Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.34 no.3 Bogotá July/Sept. 2019
https://doi.org/10.22516/25007440.335
Original articles
Clinical effectiveness of two esomeprazole presentations in a pilot trial
1Médico internista, Gastroenterólogo, Unidad de endoscopia, Fundación Valle del Lili. Cali, Colombia.
2Médico Cirujano, Gastroenterólogo, Unidad de Endoscopia, Fundación Valle del Lili. Cali, Colombia.
3Director del Centro de investigaciones clínicas, médico internista, infectólogo, epidemiólogo, Fundación Valle del Lili. Cali, Colombia.
4Coordinador de estudios clínicos, Centro de investigaciones clínicas, Fundación Valle del Lili. Cali, Colombia.
5Estadístico, Centro de investigaciones clínicas, Fundación Valle del Lili. Cali, Colombia.
Introduction:
This pilot studied the clinical effectiveness of two presentations of esomeprazole in patients with dyspepsia with undiagnosed causes.
Methods:
We conducted a pilot clinical trial of two 40 mg Esomeprazole presentations. Patients with dyspepsia of unknown cause at a gastroenterology clinic in a referral hospital were included. They received one or the other presentation daily for 28 days. Patients were initially evaluated with endoscopy and biopsy and received follow-up examinations at two and four weeks. Adverse events were recorded, and clinical symptom scales and quality of life questionnaires validated in Spanish (SODA and QoL-PEI) were used. In addition, gastric pH levels were measured continuously for 24 hours on day 14 of treatment. Serum levels of the medication administered were also measured on day 14 of treatment. A two-way repeated measures ANOVA was used to compare mean differences between the two groups. When significant differences in times were found, a Bonferroni correction was made.
Results:
A total of 33 patients were randomized into two groups: 16 patients in one group and 17 in the other. There were no differences in the percentages of gastric pH inhibition at day 14 of treatment (p = 0.9795). There were no differences in serum level concentrations on day 14 (p = 0.2199). No significant differences were found in severity and quality of life scales in the first two weeks of treatment. However, in the last two weeks of treatment the test product showed a larger decrease in pain (p = 0.0048) and superiority in compliance (p = 0.01) on the SODA subscale. There were no serious adverse events, and there were no statistical differences between the presentations of non-serious adverse events.
Conclusions:
The Test product and the Reference product showed similar effects on clinically relevant variables.
Keywords: Esomeprazole; SODA; QoL-PEI; proton pump inhibitors; dispepsia
Introducción:
el presente estudio tuvo como fin investigar la efectividad clínica de dos presentaciones de esomeprazol en pacientes con dispepsia de causa no estudiada.
Métodos:
se realizó un ensayo clínico piloto de dos presentaciones de esomeprazol de 40 mg recibidos diariamente por 28 días. Se eligieron pacientes con diagnóstico de dispepsia no estudiada que asistieron a consulta de gastroenterología en un hospital de referencia. Se evaluaron a los pacientes inicialmente con endoscopia y biopsia, el seguimiento a 2 y 4 semanas con escalas clínicas de síntomas y calidad de vida con cuestionarios validados en español (SODA y QoL-PEI) y eventos adversos. Además, se midieron los niveles de pH gástrico con pH-metrías en 24 horas al día 14 de tratamiento. Se tomaron niveles séricos del medicamento al momento de la evaluación de la pH-metría. Para las escalas clínicas se aplicó un análisis de varianza (ANOVA) de dos factores con medidas repetidas y al encontrar diferencias significativas en los tiempos se realizó una corrección de Bonferroni.
Resultados:
se aleatorizó un total de 33 pacientes, 16 y 17 pacientes en cada grupo. No hubo diferencias en el porcentaje de inhibición del pH gástrico al día 14 de tratamiento (p = 0,9795). No hubo diferencias en concentraciones de niveles séricos el día 14 (p = 0,2199). No se encontraron diferencias significativas en las escalas de gravedad y calidad de vida en las dos primeras semanas de tratamiento, pero sí en las últimas dos semanas, en las cuales el producto de prueba demostró mayor disminución del dolor (p = 0,0048) y superioridad en conformidad (p = 0,01) en la subescala SODA. No se presentaron eventos adversos serios y no hubo diferencias estadísticas entre la presentación eventos adversos no serios.
Conclusiones:
los productos de prueba y el de referencia mostraron efectos similares en variables clínicamente relevantes.
Palabras clave: Esomeprazol; SODA; QoL-PEI; inhibidores de la bomba de protones; dispepsia
Introduction
Dyspepsia is defined as chronic and recurring pain or discomfort in the central part of the upper abdomen. 1 According to the criteria of the ROMA IV consensus, there are two types. The first has a defined organic cause while the second has no specific cause and is called functional dyspepsia (FD). FD is considered to be due to physiological alterations, immunological alterations, hypersensitivity and/or brain-intestine interactions. In addition, it is associated with daily eating and life style habits and may be related to Helicobacter pylori infections which are of great importance in our environment due their prevalence of approximately 60%. 2,3,4
Fifteen to forty percent of the world’s population are thought to have dyspeptic symptoms, and of these, 70% are idiopathic. The annual incidence of dyspepsia is approximately 1%, and it is estimated that 50% of people will consult a physician because of these symptoms at some point in their lives. 1,2,3
The negative impact of dyspepsia on the quality of life has encouraged the development of assessment scales for measuring severity, disability and alterations of daily life. There are two instruments that have been validated in Spanish: the SODA (Severity of Dyspepsia Assessment) scale which assesses the intensity of pain, associated symptoms and level of compliance, and the Dyspepsia Related Health Scale (DRHS) which determines the impact that this disease has on daily life. 4-11
Part of standard dyspepsia management focuses on control of gastric acid. Proton pump inhibitors (PPIs), one of the main classes of drugs used for this purpose, have been widely used to treat both FD and organic dyspepsia. 1,5-8 Esomeprazole, a PPI, is indicated for relief of gastrointestinal symptoms, healing of gastric lesions, and maintenance of healing. 5 Esomeprazole improves dyspeptic symptoms through several mechanisms. First, patients with dyspepsia are hypersensitive to duodenal acid. Second, patients with dyspepsia have low-grade inflammation that is worsened by acid secretion. Inhibition of gastric acid secretion by esomeprazole modifies these effects.
Controlled release forms of esomeprazole have been developed to improve absorption and bioavailability thus avoiding early chemical degradation which limits effectiveness. NEXIUM-MUPS®, developed by AstraZeneca, includes a system that uses a Multiple-Unit Pellet System (MUPS) that releases micropellets of the PPI as the tablet disintegrates. In Colombia, an esomeprazole formulation has been developed that uses a polymer coating that resists acid as the tablets pass through the gastric acid medium. They disintegrate when they reach the less acidic pH (pH> 4.5) of the proximal portion of the duodenum.
The objective of this study was to compare clinical responses to these two formulations of esomeprazole by evaluating serum concentrations in the first hour after intake and measuring efficacy due to the increase in gastric pH at 24-hour follow-ups. The SODA and DRHS scales were used to assess patients’ clinical evolution and clinical safety related to adverse events of special-release esomeprazole therapy and reference esomeprazole therapy. Doses of 40 mg/day of all medications were used in dyspeptic patients in whom organic causes of dyspepsia had not been proven.
Materials and methods
A blinded two-arm, randomized, controlled trial was conducted to compare 40 mg/day doses of two different presentations of esomeprazole for 28 days. The study population consisted of the patients with abdominal and digestive symptoms suggestive of dyspepsia who came to the gastroenterology clinic at Fundación Valle del Lili between July 2016 and March 2017, and who had not undergone previous diagnostic studies. Patients who were older than 18 years with a final diagnosis of previously unstudied dyspepsia were included. Exclusion criteria included unexplained weight loss; dysphagia; anemia; bleeding; jaundice; history of gastric surgery; neoplasms; erosive esophagitis; pregnancy; lactation; known allergy to esomeprazole; use of nonsteroidal anti-inflammatory drugs (NSAIDs), PPIs or drugs with potential interactions within the two weeks prior to coming to the clinic, and prior endoscopic diagnosis of alkaline pH, digestive ulcers or malignancy.
This study was carried out at the Fundación Valle del Lili in accordance with the 2013 Helsinki Declaration, the International Conference on Harmonization (ICH) Good Clinical Practice (GCP) Guideline, Colombian Resolution 8430 of 1993 and Resolution 2378 of 2008, and the Guidelines for good clinical practice. The study was approved by the biomedical research ethics committee, and each participant consented to the study. The subjects were not compensated for their participation. This study has been included in the International Clinical Trials registry.
We sought to include at least 30 volunteers in each group in this pilot clinical trial to test normal distribution. However, due to recruitment problems, only 16 patients were included in one group and 17 patients in the other group.
The study began with screening upper endoscopies of volunteers which included routine biopsies and measurement of gastric pH an Inolab 7110® pH meter and staining with Congo red and Litmus paper to exclude patients with pH> 4 which is suggestive of hypochlorhydria. After screening, patients were randomly assigned to the two groups. One group received modified-release esomeprazole from Technochemicals (test esomeprazole), and the other group received NEXIUM-MUPS® from AstraZeneca (reference esomeprazole). Both products are registered and marketed in Colombia for the indication used in this study. The randomization sequence was performed with Randomization® software which created blocks of six participants. 12 Security envelopes relating the participant’s code and the group to which s/he was assigned were then created.
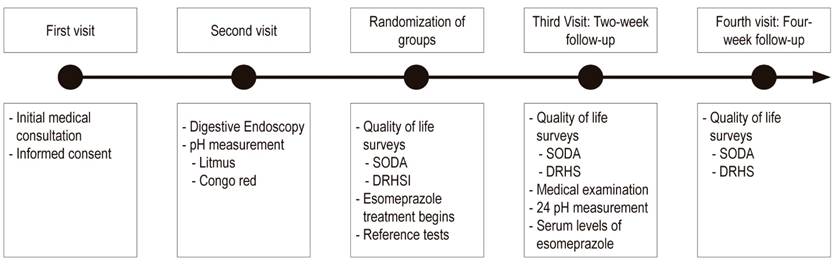
The attending physician and the research team were blind to the randomized drug throughout the study. Since the presentations of the medications were different, it was not possible to ensure the blinding of the study subjects. The treatment provided on the day of recruitment consisted of twenty-eight 40 mg tablets of esomeprazole to be taken daily at least 30 minutes before breakfast for 28 day. Patients were required to keep a daily log. Follow-up visits were scheduled at 2 and 4 weeks to evaluate study outcomes (Figure 1).
The two week follow-up also evaluated the impact on gastric pH of treatment with esomeprazole by means of 24 hour ambulatory pH monitoring with Versaflex® Z dual pH sensor catheters with 8 impedance rings. Data was was stored on a digitraper pH Z Given® Imagin device and subsequently downloaded and analyzed with the Accuview® pH-Z 5.2 program.
We decided to take drug serum levels on day 14 of treatment as a measure that could be correlated to pH measurement and clinical outcomes. Two weeks after each patient started treatment, a five ml sample of venous blood was taken within the first hour after the patient took her/his daily dose of esomeprazole. This was sufficient time to guarantee esomeprazole concentrations in the blood at equilibrium. Plasma was frozen at -20° C. Subsequently, the sample was analyzed with a UHPLC Lachrom Ultra-VWR liquid chromatograph whose diode array detector was used to determine serum esomeprazole levels.
The SOSA and DRHS surveys and assessment scales were used for clinical evaluations on the day of recruitment and at subsequent follow-up visits at two and four weeks after the start of treatment. The version of SODA validated by the Benites et al. was used together with the score adjustment for balancing subscales suggested and applied by Rabeneck et al. 10,11 For analysis, it was divided into subscales of pain intensity, associated non-painful symptoms such as belching, heartburn, and swelling, and level of compliance. The first two subscales express greater severity at higher scores while the third expresses a higher level of perceived well-being at higher scores. DRHS was used with the same methodology and at the same times as the SODA scale, but a single global score in which greater severity of symptoms is expressed at higher scores was used. Unlike SODA, one of the components of DRHS scores reflects disability associated with pain. 9
Clinical safety was monitored at each visit through analysis of patient’s daily logs in which they recorded any associated symptoms and adverse events during treatment.
Statistical Analysis
All participant information was uploaded into a database on the BD Clinic® platform. The descriptive analysis expressed continuous variables as means and standard deviations (SD) or medians and interquartile ranges (IQR). They were compared with a Student’s T test or Mann-Whitney test depending on whether the assumption of normality was fulfilled. Categorical variables were presented in proportions and correlated with the chi square (χ2) test or Fisher’s exact test depending on the observations.
Subsequently, analysis of variance (ANOVA) of two factors (drug-time) with 99 repeated measurements was performed with the clinical scales to establish differences. Upon finding significant differences in the times for the main effect of ANOVA, pairwise comparison was performed using Bonferroni correction for multiple comparisons. Analyses were performed with STATA statistical package 12.1.
Results
A total of 205 patients were screened, 55 were recruited, and the final overall sample included 33 patients. The main cause of exclusion was gastric pH suggestive of hypochlorhydria (Figure 2). 13 During follow-up, two participants who could not perform pH-metrics were excluded from the analysis.
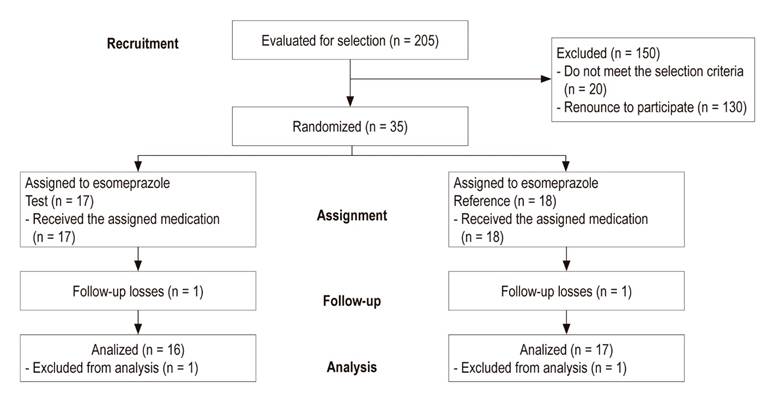
Figure 2 Flowchart of participants from recruitment between July 2016 and April 2017 through to the end of the clinical trial.
The comparison of baseline sociodemographic and clinical characteristics, summarized in Table 1 found no significant differences between the groups. The average serum concentration of the test esomeprazole was 0.67 µmol/L (0.18-1.61) and that of the reference esomeprazole was 0.28 µmol/L (0.16-0.53) but the difference was not statistically significant (p = 0.219). Similarly, the comparison of inhibition of acid secretion to a constant gastric pH> 4 showed an average time for the test esomeprazole of 19.98 h (SD ± 3.87 [83.3%]) and 19.95 h (SD ± 3.55 [83.2%]) for reference esomeprazole, p = 0.986.
Table 1 Comparison of baseline sociodemographic and clinical characteristics between groups: test esomeprazole (n = 16) and reference esomeprazole (n = 17)
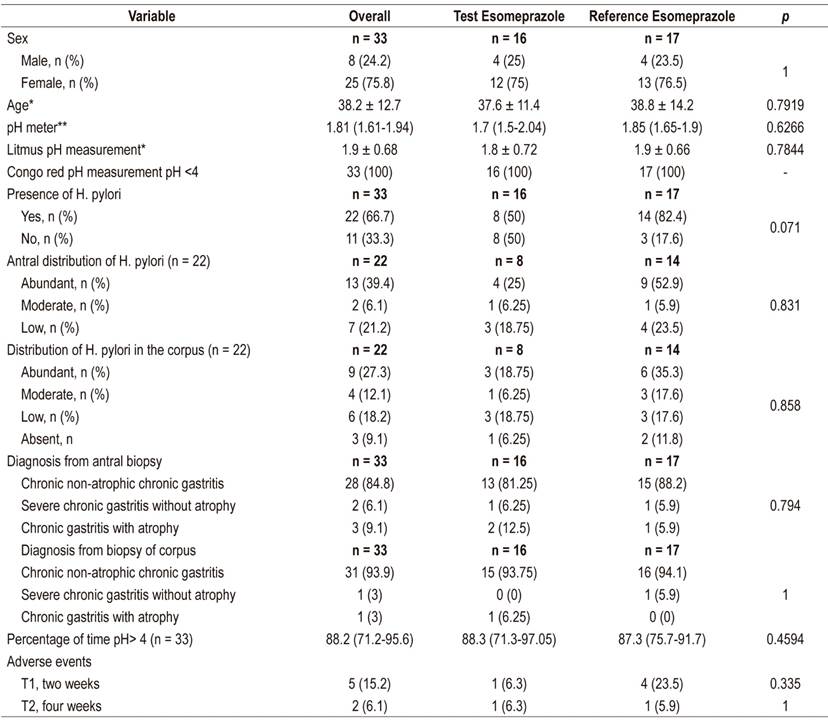
* Mean ± SD. ** Median (IQR)
Statistically significant differences were found for both survey scales at four weeks of treatment, and the pain intensity subscale scores decreased more for the test esomeprazole than they did for the reference esomeprazole. Similarly, improvement in compliance was more noticeable in the test product than in the reference product (Table 2). These trends were repeated the two factor ANOVA which found differences in the time variable, but only for interaction in the pain subscale. Differences in the compliance subscale were found between treatments (p = 0.012) (Table 3). The Bonferroni adjustment found that there are significant differences (p <0.05) between the start and end time of the treatment at 4 weeks in the SODA subscales and the DRHS scale independently for each type of esomeprazole. However, the SODA pain subscale found no significant differences in this time (p = 0.018).
Table 2 Comparison of drugs between independent times: time 0 = time of recruitment, time 1= 2 week follow-up, time 2 = 4 week follow-up.
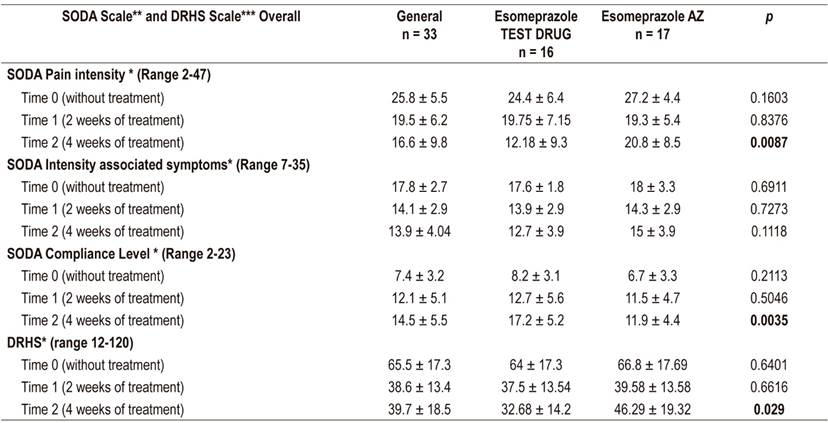
* Average ± SD Two-sample t test. ** Severity of dyspepsia assessment. *** Quality of life associated with intestinal problems questionnaire.
Table 3 Comparison between drugs by means of two factor ANOVA of repeated measures: time 0 = time of recruitment, time 1= 2 week follow-up, time 2 = 4 week follow-up.
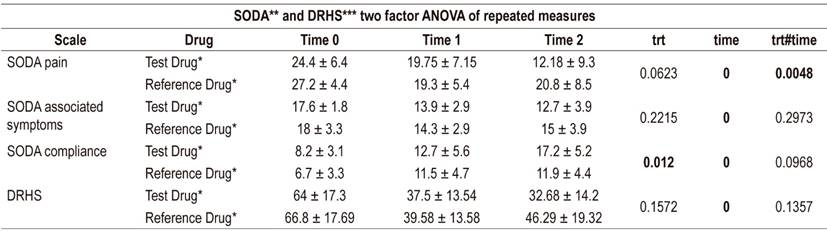
* Average ± SD. ** Severity of dyspepsia assessment. *** Quality of life questionnaire associated with intestinal problems.
The proportion of patients who experienced adverse events during the study was similar for both groups. At four weeks, it was 6.3% for the group who received the test drug (Tecnoquímicas esomeprazole) and 5.9% for the group who took the reference drug (AstraZeneca esomeprazole) (p = 0.999). There were no serious adverse effects and all adverse effects had resolved before the end of the study. All of these adverse effects are among those referred to in the technical sheets as being associated with the use of esomeprazole and include nausea, dry mouth, belching and flatulence.
Discussion
Serum concentrations measured in our patients varied considerably, similar to measurements of serum concentrations reported in the literature. These variations may be due to the designs of special release presentations, time of administration, physiological conditions at the time of drug use, and to varying abilities of individuals to metabolize PPI according to polymorphisms in their enzymes, especially CYP2C19. 6,14-20
Both drugs effectively increased gastric pH by inhibiting 83% of acid production which is at the upper limits of figures reported in similar studies which range from 50% to 85%. The minimum inhibition value in our study was 50% which is within the established range for clinical impact. 7,8,14,15. There were no differences in esomeprazole serum levels at day 14 which may partly explain this finding. However, serum levels do not necessarily correlate with intracellular levels.
Significant decreases were observed in the SODA scores of both drugs. The pain subscale for Tecnoquímicas esomeprazole fell 20.8 points while that of the AstraZeneca esomeprazole fell 12.8 points. These decreases were slightly higher than those reported by Benites et al., who described decreases of 7 points. However, measurement of symptoms not associated with pain decreased only around 2.5 points, similar to the findings of Benites et al. Similar behavior to our findings and those of Benites et al., another similar study by Rabeneck et al. found that the greatest impact was on the pain subscale. They concluded that this result was possible because of effective control of gastric acid, which is the main cause of the sensation of pain, and that questions about compliance are associated with pain control. In relation to compliance, we observed that the test esomeprazole had a higher final score for clinical improvement than did the reference esomeprazole. On average, scores improved nine points at the first follow-up and an 5.2 points at the second follow-up (p = 0.0035). These increases were also higher than those reported by Benites et al., which were 2.5 points in their study population. 10,11
The general frequencies of adverse events at four weeks in this study was 6.3% for the test esomeprazole and 5.9% for the reference esomeprazole. These frequencies are much lower than those found by Shin et al., who reported 40% in a sample of 36 patients, but the two studies are in agreement in that neither found any serious adverse events. This has also been described in various publications which have found that, when use is not prolonged, these drugs’ have good pharmacological safety profiles. 14-21.
One limitation of this present study was that diagnostic means other than clinical and endoscopic examinations were not used even though ultrasound and laboratory tests could have ruled out other causes of dyspepsia. Although dyspepsia is very prevalent, diagnosis and screening to exclude patients with organic dyspepsia is complex.
On the other hand, the statistical power of this pilot study at the time of closure was low. A representative sample for this pathology with a prevalence of 10% to 30% of the general population would have required at least 1500 patients to attain a statistical power of 80%. 22,23 Also, the follow-up time and effectiveness in the study was four weeks, so our data cannot be extrapolated to clinical situations involving longer use of these medications. In addition, patients could not be blinded to the medication they were taking which may have introduced selection bias into our study.
We conclude that the two presentations of esomeprazole had very similar outcomes of interest in terms of increasing pH and answers to symptom questionnaires. No significant differences were observed in the evolution of the clinical assessment scales for the two treatments, but the effect of the test esomeprazole was better sustained over time than was that of the reference esomeprazole. This difference was statistically significance. It could be that this difference was secondary to the greater number of positive cases for H. pylori in the reference esomeprazole group, although this difference was not statistically significant difference (p = 0.071). Both presentations of esomeprazole demonstrated that risks of use are very low for a four week course of administration in the population studied.
Acknowledgements
We would like to thank the endoscopy unit and the entire team of the clinical research center for their contributions to this study. We would also like to thank the laboratory of chemical instrumentation of ICESI University for analyses of serum concentrations
REFERENCES
1. Talley NJ, Vakil N. Guidelines for the management of dyspepsia. Am J Gastroenterol. 2005;100(10):2324-37. https://doi.org/10.1111/j.1572-0241.2005.00225.x. [ Links ]
2. Stanghellini V, Chan FK, Hasler WL, Malagelada JR, Suzuki H, Tack J, et al. Gastroduodenal disorders. Gastroenterology. 2016;150(6):1380-92. https://doi.org/10.1053/j.gastro.2016.02.011. [ Links ]
3. Talley NJ. Functional dyspepsia: new insights into pathogenesis and therapy. Korean J Intern Med. 2016;31(3):444-56. https://doi.org/10.3904/kjim.2016.091. [ Links ]
4. Bravo LE, Cortés A, Carrascal E, Jaramillo R, García LS, Bravo PE, et al. Helicobacter pylori: patología y prevalencia en biopsias gástricas en Colombia. Colomb Med. 2003;34(3):124-31. [ Links ]
5. Otero W, Zuleta MG, Otero L. Enfoque del paciente con dispepsia y dispepsia funcional: actualización. Rev Col Gastroenterol. 2014;29(2):132-8. [ Links ]
6. Shin JM, Kim N. Pharmacokinetics and pharmacodynamics of the proton pump inhibitors. J Neurogastroenterol Motil. 2013;19(1):25-35. https://doi.org/10.5056/jnm.2013.19.1.25. [ Links ]
7. Hatlebakk JG. Review article: gastric acidity − comparison of esomeprazole with other proton pump inhibitors. Alimentary Pharmacology & Therapeutics. 2003;17:10-5. https://doi.org/10.1046/j.1365-2036.17.s1.3.x. [ Links ]
8. Kirchheiner J, Glatt S, Fuhr U, Klotz U, Meineke I, Seufferlein T, et al. Relative potency of proton-pump inhibitors-comparison of effects on intragastric pH. Eur J Clin Pharmacol. 2009;65(1):19-31. https://doi.org/10.1007/s00228-008-0576-5. [ Links ]
9. Ruiz M, Villasante F, León F, González-Lara V, González C, Crespo M, et al. Cuestionario sobre calidad de vida asociada a dispepsia. Adaptación española y validación del cuestionario Dyspepsia Related Health Scale. Medicina Clínica. 2001;117(15):567-73. https://doi.org/10.1016/S0025-7753(01)72182-3. [ Links ]
10. Benites Goñi H, Cabrera Cabrejos S, Chungui Bravo J, Prochazka Zarate R, Bernabe Ortiz A, De los Ríos Senmache R, et al. Modificación y validación del instrumento SODA (severity of dyspepsia assessment) adaptada al Perú para evaluar la evolución de la severidad de los síntomas en pacientes con dispepsia. Rev Gastroenterol Perú. 2013;33(1):9-27. [ Links ]
11. Rabeneck L, Cook KF, Wristers K, Souchek J, Menke T, Wray NP. SODA (severity of dyspepsia assessment): a new effective outcome measure for dyspepsia-related health. Journal of clinical epidemiology. 2001;54(8):755-65. https://doi.org/10.1016/S0895-4356(00)00365-6. [ Links ]
12. Randomization.com. [internet] 2007 [acceso el 15 de marzo de 2017]. Disponible en: Disponible en: http://www.randomization.com/2007 . [ Links ]
13. Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. https://doi.org/10.1136/bmj.c869. [ Links ]
14. Ullah MA, Shams Ud D, Maruf AA, Azad MAK, Shohag MH, Sultana R, et al. Relative bioavailability and pharmacokinetic properties of two different enteric formulations of esomeprazole in healthy bangladeshi male volunteers: An open-label, single-dose, randomized-sequence, two-way crossover study. Clinical Therapeutics. 2010;32(7):1419-26. https://doi.org/10.1016/j.clinthera.2010.07.007. [ Links ]
15. Shin JS, Lee JY, Cho KH, Park HL, Kukulka M, Wu JT, et al. The pharmacokinetics, pharmacodynamics and safety of oral doses of ilaprazole 10, 20 and 40 mg and esomeprazole 40 mg in healthy subjects: a randomised, open-label crossover study. Alimentary Pharmacology & Therapeutics. 2014;40(5):548-61. https://doi.org/10.1111/apt.12860 [ Links ]
16. Furuta T, Ohashi K, Kosuge K, Zhao XJ, Takashima M, Kimura M, et al. CYP2C19 genotype status and effect of omeprazole on intragastric pH in humans. Clinical Pharmacology & Therapeutics. 1999;65(5):552-61. https://doi.org/10.1016/S0009-9236(99)70075-5 [ Links ]
17. Hunfeld NG, Touw DJ, Mathot RA, van Schaik RH, Kuipers EJ. A comparison of the acid-inhibitory effects of esomeprazole and rabeprazole in relation to pharmacokinetics and CYP2C19 polymorphism. Alimentary Pharmacology & Therapeutics. 2012;35(7):810-8. https://doi.org/10.1111/j.1365-2036.2012.05014.x. [ Links ]
18. Klotz U. Impact of CYP2C19 polymorphisms on the clinical action of proton pump inhibitors (PPIs). Eur J Clin Pharmacol. 2009;65(1):1-2. https://doi.org/10.1007/s00228-008-0571-x [ Links ]
19. Dean L. Esomeprazole Therapy and CYP2C19 Genotype. 2012 [updated 2016 Mar 8]. In: Pratt V, McLeod H, Rubinstein W, Dean L, Kattman B, Malheiro A, editors. Medical Genetics Summaries [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2012-. Available from http://www.ncbi.nlm.nih.gov/books/NBK100896/. [ Links ]
20. Lou H-Y, Chang C-C, Sheu M-T, Chen Y-C, Ho H-O. Optimal dose regimens of esomeprazole for gastric acid suppression with minimal influence of the CYP2C19 polymorphism. Eur J Clin Pharmacol. 2008;65(1):55-64. https://doi.org/10.1007/s00228-008-0552-0. [ Links ]
21. Menéndez JLT. Farmacología de esomeprazol. Emergencias: Revista de la Sociedad Española de Medicina de Urgencias y Emergencias. 2005;17(4):1059-66. [ Links ]
22. Mahadeva S, Goh KL. Epidemiology of functional dyspepsia: a global perspective. World J Gastroenterol. 2006;12(17):2661-6. https://doi.org/10.3748/wjg.v12.i17.2661. [ Links ]
23. Talley NJ, Vakil NB, Moayyedi P. American gastroenterological association technical review on the evaluation of dyspepsia. Gastroenterology. 2005;129(5):1756-80. https://doi.org/10.1053/j.gastro.2005.09.020. [ Links ]
Received: December 18, 2018; Accepted: May 19, 2019











 text in
text in