31, 2018 were included in this study. Patients who were not treated pharmacologically, patients who were treated before 2013, and patients whose treatment information was incomplete were excluded.
Variables
Sociodemographic information collected included patient sex, age, schooling, insurance, affiliation regime, and residence department.
Clinical information collected included HCV transmission mechanism, HCV genotype/subtype, fibrosis/cirrhosis status, co-infection with human immunodeficiency virus (HIV) and/or hepatitis B virus (HBV), previous treatment schemes, treatment with DAA, variants associated with resistance (VAR), adverse drug reactions (ADR), number of non-anti-HCV medications used by the patient, hospitalization in the HPTU related to HepC, and SVR.
Information Gathering Process
Consolidation of the medical records of patients with ICD-10 codes B182 and B171 was obtained from the investigating hepatologist. Sociodemographic and clinical variables were extracted from the electronic medical record and recorded on a form in Microsoft Access® 2010.
Results
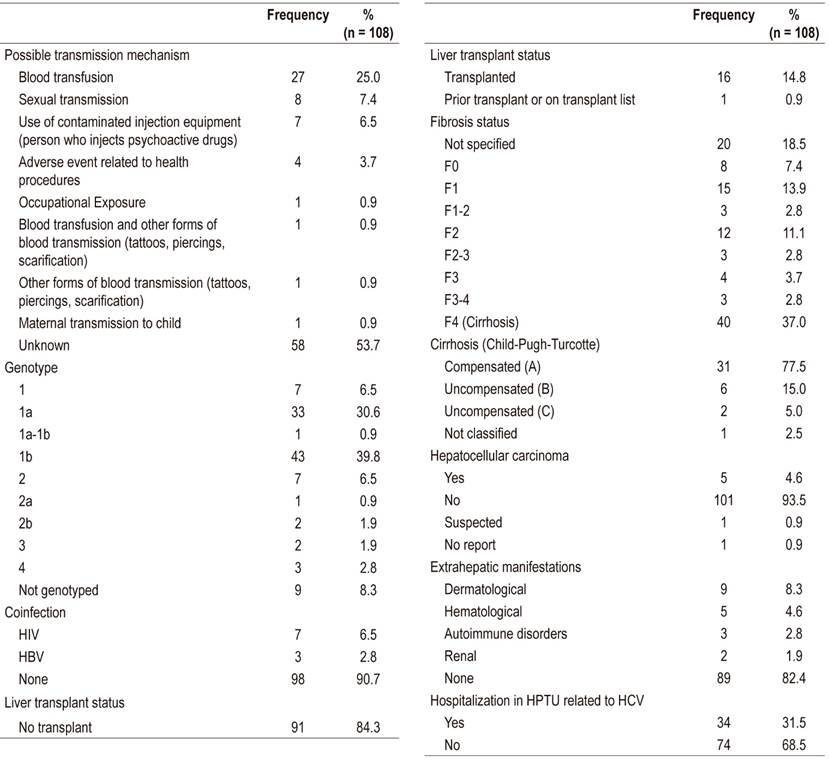
One hundred eight patients were included in the analysis (Figure 1). Of these, 51.9% were men, and the average age was 55.8 years (standard deviation [SD] 13.7) (Table 1). The most frequent transmission mechanism was a blood transfusion (25%), genotype 1 had the highest prevalence (77.8%), 39.8% of patients had advanced fibrosis/cirrhosis (F3-F4), 77.5% of patients in F4 had compensated cirrhosis, 4.6% had HCC, 90.7% had no coinfections, and 31.5% were hospitalized in the HPTU for causes related to HepC. Other clinical features can be seen in Table 2.
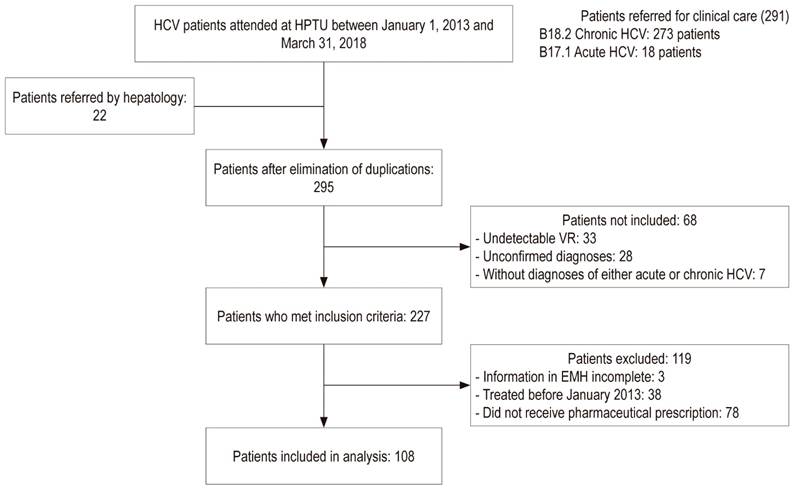
Figure 1 General diagram of the investigation. VR: viral load; EMH: electronic medical history; HPTU: Hospital Pablo Tobón Uribe.
Treatment of HCV Infections
Thirty-sever percent of the patients were treated solely with peg-INF, 24.1% were treated with peg-INF followed by rescue therapy with DAA, and 38.9% were treated only with DAA (Table 3).
Table 3 Prescribed hepatitis C treatment schemes
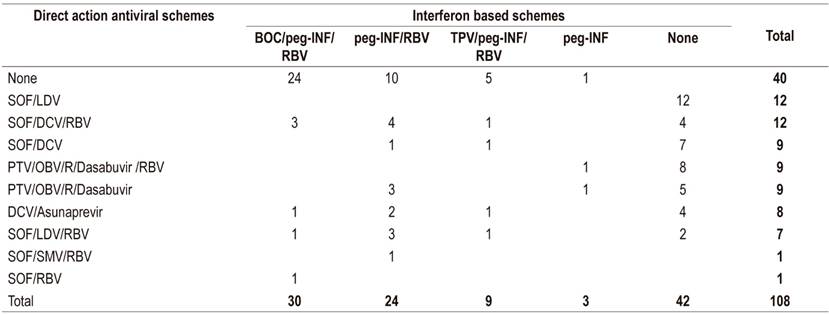
BOC: boceprevir; DCV: daclatasvir; LDV: ledipasvir; OBV: ombitasvir; peg-IFN: pegylated interferon; PTV: paritaprevir; R: ritonavir; RBV: ribavirin; SMV: simeprevir; SOF: sofosbuvir; POS: Telaprevir.
Of those treated with peg-INF (61.1%), 59.1% received boceprevir or telaprevir. Of these, 46.9% reached SVR (Figure 2). There were no SVR reports for five patients, three patients were waiting for interferon-free therapies, and one patient died due to a septic shock of urinary origin and severe hepatic encephalopathy. DAAs were prescribed for twenty-six patients who did not reach SVR.
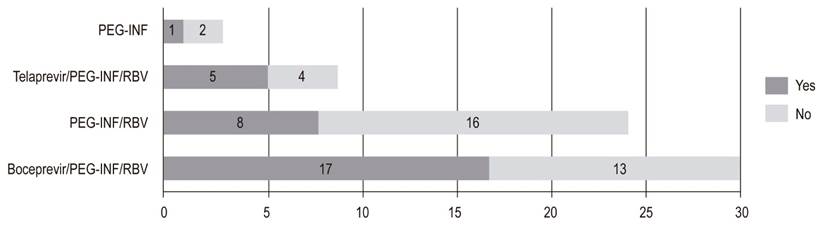
Figure 2 Sustained virological response range with interferon (n = 66). peg-INF: Pegylated interferon; RBV: ribavirin.
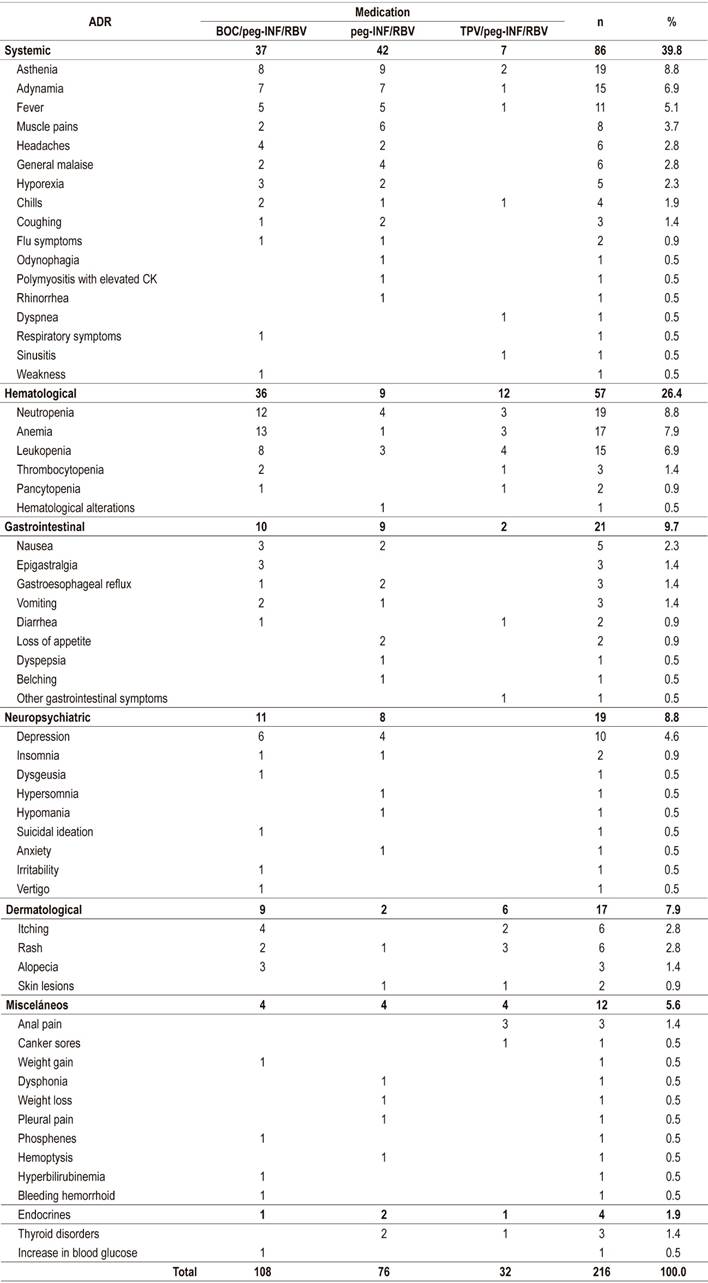
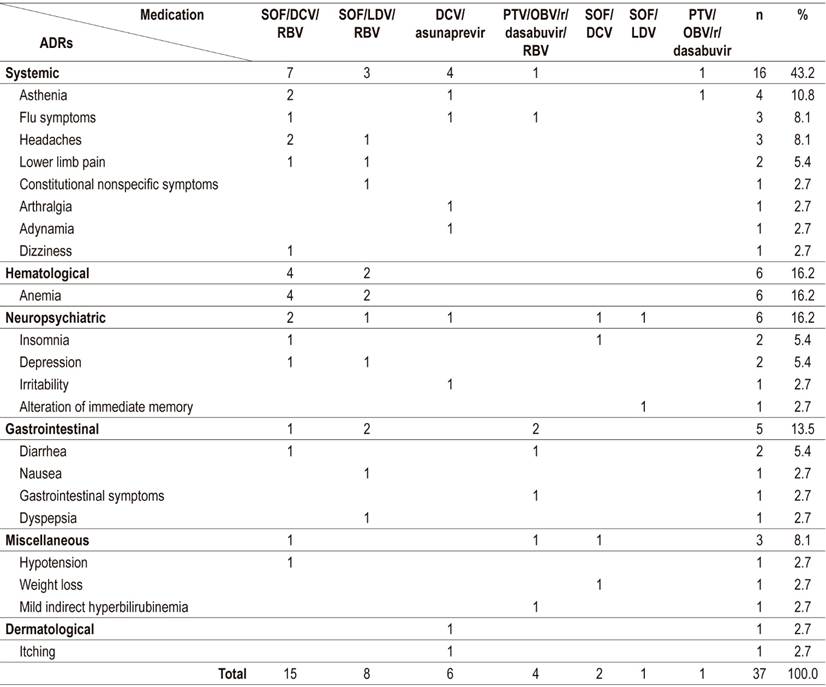
Of the patients treated with peg-IFN, 68.2% had reports of ADRs in the EMH. ADRs occurred most frequently with boceprevir schemes. A total of 216 ADRs were recorded with asthenia and neutropenia each accounting for 8.8%, anemia for 7.9%, and leukopenia and adynamia for 6.9% each (Table 4).
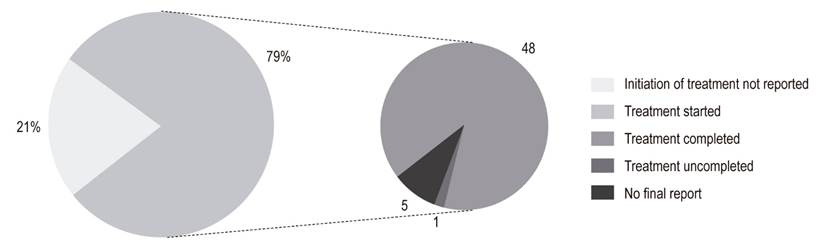
Use of Direct-Acting Antivirals
The most frequently prescribed DAAs were SOF/LDV and SOF/DCV/RBV (Table 3). Of the patients for whom DAAs were prescribed, 79.4% reportedly began treatment, and 88.9% of these completed treatment (Figure 3). Of the patients who finished treatment, 77.1% (37/48) had a viral load report at 12 weeks after the end of treatment. Of these, 94.6% achieved SVR (Figure 4). The remaining 5.4% did not achieve SVR due to a VAR, mainly related to NS5A inhibitors. The first patient took DCV/asunaprevir for 24 weeks without achieving SVR. In this case, no other scheme was initiated, given the costs and risks of side effects. The second patient received SOF/SMV/RBV for 12 weeks, but did not reach SVR. The specialist reported that the treatment was not available. It should be noted that a third patient presented VAR but achieved SVR (Table 5).
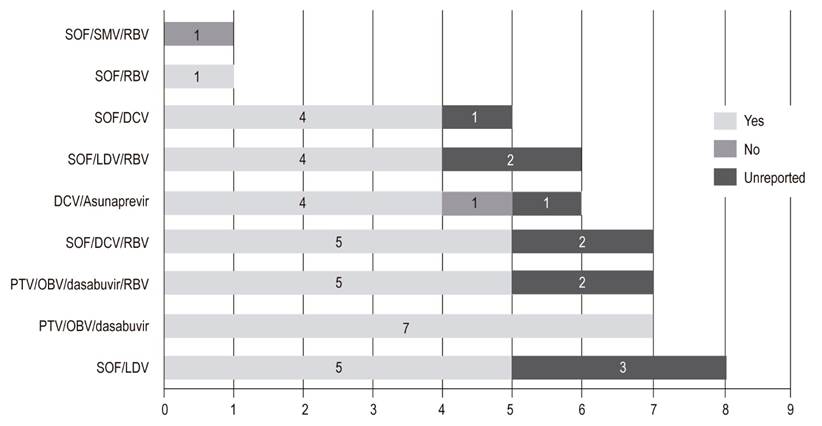
Figure 4 Scope of sustained viral response with Direct Action Antiviral schemes (n = 48). DCV: daclatasvir; LDV: ledipasvir; OBV: ombitasvir; PTV: paritaprevir; r: ritonavir; RBV: ribavirin; SMV: simeprevir; SOF: sofosbuvir.
Table 5 Patients with variants associated with resistance

DCV: daclatasvir; EBV: elbasvir; GT: genotype; LDV: ledipasvir; OBV: ombitasvir; RBV: ribavirin; SVR: sustained virological response; SMV: simeprevir; SOF: sofosbuvir; VAR: variant associated with resistance; VEL: velpatasvir.
DAA safety was analyzed for all patients who reportedly began treatment; there were records of ADRs associated with DAA for 25.9% (14/54) of these patients. Thirty-seven ADRs attributed to seven DAA schemes were identified. SOF/DCV/RBV had the highest frequency, followed by SOF/LDV/RBV. The most frequent ADRs were anemia (16.2%), asthenia (10.8%), headaches and flu symptoms (8.1% each) (Table 6). None of the reported ADRs caused treatment discontinuation.
Polypharmacy in Patients with Hepatitis C
Four or more medications in addition to anti-HCV schemes were used by 46.3% of the patients used (Table 7). No records of outpatient medications were found for 17.6% of the patients.
Discussion
This is the first study of patients with HepC at the HPTU to look at the effectiveness and safety of DAAs. The distribution of HepC by sex and age was similar to that reported by Santos et al. Based on 1538 samples collected by referral laboratories in Colombia, they found an average patient age of 53 years (SD 14) with approximately 70% of patients between 40 and 70 years. 10 Genotype 1 and subtype 1b were found in 77.8% and 39.8% of the patients analyzed, respectively. According to Santos et al., they are the predominant genotype and subtype in Colombia. 10
Advanced fibrosis/cirrhosis (F3-F4) was found in 39.8% of the patients with compensated cirrhosis in 77.5% of the cases in stage F4. An analytical cross-sectional study conducted in Cartagena for three months found that 50% of 41 patients had advanced cirrhosis/fibrosis, and 68% had compensated cirrhosis. 11 These differences may be due to the number of patients analyzed and to the short time within which information was collected in that study. The proportion of patients with cirrhosis was higher than that described by Hajarizadeh et al. (4-24%). 1 This can be explained by the level of complexity of the HPTU where patients with more advanced stages of disease are generally treated.
As in reports by other authors in Colombia and Latin America, blood transfusions constituted the main risk factor for contracting HCV. 12,13 This result was expected since screening of blood donations for HCV in Colombia only began in 1993 and only reached 99% coverage in 1995. 14 Considering that the onset of cirrhosis begins 20 years after HCV exposure, the number of HepC diagnoses could increase over the next few years as the result of transfusions from before 1993. 1
Effectiveness of Antiviral Therapy
SVR was achieved by 46.9% of patients given peg-IFN which is within the range of 6% to 56% reported in the literature. 8 For genotype 1, the most common genotype in this group, the response rate can reach 50%. 15
An SVR of 94.6% was found in the group of patients who finished treatment with DAA. The cause of therapeutic failure in the other 5.4% was found to be VAR to NS5A inhibitors. This is consistent with Buti et al. who found that 1% to 7% of patients treated with DAAs do not reach SVR. 16 Causes could be attributable to the patient, the treatment regimen and/or the virus. 17
VARs are changes in the nucleotide sequence responsible for synthesis of the proteins that are molecular targets of DAAs. This ability to generate resistance, typical of viruses, is greater in HCV than in other viruses such as HBV and HIV. 17 The VARs found in this study were L31V and Y93H which target NS5A inhibitors. VARs related to the nucleotide analog NS5B sofosbuvir were not reported. This can be explained by its high genetic resistance barrier. 18
Similar to reports by other authors, the SVR rates of patients with VAR to NS5A and without VAR to NS5A were similar in this study. 19 Some researchers disagree about the relationship of VAR and SVR, so they recommend determining these variants at baseline especially in cases that involve a null response prior to therapy. 17,18 Current Colombian guidelines for managing HepC recommend analyses of resistance to NS3 and/or NS5A only for patients who have not achieved SVR. 20
The most frequent VARs in genotype 1b are reported to be L31V/M and Y93H/N. Y93H results in high levels of resistance to drugs that act on NS5A. It is important to note that VARs to NS5A continue to be present as long as two years after the end of treatment, so it is essential to consider them before administering rescue therapy. 17
Safety of Antiviral Therapy
The availability of DAA has led to an improvement in the tolerability of treatment as in this study in which 25.9% of patients who received DAAs presented some type of ADR compared to 68.2% of those who received peg- INF. Although the analysis of the severity of ADRs was not the subject of this study, it was observed that patients with peg-INF/RBV had more severe ADRs especially hospitalizations due to anemia in which patients required blood products and infections associated with leukopenia or neutropenia.
We found that 39.8% of the patients who received peg-INF/RBV had systemic ADRs, especially asthenia, adynamia, fevers, myalgia and headaches. This is similar to reports the literature which show that these symptoms develop in 11% to 50% of cases, appear within a few hours following administration of medication, and have spontaneous remission from 24 hours to several days later. 21-25 Hematological ADRs are the most common of those due to peg-INF/RBV and are the main cause of low adherence rates, dose reductions and treatment discontinuation. 21,22,26 In this study, they occurred in 26.4% of the patients. They developed neutropenia and anemia which could be associated with bone marrow suppression by peg-IFN and RBV-induced extravascular hemolysis. 23,27
Systemic ADRs accounted for the largest portion (43.2%) of those that occurred in patients who received DAAs. They were followed by neuropsychiatric ADRs (16.2%), hematological ADRs (16.2%) and gastrointestinal ADRs (13.5%). Barrajón et. Al. reported very similar results in a retrospective analysis of 355 patients treated with DAAs. They found that 43.7% of their study population developed ADRs, mostly systemic (37.1%), gastrointestinal (18%) and neurological (15.8 %). (28) It can be inferred that the appearance of hematological and neuropsychiatric ADR swas related to the use of RBV combined with SOF/DCV or SOF/LDV. Development of anemia and depression occurred more frequently in these patiens than in those who did not receive RBV. Calleja et al. have also reported a high incidence of anemia (91%) in patients who received SOF/LDV/RBV. 7
These results show that there are still cases in which the addition of RBV or peg-INF is necessary even though the use of DAA increases tolerability to antiviral treatment. This is especially true for patients previously exposed to interferon who present with cirrhosis which increases the risk of ADRs. 4,20,28
Polypharmacy in Patients with HepC
Polypharmacy can be defined as the use of five or more daily medications. 29 Our study found that 46.3% of the patients were polymedicated. This can be explained by age (> 50 years) and HepC patients with coexisting diseases which required additional medications.
Polypharmacy can increase susceptibility to medication-related problems such as ADR, falls, hospital readmissions, and drug interactions. 29 This makes establishment of comprehensive health care programs that include pharmacotherapeutic follow-up imperative to prevent and resolve these medication-related problems.
Conclusions
Characterization of patients with HepC treated at the HPTU during the study period found a similar distribution among men and women with higher prevalences between 40 and 70 years of age and with transfusions as the most frequent transmission mechanism. DAAs were safer and more effective than schemes with peg-IFN/RBV, but RBV is still necessary in cirrhotic patients with previous exposure to treatment, and this increases the risk of ADR.
There is a need to implement comprehensive patient-centered care with access to health services and medications throughout the course of treatment and appropriate pharmacotherapeutic follow-up. Similarly, prospective studies evaluating the safety and effectiveness of DAAs in patients with chronic HepC are needed.
Limitations
This study has several limitations. Given its retrospective nature, it is directly dependent on the quality of information recorded in the electronic medical records. During data collection, incomplete records were detected which could diminish the quality of the study. Similarly, medical notes lacked uniformity indicating that the hospital’s electronic medical records need to be standardized from the start to the end dates of treatment. Reports of viral loads, concomitant treatment and possible mechanisms of transmission all need to be recorded for adequate patient follow-up as well as for the national epidemiological report.
Acknowledgements
We would like to thank the Pharmaceutical Promotion and Prevention research group of the University of Antioquia for academic support and to the HPTU Hepatology Unit for permission for this research
REFERENCES
1. Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013 Sep;10(9):553-62. https://doi.org/10.1038/nrgastro.2013.107. [ Links ]
2. Kohli A, Shaffer A, Sherman A, Kottilil S. Treatment of hepatitis C: a systematic review. JAMA. 2014 Aug 13;312(6):631-40. https://doi.org/10.1001/jama.2014.7085. [ Links ]
3. Center for Disease Analysis. Hepatitis C prevalence [Internet]. 2012 [acceso 19 de febrero de 2017]. Disponible en: Disponible en: http://www.centerforda.com/HepC/HepMap.html . [ Links ]
4. American Association for the Study of Liver Diseases and the Infectious Diseases Society of America. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C [Internet]. 2016 [acceso 23 de marzo de 2017]. Disponible en: Disponible en: http://www.hcvguidelines.org/full-report-view . [ Links ]
5. Ministerio de Salud y Protección Social, Instituto de Evaluación Tecnológica en Salud. Guía de Práctica Clínica para la tamización, diagnóstico y tratamiento de personas con infección por el virus de la hepatitis C. Bogotá, Colombia: Ministerio de Salud y Protección Social; 2016. [ Links ]
6. Strader DB, Seeff LB. A brief history of the treatment of viral hepatitis C. Clin Liver Dis (Hoboken). 2012 Mar 6;1(1):6-11. https://doi.org/10.1002/cld.1. [ Links ]
7. Calleja JL, Crespo J, Rincón D, Ruiz-Antorán B, Fernandez I, Perelló C, et al. Effectiveness, safety and clinical outcomes of direct-acting antiviral therapy in HCV genotype 1 infection: Results from a Spanish real-world cohort. J Hepatol. 2017 Jun;66(6):1138-1148. https://doi.org/10.1016/j.jhep.2017.01.028. [ Links ]
8. Yang S, Britt RB, Hashem MG, Brown JN. Outcomes of Pharmacy-Led Hepatitis C Direct-Acting Antiviral Utilization Management at a Veterans Affairs Medical Center. J Manag Care Spec Pharm. 2017 Mar;23(3):364-369. https://doi.org/10.18553/jmcp.2017.23.3.364. [ Links ]
9. Sistema de Trámites en Línea - Consultas Públicas [Internet]. [acceso 30 de julio de 2018]. Disponible en: Disponible en: http://consultaregistro.invima.gov.co:8082/Consultas/consultas/consreg_encabcum.jsp . [ Links ]
10. Santos Ó, Gómez A, Vizcaíno V, Casas MC, Ramírez MDP, Olaya P. [Hepatitis C virus genotypes circulating in Colombia]. Biomedica. 2017 Jan 24;37(1):22-27. https://doi.org/10.7705/biomedica.v37i1.3173. [ Links ]
11. Yepes I de J, Carmona ZA, Múnera MN. Calidad de vida en pacientes con hepatitis C crónica en Colombia. Rev Colomb Gastroenterol. 2017;32(2):112. https://doi.org/10.22516/25007440.139. [ Links ]
12. Yepes I de J, Lince B, Caez C, Vuono G de. Factores de riesgo para la infección por el virus de la hepatitis C en la Costa Caribe colombiana: un estudio de casos y controles. Biomédica. 2016;36(4):564-71. https://doi.org/10.7705/biomédica.v36i4.3105. [ Links ]
13. Claudino Botero R, Tagle M. Los nuevos tratamiento de hepatitis C: Perspectivas latinoamericanas. Clin Liver Dis (Hoboken). 2015 Mar 4;5(1):11-13. https://doi.org/10.1002/cld.466. [ Links ]
14. Beltrán M. Riesgo de infección transfusional de hepatitis C en Colombia. Iatreia. 2004;17(3-S):305. [ Links ]
15. Saludes V, Ausina V, Martró E. Posibilidades actuales para predecir la respuesta a la terapia en pacientes con hepatitis C crónica por el genotipo 1 del virus de la hepatitis C. Enfermedades Infecc Microbiol Clínica. 2011;29:51-8. https://doi.org/10.1016/S0213-005X(11)70044-1. [ Links ]
16. Buti M, Riveiro-Barciela M, Esteban R. Management of direct-acting antiviral agent failures. J Hepatol. 2015 Dec;63(6):1511-22. https://doi.org/10.1016/j.jhep.2015.08.010. [ Links ]
17. Llerena S, Cabezas J, Iruzubieta P, Crespo J. Resistencias al virus de la hepatitis C. Implicaciones y posibilidades terapéuticas. Gastroenterol Hepatol. 2017;484-94. https://doi.org/10.1016/j.gastrohep.2017.04.007. [ Links ]
18. Dietz J, Susser S, Berkowski C, Perner D, Zeuzem S, Sarrazin C. Consideration of Viral Resistance for Optimization of Direct Antiviral Therapy of Hepatitis C Virus Genotype 1-Infected Patients. PLoS One. 2015 Aug 28;10(8):e0134395. https://doi.org/10.1371/journal.pone.0134395. [ Links ]
19. Sarrazin C, Dvory-Sobol H, Svarovskaia ES, Doehle B, Martin R, Zeuzem S, et al. The prevalence and the effect of HCV NS5A resistance associated variants in subjects with compensated cirrhosis treated with ledipasvir/sofosbuvir +/- RBV. J Hepatol. 2015;62:S620. https://doi.org/10.1016/S0168-8278(15)30976-4. [ Links ]
20. Ministerio de Salud y Protección Social, Instituto de Evaluación Tecnológica en Salud. Vía clínica para el tratamiento de hepatitis C. Bogotá, Colombia: Ministerio de Salud y Protección Social; 2017. p. 41. [ Links ]
21. Santos OM, Orrego M. Tratamiento: Efectos adversos del tratamiento de hepatitis C. Rev Colomb Gastroenterol. 2012;27:37-40. [ Links ]
22. Mulet Pérez A, Pullés Labadié M, Gámez Escalona M, Mulet Gámez A, Díaz Santos O, Infante Velázquez M. Efectos adversos del tratamiento con interferón alfa-2b humano recombinante y rivabirina en pacientes con hepatitis crónica C. Rev Cuba Med Mil. 2011;40(1):76-84. [ Links ]
23. Sulkowski MS, Cooper C, Hunyady B, Jia J, Ogurtsov P, Peck-Radosavljevic M, Shiffman ML, Yurdaydin C, Dalgard O. Management of adverse effects of Peg-IFN and ribavirin therapy for hepatitis C. Nat Rev Gastroenterol Hepatol. 2011 Apr;8(4):212-23. https://doi.org/10.1038/nrgastro.2011.21. [ Links ]
24. Huang YM, Wang H, Wang C, Chen M, Zhao MH. Promotion of hypercoagulability in antineutrophil cytoplasmic antibody-associated vasculitis by C5a-induced tissue factor-expressing microparticles and neutrophil extracellular traps. Arthritis Rheumatol. 2015 Oct;67(10):2780-90. https://doi.org/10.1002/art.39239. [ Links ]
25. Hadziyannis SJ, Sette H Jr, Morgan TR, Balan V, Diago M, Marcellin P, Ramadori G, Bodenheimer H Jr, Bernstein D, Rizzetto M, Zeuzem S, Pockros PJ, Lin A, Ackrill AM; PEGASYS International Study Group. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004 Mar 2;140(5):346-55. https://doi.org/10.7326/0003-4819-140-5-200403020-00010. [ Links ]
26. Nachnani JS, Rao GA, Bulchandani D, Pandya PK, Alba LM. Predictors of hematological abnormalities in patients with chronic hepatitis C treated with interferon and ribavirin. Ann Hematol. 2010 Feb;89(2):121-5. https://doi.org/10.1007/s00277-009-0774-y. [ Links ]
27. UpToDate Inc. Ribavirin (systemic): Drug information [Internet]. [acceso 10 de agosto de 2018]. Disponible en: Disponible en: http://www.uptodate.com [ Links ]
28. Barrajón L, Soler E, Lorente L, Pérez J. Efectividad y seguridad de los antivirales de acción directa frente al virus de la hepatitis C. Rev OFIL. 2016;26(4):243-50. [ Links ]
29. Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017 Oct 10;17(1):230. https://doi.org/10.1186/s12877-017-0621-2. [ Links ]
Received: December 10, 2018; Accepted: March 04, 2019











 text in
text in