Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.34 no.3 Bogotá July/Sept. 2019
https://doi.org/10.22516/25007440.322
Review articles
Pathophysiology of Hepatitis C and Diabetes Mellitus: Towards the cure of two epidemics in the 21 st century
1Internista. Universidad Nacional de Colombia. Hospital Universitario Nacional de Colombia. Bogotá D. C., Colombia.
2Internista, Gastroenterólogo. Universidad Nacional de Colombia. Centro de Enfermedades Digestivas, GutMédica. Bogotá D. C., Colombia.
3Internista, Universidad Javeriana. Gastroenterólogo, Universidad Nacional de Colombia. Fundación Santa Fe de Bogotá, Centro de Enfermedades Digestivas, GutMédica. Bogotá D. C., Colombia.
Chronic hepatitis C virus (HCV) and diabetes mellitus (DM) are two public health problems that impact health care systems with overall high costs. HCV infections cause liver manifestations such as hepatitis, cirrhosis and hepatocellular carcinoma. They have also been involved in the pathogenesis of extrahepatic manifestations among which are metabolic disorders such as DM. Longitudinal and cross-sectional studies have reported a higher incidence and prevalence of DM in patients with chronic HCV infections. DM accelerates histological and clinical progression of chronic HCV infections and leads to cardiovascular complications. Recently, progress has been made in treatment with the introduction of new medications such as direct-acting antiviral drugs that improve glycemic control in these patients.
Keywords: Hepatitis C; chronic hepatitis C; diabetes mellitus
La infección crónica por virus de la hepatitis C (VHC) y la diabetes mellitus (DM) son dos problemas de salud pública que impactan los sistemas de salud, con una alta carga económica global. La infección por VHC produce manifestaciones hepáticas tales como hepatitis, cirrosis y carcinoma hepatocelular; asimismo, se ha involucrado en la patogénesis de manifestaciones extrahepáticas, entre las cuales se ha asociado con alteraciones metabólicas como la DM. Estudios longitudinales y transversales han reportado mayor incidencia y prevalencia de DM en pacientes con infección crónica por VHC. La DM acelera la progresión histológica y clínica en pacientes con infección crónica por VHC y las complicaciones cardiovasculares. Recientemente se ha avanzado en el tratamiento y la introducción de nuevos medicamentos como los antivirales de acción directa, que mejoran el control glucémico en estos pacientes.
Palabras clave: Hepatitis C; hepatitis C crónica; diabetes mellitus
Introduction
Worldwide, hepatitis C virus (HCV) infections and diabetes mellitus (DM) are two of the main public health problems facing health care systems. They place a heavy overall burden on health care finance, especially in developing countries. 1,2
Currently, DM is defined as a group of metabolic diseases whose common characteristic is hyperglycemia caused by a deficit in the secretion and/or action of insulin. 3 Chronic hyperglycemia in DM has been associated with long term damage to, and dysfunction and failure of eyes, kidneys, nerves, heart and blood vessels. This affects the quality of life and increases mortality rates. 3,4
DM is classified as either type 1 or type 2 (TYPE 2). Type 1 accounts for 5% to 10% of DM cases and is characterized by the destruction β cells in the pancreas due to an autoimmune mechanism associated with the HLA-DR/DQ haplotype. It leads to absolute insulin deficits. Islet cell cytoplasmic autoantibodies (ICA) are predictive and diagnostic markers for T1D. Other autoantibodies found include anti-glutamic acid decarboxylase (anti-GAD), anti-insulin antibodies (anti-IA) and anti-protein tyrosine phosphatase (PTP) antibodies (IA-2, IA-2 beta). 5,6 Type 2 diabetes accounts for 90% to 95% of the patients with DM. It is characterized by insulin resistance and varying degrees of insulin deficit. It can also lead to increased glucose production in the liver. There are probably several causes of this type of DM, but its specific etiology is not known exactly. 7
The prevalence of chronic HCV infections ranges between 1.2% and 3.8% depending on geographic region. 8 Approximately 130 to 175 million people are currently infected: 3 to 4 million more are infected each year, and 350,000 people die from the disease every year. 9 It is the main cause of liver transplantation in developed countries and is the main cause of morbidity and mortality related to the liver. 10,11
HCV is a common cause of chronic liver diseases including hepatitis, cirrhosis and hepatocellular carcinoma. It also involved in the pathogenesis of several autoimmune and rheumatologic diseases including arthritis, vasculitis, sicca syndrome, late cutaneous porphyria, lichen planus, kidney disease, thyroid diseases and pulmonary fibrosis. It is also involved in the development of lymphoproliferative disorders of β cells 12,13 and has been associated with extrahepatic manifestations including metabolic alterations including DM. 14
The objective of this work is to review the evidence of association of chronic HCV infections and DM related to epidemiology, pathogenesis, clinical symptoms, treatment and prevention.
Methodology
We conducted a search of the web using the following MeSH terms and keywords: hepatitis C, chronic hepatitis C, diabetes mellitus, epidemiology, physiopathology, diagnosis, therapeutics, and antiviral agents.
The search was limited to studies conducted in humans that were published in English or Spanish from the initial description of the association in 1994 to November 2018. The Cochrane, Central Controlled Trials, Medline, Embase and Science Citation Index electronic databases were used and then supplemented with manual searches. Publications considered to be most relevant by the authors were chosen.
Overview
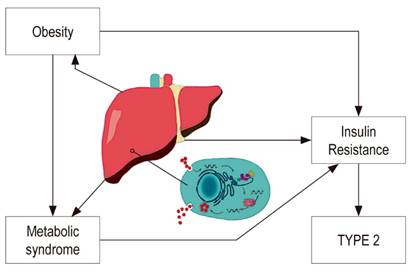
The liver plays an important role in carbohydrate metabolism. Chronic liver diseases frequently involve alterations in glucose homeostasis, carbohydrate intolerance and insulin resistance which may eventually lead to DM (Figure 1). 15,16,17 Moderate asymptomatic elevation of aminotransferases is also common in DM patients, especially those with TYPE 2, and has been associated with fat infiltration of the liver. 18
Progression of liver fibrosis is responsible for the development of insulin resistance and type 2 diabetes, but DM can occur in early stages of liver disease. 19,20 Initially, long-term hepatocyte damage was considered to be a cause of alterations in glucose homeostasis, but various studies have shown that patients with other chronic liver diseases such as hepatitis B virus (HBV) infections have lower prevalences of DM than do patients with chronic hepatitis C. 21-24
The prevalence of HCV antibodies in the population with type 2 diabetes varies between 1.78% and 12.1%. 25,26 Cross-sectional studies with a control groups of non-diabetic individuals have established that patients with type 2 diabetes have a higher prevalence of HCV antibodies than does the general population. 27-30 In contrast, the prevalence of HCV antibodies in patients with type 1 diabetes does not exceed the prevalence in the general population. 31 The NHANES III study found that people over 40 years of age with HCV infections have three times the probability of having type 2 as do those who are not infected. Non association with TYPE 1 was detected. 32 Another large community-based study has established that people between 39 and 49 years of age who have type 2 are very likely to test positive for the HCV antibody. 33
Evaluations of patients with HCV and cirrhosis show that the prevalence of type 2 varies from 19.6% to 50%, a higher range than that reported for patients with chronic hepatitis. 34 A prospective study that compared 50 patients with cirrhosis due to HCV and 50 patients with cirrhosis without HCV found a higher prevalence of DM and higher baseline blood insulin levels in patients with HCV. It would appear that the presence of advanced liver disease is a strong diabetogenic factor, like HCV itself. 34.
The clinical consequence of the high prevalence of HCV infections in type 2 patients is that mild elevations of serum aminotransferases should not automatically be attributed to nonalcoholic fatty liver disease, and tests for HCV should be mandatory for diabetic patients with altered liver profiles. 35
There is insufficient information about the duration of HCV in patients with DM to allow evaluation of their temporal relationship. One study has reported that all patients with HCV and type 2 had histories of blood transfusions 10 to 20 years prior to the onset of DM. 31 Another study found that type 2 was diagnosed 18 years after HCV infections. 36 A study of the temporal sequence in diabetic HCV positive patients found that HCV diagnoses preceded those of DM in 73% of cases. 37
There are risk factors for HCV before the onset of DM in 52% of people who have both HCV and type 2, while none had risk factors for HCV after the onset of DM. 15 The absence of any epidemiological factor for HCV infections among diabetic patients and the evidence suggesting that the infection precedes DM supports the idea that the virus can cause or predispose HCV patients to develop DM. Nevertheless, definitive conclusions should be made on the basis of prospective studies. Age, obesity, family histories of DM, African-American origins, and coinfections of HCV and human immunodeficiency virus (HIV) are all risk factors associated with the development of DM. 38,39 In contrast, the hypothesis that some specific HCV genotype predisposes those infected to the development of DM, or somehow protects the development of DM, remains controversial. 40
Post-transplant diabetes mellitus (PTDM) is a medical condition that can arise following kidney and liver transplantation. Its incidence has increased in recent decades. 41 Independent risk factors for PTDM include administration of immunosuppressive agents to prevent and treat rejection, donor origin, and factors related to the recipient. 42 Chronic HCV infection is one current indication for orthotopic liver transplantation. The prevalence of PTDM in liver transplant recipients infected with HCV is between 40% and 64% which is significantly higher than the prevalence reported in patients transplanted for other causes of liver failure. In addition, it has been established that HCV is an independent risk factor for development of PTDM. 42 The incidence of HCV can reach 50% in patients with end-stage renal disease and has been identified as an independent risk factor for development of PTDM after kidney transplantation. 43 All these data reinforce the hypothesis that HCV is more likely to be a cause than a consequence of DM. In addition, the relationship between HCV and DM can contribute substantially to the harmful effects of the virus on patient and graft survival after liver or kidney transplantation. 43
The link between HCV infections and DM has been explored both by assessing the prevalence DM and by studying impaired fasting glucose (IFG). A cohort of patients with chronic HCV hepatitis was observed to have almost three times more glucose abnormalities than did HCV negative patients including those with other liver diseases (32% versus 12%). Among the patients with HCV infections, a higher prevalence of DM and IFG was found (17% versus 7%, and 15% versus 5%, respectively). No differences were observed in cirrhotic patients with or without HCV infections. These findings suggest that the genuine connection between HCV infections and DM begins in the early stages of liver disease. 40
The high prevalence of impaired glucose metabolism found in patients with HCV infections suggests that they should be considered a high-risk group who should be screened for DM and IFG. Lecube et al. performed oral glucose tolerance tests (OGTT) on 50 patients with chronic hepatitis C and 50 HCV negative patients in whom DM had not been diagnosed. Both groups were matched by age, body mass indexes (BMI) and sex. OGTT diagnosed new cases of DM in 18% of the HCV patients and diagnosed new cases of IFG in 30 % of these patients while only 4% and 18% of the HCV negative patients were found to have DM and IFG, respectively. 44
Table 1 defines criteria for diagnosis of DM according to the American Diabetes Association (ADA). 45
Pathogenic mechanisms involved in HCV diabetogenic action
HCV Effects and Insulin Resistance
Hepatitis C is hepatotropic non-cytopathic virus whose genome has been identified in tissues beyond the liver including pancreatic acinar cells and epithelial cells of the pancreatic duct. 46 Post-mortem studies reveal that HCV replicates in the pancreas, and animal models suggest a direct effect of infection on insulin resistance in the liver. 47
The virus has a 9.6 Kb ribonucleic acid (RNA) genome which encodes approximately 3,010 amino acids and is transported in structural proteins (core, E1, E2) and non-structural proteins (NS3-NS5B). These proteins play important roles in the development of insulin resistance and oxidative stress at the cellular level through reactive oxygen species. 48 The core protein, alone or in combination with other viral proteins, increases phosphorylation of the insulin receptor substrate-1 (IRS1) which is the basis of insulin resistance. 49 Phosphorylated IRS1 activates phosphatidylinositol 3 kinase (PI3K). Activation of PI3K and Akt is essential for many of the metabolic effects of insulin (Figure 2). 50 Therefore, defective association of PI3K with IRS1 and loss of activation of PI3K may contribute to insulin resistance and increased prevalence of DM in subjects infected with HCV. Finally, this mechanism promotes translocation of glucose transporter 4 (GLUT4) to the plasma membrane to improve glucose uptake. 51 In addition, the core protein can directly activate insulin signaling inhibitors such as the mammalian target of rapamycin (mTOR), the suppressor of cytokine signaling 3 (SOCS3), and c-Jun N-terminal kinases (JNKs). 52 In addition, HCV increases stress on the endoplasmic reticulum which activates protein phosphatase 2 (PP2) which inhibits two adenosine monophosphate activated protein kinase (AMPK) and Akt which are key gluconeogenesis regulators. 52
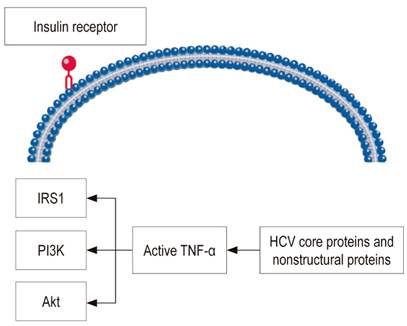
Figure 2 Mechanism by which HCV interferes with insulin signaling. TNF-α: tumor necrosis factor alpha.
Recent evidence supports the existence of a virus induced extrahepatic component of insulin resistance, an indication that the molecular pathogenesis of glucose metabolism abnormalities observed in HCV infections is much more complex than previously thought. 51
Inflammatory Cytokines
Innate viral evasion strategies and human genetic determinants are the basis of the transition from acute infections to viral persistence and chronic infection. Host genetic factors can influence infection outcomes and responses to antiviral therapy. Recent studies reveal a complex interaction between each patient’s genetic context, viral factors, and host factors related to the innate immune trigger which dictates control of HCV infections and immunity. 53
Beyond the direct effects of HCV on IRS1 and PI3K, the core protein can induce insulin resistance indirectly through stimulating secretion of inflammatory cytokines. In patients with chronic HCV, inflammation induced by the virus causes hypersecretion of insulin-resistant inflammatory cytokines such as interleukin 6 (IL-6) and TNF-α. 52,54,55 Inflammatory cytokines also regulate protein suppressors of the cytokine signal as part of a negative feedback circuit by attenuating their signal. 56 This phenomenon may contribute to increases in gluconeogenesis due to the loss of Akt-mediated inhibition of the expression of the phosphoenolpyruvate carboxykinase (PEPCK) gene. In this context, it is interesting to note that leptin can modulate the action of insulin in liver cells by antagonizing phosphorylation of insulin-stimulated IRS1. This increases expression of the FEPCK gene and decreases expression of glucokinase resulting in increased gluconeogenesis. 57 Increased gluconeogenesis after HCV infection results in increased production and accumulation of lipids mediated by inhibition of AMPK. 58 The lipolysis stimulating effect of TNF-α leads to elevated serum levels of free fatty acids which reduce insulin sensitivity. 59,60
Cytokines are intercellular mediators involved in viral control and HCV-induced liver damage. The complex cytokine network that operates during an initial infection allows coordinated and effective development of innate and adaptive immune responses, but the virus interferes with cytokines at various levels and escapes the immune response by inducing a Th2 profile. Inability to control infection leads to recruitment of inflammatory infiltrates in the hepatic parenchyma by interferon gamma (IFN-γ) and induces chemokines CXCL9, CXCL10 and CXCL11 which results in sustained liver damage and eventually cirrhosis. 61,62 Eslam et al. have found polymorphisms in the IFNL3 region (IL-28B) that are associated with spontaneous recovery and which were induced by treatment of infection. 63
The most important extrahepatic systemic diseases related to HCV (mixed cryoglobulinemia, lymphoproliferative disorders, autoimmune thyroid diseases, and Type 2 DM) are associated with alterations in the complex regulation of the cytokine/chemokine network involving inflammatory chemokines and the Th1 response. 61,62
HCV and Type 1 DM
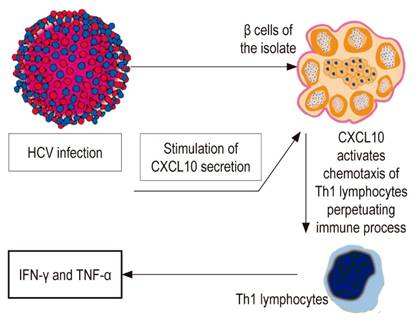
Various mechanisms have been postulated. Although HCV can infect extrahepatic tissues, its participation in the onset of type 1 DM has not yet been clarified. 64 Aside from direct mechanisms which have not been demonstrated, HCV infection initiates or accelerates an immune reaction against β cells. HCV β cell infection can regulate the expression and secretion of the CXCL10 gene and recruitment of Th1 lymphocytes which secrete IFN-γ and TNF-α. They induce secretion of CXCL10 by β cells and thus perpetuate the immune cascade. This cascade can lead to β cell dysfunction in genetically predisposed subjects (Figure 3). 65 In addition, molecular mimicry with an HCV-related autoimmune trigger involving glutamic acid decarboxylase (GAD), which shares a similar 65 amino acid sequence with antigenic regions of the virus polyprotein, has been suggested. 66 Another possibility is induction of antibodies that react against GAD and the development of DM mediated by interleukin 18 (IL-18) and other inflammatory cytokines. 67 IL-18 plays a pathogenic role in type 1 DM because it is involved in acceleration of the development of manifest disease. It can induce Th1 and/or Th2 responses depending on the surrounding cytokines. In addition, it plays a pathogenic role in various diseases including acute liver failure. 67,68 Other inflammatory cytokines such as TNF-α and IL-1B, which are elevated in patients with acute hepatitis, can also induce autoimmune diabetes. 69
Iron Overload
High concentrations of ferritin are associated with insulin resistance and with increased risk of type 2 DM in healthy people, and increased ferritin levels have been reported in HCV patients. 70 Ferritin is an acute phase reactant that may be altered by inflammatory processes, and evidence suggests that there is a low degree of inflammation in type 2 DM. Patients with HCV infections without DM do not have high levels of ferritin, so it has been suggested that DM, and not the HCV infection, is a risk factor for high iron concentrations. However, more prospective studies are needed to evaluate this relationship. 70 In addition, hepatic iron deposits can cause insulin resistance by interfering with the ability of insulin to suppress hepatic glucose production. 70
Hepatic Steatosis
Hepatic steatosis occurs more commonly in HCV patients than in HBV patients: it occurs in more than 50% of chronic HCV patients. 71 Mild steatosis is associated with elevated BMI and visceral obesity while moderate to severe steatosis is directly caused by the virus. 52 Genotypic differences related to progression of liver disorders have also been described. Steatosis in HCV genotype 4 infection is an expression of metabolic syndrome caused by activation of inflammatory mechanisms as well as obesity and insulin resistance. The degree of steatosis in this genotype is independent of viral load and antiviral therapy does not improve it. 72 Genotype 3a is mainly related to severe steatosis. 52
Hepatic steatosis may contribute to DM associated with HCV due to damage of the ability of insulin to decrease gluconeogenesis and promote liver fibrosis. 71
DM in HCV Infected Patients Treated with Interferon Alfa (IFN-α)
Some studies have shown a high prevalence of pancreatic autoimmune markers in patients with HCV during or after IFN-α therapy which is probably due to the immunostimulatory effects of these cytokines. 73 IFN-α has antiviral, antiproliferative and immunomodulatory activity. In predisposed individuals, it can induce a diabetogenic process. For this reason, islet cell and anti-GAD autoantibodies should be investigated before and during treatment to identify individuals who are at high risk of developing type 1 DM. 73 Some patients may develop de novo pancreatic autoimmunity and be at risk of developing DM. Patients who are initially positive for organ specific autoantibodies, especially specific pancreas and thyroid autoantibodies, and those who undergo seroconversion, are at high risk of developing clinical autoimmune disease after treatment. 74 IFN-α increases the expression of HLA class I antigens, activates natural killer cells and T lymphocytes and can be an important cofactor in the development of a Th1 immune reactions. 75 Timely suspension of therapy is rarely accompanied by clinical regression of DM. 74
Cancer in HCV and DM infection
The main characteristic of diabetic patients is insulin resistance which is crucial for progression of fibrosis and which negatively impacts responses to antiviral treatment in subjects with chronic HCV. 76 Reduced insulin sensitivity is the basis of compensatory hyperinsulinemia and elevated levels of insulin-like growth factor type 1 (IGF-1) which stimulates cell proliferation and inhibits apoptosis. This phenomenon has strong mitogenic effects on a wide variety of cancer cell lines. 77 At the same time, insulin activates the IGF-1 receptor which promotes growth and modulates cell cycle progression. Excess insulin can indirectly lead to the development of cancer by increasing the amount and bioavailability of circulating IGF-1. Obesity and physical inactivity also cause hyperinsulinemia and therefore are also associated with accelerated cancer progression. 78
Chronic HCV is a progressive form of liver disease that leads to cirrhosis and HCC. 13 Patients with diabetes and HCV infections have higher risks of HCC than do non-diabetic individuals, so DM seems to have a selective impact on the development of HCC. 79 Patients with type 2 DM who achieve good glycemic control by reaching HbA1c levels of less than 7% can reduce the risk of HCC. 80
Treatment and prevention
Clinical studies of HCV patients have reported improvement in glycemic control and insulin resistance with direct-acting antiviral agents (DAA) in patients who have DM as well as in those who do not. 81,82,83 Diabetic patients who receive DAA should be strictly monitored so that diabetes medications, mainly insulin and sulfonylureas, can be reduced to avoid hypoglycemia. 84
The advent of HCV therapies has helped us learn that the first interferon-based treatments acted as facilitators in the development of DM. 85 However, oral antiviral therapies have been accompanied by decreasing incidence of DM. A study of 5,127 patients treated for HCV found an incidence of 6.2% among those who achieved sustained virological response in contrast to 21.7 % of the patients who had therapeutic failure after 3.7 years of follow-up (adjusted hazard ratio: 0.79; 95% CI: 0.65-0.96). 86 Li et al. followed 1,395 HCV patients who also had type 2 DM who maintained sustained virological responses for an average period of 2.7 years. They found significant less complications such as acute coronary syndrome (Hazard ratio: 0.36; p <0.001), terminal chronic kidney disease (Hazard ratio: 0.46; p <0.001), cerebrovascular events (sub-hazard ratio: 0.34; p <0.001), and retinopathy (sub-hazard ratio: 0.24; p <0.001), when than in untreated patients. 87
Other variables such as HbA1c that are related to DM have also been identified. Recent studies have shown that HbA1c decreased more in patients who achieved sustained virological response (0.6% to 0.98%) than in those for whom treatment failed. 88,89 These endocrine benefits provide additional justification for considering antiviral treatment for all patients with HCV and DM. 89 In addition, the treatment of HCV in patients with DM could decrease the prevalence of complications including chronic nephrology. 90
Various factors including viral genotype, host genetic factors and comorbidities can alter this response. 91 Some research has reported that obesity and hypercholesterolemia may interfere with the sustained viral response suggesting additional therapeutic options for HCV. These include dietary changes, antidiabetic medications and statins although it is not yet clear if biguanides such as metformin are the best oral diabetes treatments while statins have not been able to inhibit HCV replication in vivo even though they can in vitro. 92,93,94
The potential relationship between HCV infections and the development of DM increases the need to implement prevention measures. These should be directed to lifestyle changes that can reduce the risk of developing DM and HCV infections, and should include regular screening for DM in patients with HCV infections plus analysis of other risk factors such as obesity, dyslipidemia and alcohol consumption that can accelerate the progression of both. 95
Additional studies are necessary to improve prevention policies and promote adequate and cost-effective programs for monitoring and treating diabetic patients with chronic HCV. Multifactorial treatment should be implemented to cure two diseases: DM and chronic HCV.
Conclusions
HCV and DM infection are two disorders which have large high impacts on health and health care throughout the world. The high prevalence of type 2 DM in HCV patients with chronic hepatitis correlates with the increasing amount of evidence that this infection is a risk factor for developing DM and other alterations in carbohydrate metabolism. The specific mechanisms through which HCV is associated with DM appear to involve direct viral effects, insulin resistance, inflammatory cytokines, chemokines, cytokine signaling suppressors and other immune mediated mechanisms. These mechanisms are initiated in the early stages of liver disease.
Age, obesity, family histories of DM, African-American origins and HCV-HIV coinfections are risk factors associated with the development of DM among people infected with HCV. Studies should be carried out to evaluate alterations in the carbohydrate metabolism of these patients. Data regarding associations of chronic HCV and type 1 DM are scarce, but it has been reported that IFN-α therapy can stimulate pancreatic autoimmunity and, in certain cases, lead to development of type 1 DM. Diabetic patients with chronic HCV have greater risks of developing cirrhosis and HCC than do non-diabetic patients with chronic HCV. DAA treatment improves glycemic control and insulin resistance. Additional studies are needed to improve prevention policies and promote adequate and cost-effective programs for diagnosis, treatment and monitoring of diabetic patients with chronic HCV.
Acknowledgements
None declared by the authors
REFERENCES
1. Stepanova M, Younossi ZM. Economic burden of hepatitis C. Clin Liver Dis. 2017;21:579-94. https://doi.org/10.1016/j.cld.2017.03.012. [ Links ]
2. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4-14. https://doi.org/10.1016/j.diabres.2009.10.007. [ Links ]
3. Fox CS, Golden SH, Anderson C, Bray GA, Burke LE, de Boer IH, et al. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2015;38:1777-803. https://doi.org/10.2337/dci15-0012. [ Links ]
4. Vaidya V, Gangan N, Sheehan J. Impact of cardiovascular complications among patients with type 2 diabetes mellitus: a systematic review. Expert Rev Pharmacoecon Outcomes Res. 2015;15:487-97. https://doi.org/10.1586/14737167.2015.1024661. [ Links ]
5. Barbeau WE. What is the key environmental trigger in type 1 diabetes--is it viruses, or wheat gluten, or both? Autoimmun Rev. 2012;12:295-9. https://doi.org/10.1016/j.autrev.2012.05.003. [ Links ]
6. Askenasy EM, Askenasy N. Is autoimmune diabetes caused by aberrant immune activity or defective suppression of physiological self-reactivity? Autoimmun Rev 2013;12:633-7. https://doi.org/10.1016/j.autrev.2012.12.004. [ Links ]
7. Ferrannini E. Physiology of glucose homeostasis and insulin therapy in type 1 and type 2 diabetes. Endocrinol Metab Clin North Am. 2012;41:25-39. https://doi.org/10.1016/j.ecl.2012.01.003. [ Links ]
8. Thrift AP, El-Serag HB, Kanwal F. Global epidemiology and burden of HCV infection and HCV-related disease. Nat Rev Gastroenterol Hepatol. 2017;14:122-32. https://doi.org/10.1038/nrgastro.2016.176. [ Links ]
9. Mohamed AA, Elbedewy TA, El-Serafy M, El-Toukhy N, Ahmed W, Ali El Din Z. Hepatitis C virus: A global view. World J Hepatol. 2015;7:2676-80. https://doi.org/10.4254/wjh.v7.i26.2676. [ Links ]
10. Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13:2436-41. https://doi.org/10.3748/wjg.v13.i17.2436. [ Links ]
11. Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333-42. https://doi.org/10.1002/hep.26141. [ Links ]
12. Antonelli A, Ferri C, Galeazzi M, Giannitti C, Manno D, Mieli-Vergani G, et al. HCV infection: pathogenesis, clinical manifestations and therapy. Clin Exp Rheumatol. 2008;26(1 Suppl 48): S39-47. [ Links ]
13. Ferri C, Antonelli A, Mascia MT, Sebastiani M, Fallahi P, Ferrari D, et al. HCV-related autoimmune and neoplastic disorders: the HCV syndrome. Dig Liver Dis. 2007;39(Suppl 1):S13-21. https://doi.org/10.1016/S1590-8658(07)80005-3. [ Links ]
14. Nocente R, Ceccanti M, Bertazzoni G, Cammarota G, Silveri NG, Gasbarrini G. HCV infection and extrahepatic manifestations. Hepatogastroenterology. 2003;50:1149-54. [ Links ]
15. Mason AL, Lau JY, Hoang N, Qian K, Alexander GJ, Xu L, et al. Association of diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;29:328-33. https://doi.org/10.1002/hep.510290235. [ Links ]
16. Weinman SA, Belalcazar LM. Hepatitis C: a metabolic liver disease. Gastroenterology 2004; 126: 917-9. https://doi.org/10.1053/j.gastro.2003.01.001. [ Links ]
17. Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology. 2005;42:987-1000. https://doi.org/10.1002/hep.20920. [ Links ]
18. Vozarova B, Stefan N, Lindsay RS, Saremi A, Pratley RE, Bogardus C, et al. High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51(6):1889-95. https://doi.org/10.2337/diabetes.51.6.1889. [ Links ]
19. Romero-Gomez M. Insulin resistance and hepatitis C. World J Gastroenterol. 2006;12:7075-80. https://doi.org/10.3748/wjg.v12.i44.7075. [ Links ]
20. Petit JM, Bour JB, Galland-Jos C, Minello A, Verges B, Guiguet M, et al. Risk factors for diabetes mellitus and early insulin resistance in chronic hepatitis C. J Hepatol. 2001;35:279-83. https://doi.org/10.1016/S0168-8278(01)00143-X. [ Links ]
21. Mehta SH, Brancati FL, Strathdee SA, Pankow JS, Netski D, Coresh J, et al. Hepatitis C virus infection and incident type 2 diabetes. Hepatology. 2003;38:50-6. https://doi.org/10.1053/jhep.2003.50291. [ Links ]
22. Huang JF, Dai CY, Hwang SJ, Ho CK, Hsiao PJ, Hsieh MY, et al. Hepatitis C viremia increases the association with type 2 diabetes mellitus in a hepatitis B and C endemic area: an epidemiological link with virological implication. Am J Gastroenterol. 2007;102:1237-43. https://doi.org/10.1111/j.1572-0241.2007.01181.x. [ Links ]
23. Boluda Monzo S, Mesa Manteca J, Obiols Alfonso G, Simo Canonge R. Surface antigen of hepatitis B in diabetes mellitus. Med Clin (Barc). 1989;92:397. [ Links ]
24. Mangia A, Schiavone G, Lezzi G, Marmo R, Bruno F, Villani MR, et al. HCV and diabetes mellitus: evidence for a negative association. Am J Gastroenterol. 1998;93:2363-7. https://doi.org/10.1111/j.1572-0241.1998.00688.x. [ Links ]
25. Ozyilkan E, Erbas T, Simsek H, Telatar F, Kayhan B, Telatar H. Increased prevalence of hepatitis C virus antibodies in patients with diabetes mellitus. J Intern Med. 1994;235:283-4. https://doi.org/10.1111/j.1365-2796.1994.tb01075.x. [ Links ]
26. Gray H, Wreghitt T, Stratton IM, Alexander GJ, Turner RC, O’Rahilly S. High prevalence of hepatitis C infection in Afro-Caribbean patients with type 2 diabetes and abnormal liver function tests. Diabet Med. 1995;12:244-9. https://doi.org/10.1111/j.1464-5491.1995.tb00466.x. [ Links ]
27. Fabiani S, Fallahi P, Ferrari SM, Miccoli M, Antonelli A. Hepatitis C virus infection and development of type 2 diabetes mellitus: Systematic review and meta-analysis of the literature. Rev Endocr Metab Disord. 2018;19(4):405-20. https://doi.org/10.1007/s11154-017-9440-1. [ Links ]
28. Sotiropoulos A, Peppas TA, Skliros E, Apostolou O, Kotsini V, Pappa SI. Low prevalence of hepatitis C virus infection in Greek diabetic patients. Diabet Med. 1999;16:250-2. https://doi.org/10.1046/j.1464-5491.1999.00009.x. [ Links ]
29. Ryu JK, Lee SB, Hong SJ, Lee S. Association of chronic hepatitis C virus infection and diabetes mellitus in Korean patients. Korean J Intern Med. 2001;16:18-23. https://doi.org/10.3904/kjim.2001.16.1.18. [ Links ]
30. Okan V, Araz M, Aktaran S, Karsligil T, Meram I, Bayraktaroglu Z, et al. Increased frequency of HCV but not HBV infection in type 2 diabetic patients in Turkey. Int J Clin Pract. 2002;56:175-7. [ Links ]
31. Cerutti F, Palomba E, Sacchetti C, Gay V, Versace A, Tovo PA. Anti-HCV antibodies in a population of insulin-dependent diabetic children and adolescents. Diabetes Care. 1999;22:1587-8. https://doi.org/10.2337/diacare.22.9.1587. [ Links ]
32. Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. 2000;133:592-9. https://doi.org/10.7326/0003-4819-133-8-200010170-00009. [ Links ]
33. Wang CS, Wang ST, Yao WJ, Chang TT, Chou P. Community-based study of hepatitis C virus infection and type 2 diabetes: an association affected by age and hepatitis severity status. Am J Epidemiol. 2003;158:1154-60. https://doi.org/10.1093/aje/kwg259. [ Links ]
34. Garrido Serrano A, Guerrero Igea FJ, Lepe Jimenez JA, Palomo Gil S, Grilo Reina A. Hyperinsulinemia in cirrhotic patients infected with hepatitis C virus infection. Gastroenterol Hepatol. 2001;24:127-31. https://doi.org/10.1016/S0210-5705(01)70138-0. [ Links ]
35. Simo R, Hernandez C, Genesca J, Jardi R, Mesa J. High prevalence of hepatitis C virus infection in diabetic patients. Diabetes Care 1996;19:998-1000. https://doi.org/10.2337/diacare.19.9.998. [ Links ]
36. Grimbert S, Valensi P, Levy-Marchal C, Perret G, Richardet JP, Raffoux C, et al. High prevalence of diabetes mellitus in patients with chronic hepatitis C: a case-control study. Gastroenterol Clin Biol. 1996;20:544-8. [ Links ]
37. Knobler H, Schihmanter R, Zifroni A, Fenakel G, Schattner A. Increased risk of type 2 diabetes in noncirrhotic patients with chronic hepatitis C virus infection. Mayo Clin Proc. 2000;75:355-9. https://doi.org/10.4065/75.4.355. [ Links ]
38. Thuluvath PJ, John PR. Association between hepatitis C, diabetes mellitus, and race: a case-control study. Am J Gastroenterol. 2003;98:438-41. [ Links ]
39. Mehta SH, Moore RD, Thomas DL, Chaisson RE, Sulkowski MS. The effect of HAART and HCV infection on the development of hyperglycemia among HIV-infected persons. J Acquir Immune Defic Syndr. 2003;33:577-84. https://doi.org/10.1097/00126334-200308150-00005. [ Links ]
40. Hui JM, Sud A, Farrell GC, Bandara P, Byth K, Kench JG, et al. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression. Gastroenterology. 2003;125:1695-704. https://doi.org/10.1053/j.gastro.2003.08.032. [ Links ]
41. Cosio FG, Pesavento TE, Osei K, Henry ML, Ferguson RM. Post-transplant diabetes mellitus: increasing incidence in renal allograft recipients transplanted in recent years. Kidney Int. 2001;59:732-7. https://doi.org/10.1046/j.1523-1755.2001.059002732.x. [ Links ]
42. Baid S, Cosimi AB, Farrell ML, Schoenfeld DA, Feng S, Chung RT, et al. Posttransplant diabetes mellitus in liver transplant recipients: risk factors, temporal relationship with hepatitis C virus allograft hepatitis, and impact on mortality. Transplantation. 2001;72:1066-72. https://doi.org/10.1097/00007890-200109270-00015. [ Links ]
43. Abbott KC, Lentine KL, Bucci JR, Agodoa LY, Koff JM, Holtzmuller KC, et al. Impact of diabetes and hepatitis after kidney transplantation on patients who are affected by hepatitis C virus. J Am Soc Nephrol. 2004;15:3166-74. https://doi.org/10.1097/01.ASN.0000145439.48387.BF. [ Links ]
44. Lecube A, Hernandez C, Genesca J, Esteban JI, Jardi R, Simo R. High prevalence of glucose abnormalities in patients with hepatitis C virus infection: a multivariate analysis considering the liver injury. Diabetes Care. 2004;27:1171-5. https://doi.org/10.2337/diacare.27.5.1171. [ Links ]
45. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S13-S28. https://doi.org/10.2337/dc19-S002. [ Links ]
46. Masini M, Campani D, Boggi U, Menicagli M, Funel N, Pollera M, et al. Hepatitis C virus infection and human pancreatic beta-cell dysfunction. Diabetes Care. 2005;28:940-1. https://doi.org/10.2337/diacare.28.4.940. [ Links ]
47. Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, et al. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840-8. https://doi.org/10.1053/j.gastro.2003.11.056. [ Links ]
48. Bureau C, Bernad J, Chaouche N, Orfila C, Béraud M, Gonindard C, et al. Nonstructural 3 protein of hepatitis C virus triggers an oxidative burst in human monocytes via activation of NADPH oxidase. J Biol Chem. 2001;276:23077-83. https://doi.org/10.1074/jbc.M100698200. [ Links ]
49. Banerjee S, Saito K, Ait-Goughoulte M, Meyer K, Ray RB, Ray R. Hepatitis C virus core protein upregulates serine phosphorylation of insulin receptor substrate-1 and impairs the downstream akt/protein kinase B signaling pathway for insulin resistance. J Virol. 2008;82:2606-12. https://doi.org/10.1128/JVI.01672-07. [ Links ]
50. Burén J, Liu HX, Jensen J, Eriksson JW. Dexamethasone impairs insulin signalling and glucose transport by depletion of insulin receptor substrate-1, phosphatidylinositol 3-kinase and protein kinase B in primary cultured rat adipocytes. Eur J Endocrinol. 2002;146:419-29. https://doi.org/10.1530/eje.0.1460419. [ Links ]
51. Negro F. Mechanisms of hepatitis C virus-related insulin resistance. Clin Res Hepatol Gastroenterol. 2011;35:358-63. https://doi.org/10.1016/j.clinre.2011.01.011. [ Links ]
52. Gastaldi G, Goossens N, Clément S, Negro F. Current level of evidence on causal association between hepatitis C virus and type 2 diabetes: A review. J Adv Res. 2017;8:149-59. https://doi.org/10.1016/j.jare.2016.11.003. [ Links ]
53. Horner SM, Gale M Jr. Regulation of hepatic innate immunity by hepatitis C virus. Nat Med. 2013;19:879-88. https://doi.org/10.1038/nm.3253. [ Links ]
54. Bastard JP, Maachi M, Van Nhieu JT, Jardel C, Bruckert E, Grimaldi A, et al. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J Clin Endocrinol Metab. 2002;87:2084-9. https://doi.org/10.1210/jcem.87.5.8450. [ Links ]
55. Nelson DR, Lim HL, Marousis CG, Fang JW, Davis GL, Shen L, et al. Activation of tumor necrosis factor-alpha system in chronic hepatitis C virus infection. Dig Dis Sci. 1997;42:2487-94. https://doi.org/10.1023/A:1018804426724. [ Links ]
56. Krebs DL, Hilton DJ. SOCS proteins: negative regulators of cytokine signaling. Stem Cells 2001;19:378-87. https://doi.org/10.1634/stemcells.19-5-378. [ Links ]
57. Oncül O, Top C, Cavuplu T. Correlation of serum leptin levels with insulin sensitivity in patients with chronic hepatitis-C infection. Diabetes Care 2002; 25: 937. https://doi.org/10.2337/diacare.25.5.937. [ Links ]
58. Mankouri J, Tedbury PR, Gretton S, Hughes ME, Griffin SD, Dallas ML, et al. Enhanced hepatitis C virus genome replication and lipid accumulation mediated by inhibition of AMP-activated protein kinase. Proc Natl Acad Sci USA. 2010;107:11549-11554. https://doi.org/10.1073/pnas.0912426107. [ Links ]
59. Cheung AT, Wang J, Ree D, Kolls JK, Bryer-Ash M. Tumor necrosis factor-alpha induces hepatic insulin resistance in obese Zucker (fa/fa) rats via interaction of leukocyte antigen-related tyrosine phosphatase with focal adhesion kinase. Diabetes 2000; 49: 810-9. https://doi.org/10.2337/diabetes.49.5.810. [ Links ]
60. Ruan H, Lodish HF. Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-alpha. Cytokine Growth Factor Rev. 2003;14:447-55. https://doi.org/10.1016/S1359-6101(03)00052-2. [ Links ]
61. Fallahi P, Ferri C, Ferrari SM, Corrado A, Sansonno D, Antonelli A. Cytokines and HCV-related disorders. Clin Dev Immunol. 2012;2012:468107. https://doi.org/10.1155/2012/468107. [ Links ]
62. Antonelli A, Ferri C, Fallahi P, Ferrari SM, Sebastiani M, Ferrari D, et al. High values of CXCL10 serum levels in mixed cryoglobulinemia associated with hepatitis C infection. Am J Gastroenterol. 2008;103:2488-94. https://doi.org/10.1111/j.1572-0241.2008.02040.x. [ Links ]
63. Eslam M, Booth DR, George J, Ahlenstiel G. Interaction of IFNL3 with insulin resistance, steatosis and lipid metabolism in chronic hepatitis C virus infection. World J Gastroenterol. 2013;19:7055-61. https://doi.org/10.3748/wjg.v19.i41.7055. [ Links ]
64. Yan FM, Chen AS, Hao F, Zhao XP, Gu CH, Zhao LB, et al. Hepatitis C virus may infect extrahepatic tissues in patients with hepatitis C. World J Gastroenterol. 2000;6:805-11. https://doi.org/10.3748/wjg.v6.i6.805. [ Links ]
65. Antonelli A, Ferri C, Ferrari SM, Colaci M, Sansonno D, Fallahi P. Endocrine manifestations of hepatitis C virus infection. Nat Clin Pract Endocrinol Metab. 2009;5:26-34. https://doi.org/10.1038/ncpendmet1027. [ Links ]
66. Bogdanos DP, Rigopoulou EI. Viral/self-mimicry and immunological cross-reactivity as a trigger of hepatic C virus associated autoimmune diabetes. Diabetes Res Clin Pract. 2007;77:155-6. https://doi.org/10.1016/j.diabres.2006.10.012. [ Links ]
67. Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine Growth Factor Rev. 2001;12:53-72. https://doi.org/10.1016/S1359-6101(00)00015-0. [ Links ]
68. Yumoto E, Higashi T, Nouso K, Nakatsukasa H, Fujiwara K, Hanafusa T, et al. Serum gamma-interferon-inducing factor (IL-18) and IL-10 levels in patients with acute hepatitis and fulminant hepatic failure. J Gastroenterol Hepatol. 2002;17:285-94. https://doi.org/10.1046/j.1440-1746.2002.02690.x. [ Links ]
69. Lee LF, Xu B, Michie SA, Beilhack GF, Warganich T, Turley S, et al. The role of TNF-alpha in the pathogenesis of type 1 diabetes in the nonobese diabetic mouse: analysis of dendritic cell maturation. Proc Natl Acad Sci USA. 2005;102:15995-6000. https://doi.org/10.1073/pnas.0508122102. [ Links ]
70. Lecube A, Hernandez C, Genesca J, Esteban JI, Jardi R, Garcia L, et al. Diabetes is the main factor accounting for the high ferritin levels detected in chronic hepatitis C virus infection. Diabetes Care. 2004;27:2669-75. https://doi.org/10.2337/diacare.27.11.2669. [ Links ]
71. Ramalho F. Hepatitis C virus infection and liver steatosis. Antiviral Res. 2003;60:125-7. https://doi.org/10.1016/j.antiviral.2003.08.007. [ Links ]
72. Tsochatzis E, Papatheodoridis GV, Manesis EK, Chrysanthos N, Kafiri G, Petraki K, et al. Hepatic steatosis in genotype 4 chronic hepatitis C is mainly because of metabolic factors. Am J Gastroenterol. 2007;102:634-41. https://doi.org/10.1111/j.1572-0241.2006.01025.x. [ Links ]
73. Schreuder TC, Gelderblom HC, Weegink CJ, Hamann D, Reesink HW, Devries JH, et al. High incidence of type 1 diabetes mellitus during or shortly after treatment with pegylated interferon alpha for chronic hepatitis C virus infection. Liver Int. 2008;28:39-46. https://doi.org/10.1111/j.1478-3231.2007.01610.x. [ Links ]
74. Betterle C, Fabris P, Zanchetta R, Pedini B, Tositti G, Bosi E, et al. Autoimmunity against pancreatic islets and other tissues before and after interferon-alpha therapy in patients with hepatitis C virus chronic infection. Diabetes Care. 2000;23:1177-181. https://doi.org/10.2337/diacare.23.8.1177. [ Links ]
75. Chakrabarti D, Hultgren B, Stewart TA. IFN-alpha induces autoimmune T cells through the induction of intracellular adhesion molecule-1 and B7.2. J Immunol. 1996;157:522-8. [ Links ]
76. Romero-Gómez M, Del Mar Viloria M, Andrade RJ, Salmerón J, Diago M, Fernández-Rodríguez CM, et al. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology. 2005;128:636-41. https://doi.org/10.1053/j.gastro.2004.12.049. [ Links ]
77. Alexia C, Fallot G, Lasfer M, Schweizer-Groyer G, Groyer A. An evaluation of the role of insulin-like growth factors (IGF) and of type-I IGF receptor signalling in hepatocarcinogenesis and in the resistance of hepatocarcinoma cells against drug induced apoptosis. Biochem Pharmacol. 2004;68:1003-15. https://doi.org/10.1016/j.bcp.2004.05.029. [ Links ]
78. Stuver SO, Kuper H, Tzonou A, Lagiou P, Spanos E, Hsieh CC, et al. Insulin-like growth factor 1 in hepatocellular carcinoma and metastatic liver cancer in men. Int J Cancer. 2000;87:118-21. https://doi.org/10.1002/1097-0215(20000701)87:1<118::aid-ijc17>3.0.co;2-w. [ Links ]
79. Dyal KH, Aguilar M, Bartos G, Holt WE, Bhuket T, Liu B, et al. Diabetes Mellitus increases risk of hepatocellular carcinoma in chronic hepatitis C virus patients: a systematic review. Dig Dis Sci. 2016;61:636-45. https://doi.org/10.1007/s10620-015-3983-3. [ Links ]
80. Arase Y, Kobayashi M, Suzuki F, Suzuki Y, Kawamura Y, Akuta N, et al. Effect of type 2 diabetes on risk for malignancies includes hepatocellular carcinoma in chronic hepatitis C. Hepatology. 2013;57:964-73. https://doi.org/10.1002/hep.26087. [ Links ]
81. Ikeda A, Ikeda K, Takai A, Takahashi K, Ueda Y, Marusawa H, et al. Hepatitis C treatment with sofosbuvir and ledipasvir accompanied by immediate improvement in hemoglobin A1C. Digestion. 2017;96:228-30. https://doi.org/10.1159/000484237. [ Links ]
82. Adinolfi LE, Nevola R, Guerrera B, D’Alterio G, Marrone A, Giordano M, et al. Hepatitis C virus clearance by direct-acting antiviral treatments and impact on insulin resistance in chronic hepatitis C patients. J Gastroenterol Hepatol. 2018;33:1379-82. https://doi.org/10.1111/jgh.14067. [ Links ]
83. Ciancio A, Bosio R, Bo S, Pellegrini M, Sacco M, Vogliotti E, et al. Significant improvement of glycemic control in diabetes patients with HCV infection responding to direct-acting antiviral agents. J Med Virol. 2018;90:320-7. https://doi.org/10.1002/jmv.24954. [ Links ]
84. Dawood AA, Nooh MZ, Elgamal AA. Factors associated with improved glycemic control by direct-acting antiviral agent treatment in Egyptian type 2 diabetes mellitus patients with chronic hepatitis C genotype 4. Diabetes Metab J. 2017;41:316-21. https://doi.org/10.4093/dmj.2017.41.4.316. [ Links ]
85. Abdel-Hamid N, Jubori TA, Farhan A, Mahrous M, Gouri A, Awad E, et al. Underlying pathways for interferon risk to type II diabetes mellitus. Curr Diabetes Rev. 2013;9(6):472-7. [ Links ]
86. Li J, Zhang T, Gordon SC, Rupp LB, Trudeau S, Holmberg SD, et al. Impact of sustained virologic response on risk of type 2 diabetes among hepatitis C patients in the United States. J Viral Hepat. 2018;25(8):952-958. https://doi.org/10.1111/jvh.12887. [ Links ]
87. Li J, Gordon SC, Rupp LB, Zhang T, Trudeau S, Holmberg SD, et al. Sustained virological response to hepatitis C treatment decreases the incidence of complications associated with type 2 diabetes. Aliment Pharmacol Ther. 2019;49:599-608. https://doi.org/10.1111/apt.15102. [ Links ]
88. Gilad A, Fricker ZP, Hsieh A, Thomas DD, Zahorian T, Nunes DP. Sustained Improvement in Type 2 Diabetes Mellitus is Common After Treatment of Hepatitis C Virus With Direct-acting Antiviral Therapy. J Clin Gastroenterol. 2019;53(8):616-20. https://doi.org/10.1097/MCG.0000000000001168. [ Links ]
89. Hum J, Jou JH, Green PK, Berry K, Lundblad J, Hettinger BD, et al. Improvement in glycemic control of type 2 diabetes after successful treatment of hepatitis C virus. Diabetes Care. 2017;40:1173-80. https://doi.org/10.2337/dc17-0485. [ Links ]
90. Crook ED, Penumalee S, Gavini B, Filippova K. Hepatitis C is a predictor of poorer renal survival in diabetic patients. Diabetes Care 2005;28:2187-91. https://doi.org/10.2337/diacare.28.9.2187. [ Links ]
91. Walsh MJ, Jonsson JR, Richardson MM, Lipka GM, Purdie DM, Clouston AD, et al. Non-response to antiviral therapy is associated with obesity and increased hepatic expression of suppressor of cytokine signalling 3 (SOCS-3) in patients with chronic hepatitis C, viral genotype 1. Gut. 2006;55:529-35. https://doi.org/10.1136/gut.2005.069674. [ Links ]
92. Sanyal AJ. Role of insulin resistance and hepatic steatosis in the progression of fibrosis and response to treatment in hepatitis C. Liver Int. 2011;31(Suppl 1):23-8. https://doi.org/10.1111/j.1478-3231.2010.02397.x. [ Links ]
93. Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642-6. https://doi.org/10.1126/science.1120781. [ Links ]
94. O’Leary JG, Chan JL, McMahon CM, Chung RT. Atorvastatin does not exhibit antiviral activity against HCV at conventional doses: a pilot clinical trial. Hepatology. 2007;45:895-8. https://doi.org/10.1002/hep.21554. [ Links ]
95. Wang CS, Yao WJ, Chang TT, Wang ST, Chou P. The impact of type 2 diabetes on the development of hepatocellular carcinoma in different viral hepatitis statuses. Cancer Epidemiol Biomarkers Prev. 2009;18:2054-60. https://doi.org/10.1158/1055-9965.EPI-08-1131. [ Links ]
Received: November 21, 2018; Accepted: March 28, 2019











 text in
text in