Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.34 no.4 Bogotá oct./dic. 2019
https://doi.org/10.22516/25007440.333
Original articles
Thyrogastric syndrome: Case Series
1Médico docente Facultad de Medicina, Universidad Nacional de Colombia, Bogotá, Colombia
A significant percentage of patients with chronic autoimmune atrophic body gastritis (type A gastritis) develop thyroid autoimmune disease (Graves’ disease or Hashimoto’s disease) and vice versa. This situation is known as thyrogastric syndrome. Its prevalence is unknown, due to incomplete diagnoses. Since the development of atrophic gastritis limits the absorption of vitamin B12 leading to hematological, neurological and metabolic alterations, it is important to perform necessary diagnostic tests and to closely monitor the evolution of patients. Serological detection of autoantibodies against the thyroid gland and the gastric body show the autoimmune etiology and an inflammatory state with tissue damage. Every patient with autoimmune disease should be evaluated to rule out the presence of other pathologies of immunological etiology.
Keywords: Autoimmune diseases; thyroiditis; gastritis; atrophy; antibodies; helicobacter pylori (DeSC)
Un porcentaje importante de pacientes con gastritis crónica atrófica corporal autoinmune, o gastritis tipo A, desarrollan enfermedad autoinmune tiroidea (enfermedad de Graves o de Hashimoto) y viceversa, situación conocida como síndrome autoinmune tirogástrico (SAT), pero no se conoce su prevalencia, por lo que puede pasarse sin el diagnóstico completo. El desarrollo de la gastritis atrófica limita la absorción de la vitamina B12, lo que lleva a alteraciones hematológicas, neurológicas y metabólicas, por tanto, es importante realizar las pruebas necesarias para su diagnóstico y seguir de cerca la evolución de los pacientes. La detección serológica de los autoanticuerpos contra la glándula tiroides y el cuerpo gástrico muestran la etiología autoinmune y un estado inflamatorio con daño tisular. Todo paciente con enfermedad autoinmune debe ser valorado para descartar la presencia de otras patologías de etiología inmunológica.
Palabras clave: Enfermedades autoinmunes; tiroiditis; gastritis; atrofia; anticuerpos; Helicobacter pylori; DeSC.
Introduction
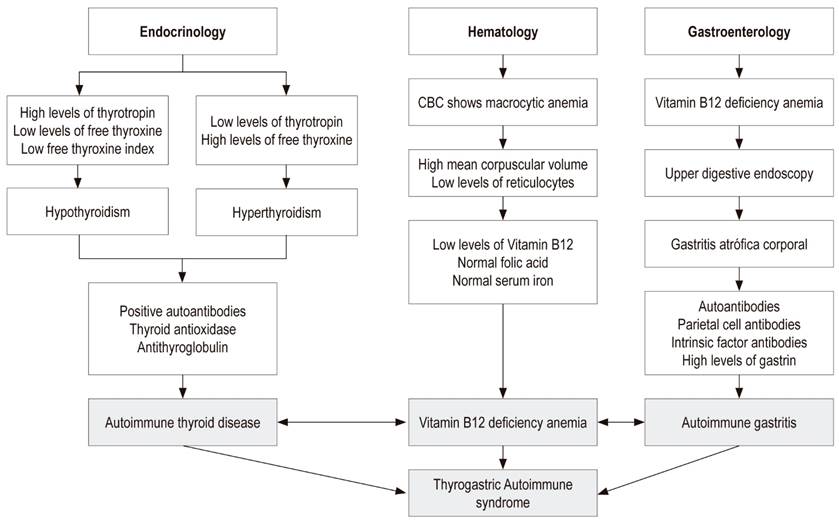
Thyrogastric autoimmune syndrome (TAS) is defined as thyroid disease of autoimmune etiology (Hashimoto’s thyroiditis or Graves’ disease) and diffuse chronic atrophic gastritis of the corpus, also called type A gastritis or autoimmune gastritis. It was initially described by Tudhope and Wilson in 1960. 1
The main cause of vitamin B12 malabsorption is hypochlorhydria or achlorhydria, secondary to atrophy of the gastric oxyntic mucosa. This has been reported in about one third of all people over 50 years of age. There are multiple causes of decreased or absent acid secretion by parietal cells including aggression by autoantibodies against these cells, Helicobacter pylori infections and prolonged use of medications that inhibit gastric secretion (proton pump inhibitors), especially in elderly people. 2,3
Diagnosis of TAS depends on clinical suspicion by endocrinologists, hematologists and gastroenterologists. Failure to diagnose can cause serious health problems related chronic malabsorption of essential nutrients such as iron and vitamin B12 (Figure 1). 4
Case presentations
Case 1
The patient was a 60-year-old woman with megaloblastic anemia who had been receiving a 1 mg monthly cyanocobalamin supplement to treat vitamin B12 deficiency that had been diagnosed three months earlier. (Table 1). She had developed hypothyroidism three years earlier and was being treated with 75 μg/day of levothyroxine. Esophagogastroduodenoscopy showed atrophic gastritis of the corpus and antrum. Biopsies from the corpus, angular incisures and antrum indicated chronic atrophic gastritis with complete intestinal metaplasia, without dysplasia and without H. pylori. The patient tested positive for parietal cell antibodies (Table 2). The diagnosis of pernicious anemia plus her history of hypothyroidism antithyroid antibodies (Table 2) result in a diagnosis of TAS.
Table 1 Patients’ laboratory test results
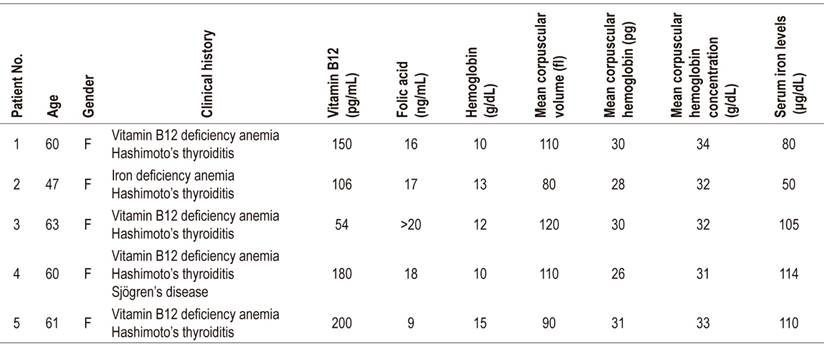
Normal ranges: serum iron: 60-170 μg/dL; Vitamin B12: 200-900 pg/mL (chemiluminescence); hemoglobin: 12-16 g/dL; folic acid: 2.7-17 ng/mL; mean corpuscular volume: 82-98 fl; mean corpuscular hemoglobin: 27-31 pg; mean corpuscular hemoglobin concentration: 33-37 g/dL.
Table 2 Patient autoantibody and gastric biopsy test results
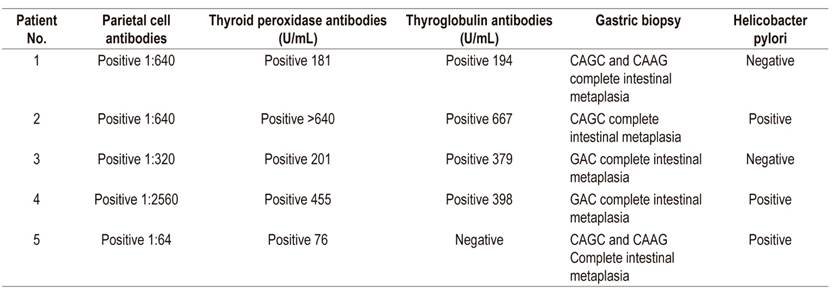
Normal ranges: thyroid peroxidase antibodies: 0-34 U/mL (chemiluminescence); thyroglobulin antibodies: 0-115 U/mL (chemiluminescence); parietal antibodies: negative (indirect immunofluorescence). CAAG: chronic atrophic antral gastritis; CAGC: chronic atrophic gastritis of the corpus.
Case 2
The patient was a 47-year-old woman who had been diagnosed with iron deficiency anemia without a specific cause and who needed ferrous replacement (Table 1). She had no history of digestive bleeding and was not menstruating. She had developed hypothyroidism 7 years earlier which was being treated with 100 μg/day of levothyroxine. Esophagogastroduodenoscopy showed atrophy in the fundus and corpus (Table 2). Two weeks of helicobacter pylori eradication treatment with esomeprazole, clarithromycin and amoxicillin was prescribed. Because of coexistence of hypothyroidism, vitamin B12 deficiency and atrophic gastritis of the corpus, she was tested for thyroid autoantibodies and parietal antibodies (Table 2). A diagnosis of TAS was made and cyanocobalamin treatment was initiated.
Case 3
The patient was a 63-year-old woman who had been referred because of megaloblastic vitamin B12 deficiency anemia. She had been receiving cyanocobalamin due to of 3 months. In addition, she had suffered from hypothyroidism for 10 years and was being treated with 75 μg/day of levothyroxine (Table 1). Esophagogastroduodenoscopy showed atrophy of the corpus and the gastric antrum. Because of the combination of atrophic gastritis and vitamin B12 deficiency, immunological tests for parietal cell antibodies and thyroid antibodies were performed (Table 2) and the diagnosis of TAS was made.
Case 4
The patient was a 60-year-old woman who had been undergoing treatment of Sjögren’s syndrome with pilocarpine for five years. The patient had had a combination of megaloblastic anemia (Table 1) and autoimmune hypothyroidism for one year and was receiving 100 μg/day of levothyroxine and 1 mg of cyanocobalamin. Esophagogastroduodenoscopy showed chronic atrophic gastritis and Helicobacter pylori (Table 2). Eradication treatment was prescribed and she was tested for parietal cell antibodies and thyroid antibodies (Table 2). Her results of anemia, hypothyroidism and autoimmune gastritis resulted in a diagnosis of TAS.
Case 5
The patient was a 61-year-old woman with vitamin B12 deficiency anemia who had had hypothyroidism for 10 years. She was being treated with levothyroxine. Her blood tests were positive for thyroid peroxidase antibodies (Tables 1and2). Esophagogastroduodenoscopy showed multifocal atrophic chronic gastritis with complete metaplasia, without dysplasia. She was positive for H. pylori. Eradication treatment was prescribed and followed up with a breath test which was negative. She tested positive for parietal cells antibodies (Table 2) and a diagnosis of TAS was made.
Comments
Vitamin B12 deficiency, defined by serum levels of less than 200 pg/dL, is often found in elderly patients. It has been observed in one in twenty people over the age of 65. 5-7
Hematological, neurological and metabolic alterations secondary to vitamin B12 deficiency are known. An English study over 75 years found vitamin B12 deficiencies in 13% of 1,000 patients and found a clear association between low levels of vitamin B12 and cognitive alterations observed in these individuals, OR = 3.0 (95% CI 1 , 3-6.9). 8
Vitamin B12 deficiency secondary to autoimmune-caused atrophy of the gastric oxyntic mucosa, also called chronic atrophic corpus gastritis or type A gastritis, causes a type of megaloblastic anemia (classically referred to as pernicious anemia). It is responsible for 25% of all cases of vitamin B12 deficiency. These patients have autoantibodies against parietal cell canaliculi and against the intrinsic factor which leads to the destruction of oxygen glands and induces atrophy. This is evidenced by hypochlorhydria or achlorhydria, G-cell hyperplasia and decreased serum pepsinogen I. It inhibits absorption of vitamin B12. 9,10
Vitamin B12 deficiency is also commonly found in patients with chronic multifocal atrophic gastritis (type B gastritis) who do not have any autoimmune etiology. These cases are associated with Helicobacter pylori infections. In contrast to patients with type A gastritis, secretion of intrinsic factor is adequate, but insufficient acid secretion prevents normal absorption of the vitamin. This type of gastritis is common in adults, becoming more common with aging as do neurological and cardiovascular diseases. 11,12 A study of 75 adult Colombian patients (average age of 56 years) with chronic multifocal atrophic corpus and antral gastritis found vitamin B12 deficiencies in 28%. A third of them tested positive for parietal cell antibodies against. 13
H. pylori has been found to induce an autoimmune response which causes atrophy of the mucosa of the gastric corpus. Autoantibodies against the gastric mucosa have been detected in 50% to 60% of patients infected with H pylori. Autoantibodies against the luminal membrane of the epithelial cells of the foveoles of the mucosa of both the antrum and the corpus as well as against the membrane canaliculi of the parietal cells of the oxygen mucosa have also been identified. The most important autoantigen is the proton pump H + K + ATPase or acid pump alpha and beta subunits. 14,15
Gastric autoantibodies, especially H. pylori-induced anti-canalicular antibodies, are associated with histological changes such as increased acute inflammatory activity, hyperplasia of gastrin-producing enterochromaffin cells, decreased production of hydrochloric acid, impaired pepsinogen types I and II, and decreased absorption of vitamin B12. It is known that the majority of patients with autoimmune gastritis are, or were, infected with H. pylori. H. pylori eradication in early stages improves histological changes and decreases autoantibody concentrations. It has been observed that gastritis in the corpus is more severe when anti-canalicular antibodies are identified. 16
The main tissue damage mechanism is CD4 + T lymphocytes. H. pylori infections induce expression of the major histocompatibility class II complex and costimulation molecules by gastric mucosa cells. This activates intraepithelial lymphocytes. Dendritic cells induce a specific T response against the beta subunit of ATPase which releases cytokines and chemokines (TNF, IL-2, interferon gamma, CXCL8) and increases the number of inflammatory cells. 14 It has been suggested that this autoimmune response may be due to a failure of central immune tolerance. Autoreactive T lymphocytes appear to act on ATPase beta during H. pylori infections or during cell turnover. An increase in gastric cell apoptosis through Fas-FasL molecules has been observed in-vitro when inflammatory cytokines are present. Increased Fas-FasL expression has also been observed in patients with H. pylori where it leads to parietal cell death and where it causes mucosal atrophy. 17
Several studies have linked H. pylori infections with development of autoimmune diseases such as autoimmune thyroid disease, type 1 diabetes mellitus (DM1), rheumatoid arthritis, Sjögren’s syndrome and autoimmune gastritis. 18 Genetic, environmental, and immunological factors are all involved in the etiology of autoimmune diseases. Genetic factors include HLA alleles, mutations, and nucleotide polymorphisms, environmental factors include ultraviolet light, smoking, medications and infections, and immunological factors include T lymphocytes and self-reactive B lymphocytes. 17
Autoimmune thyroiditis is one of the most frequently diagnosed endocrinopathies. Antibodies against thyroglobulin and thyroid peroxidase have been identified as serological markers of the disease. Molecular mimicry has been postulated as a possible autoimmune mechanism in patients infected with Yersinia enterocolitica bacteria, hepatitis C virus and Helicobacter pylori. Sequences of Helicobacter Cag A cytotoxin and thyroid peroxidase are similar. A reduction in antimicrosomal antibody and H. pylori antibody concentrations has also been observed after eradication treatment. Inflammatory cells, plasma cells that produce autoantibodies against thyroglobulin, peroxidase and the thyrotropin (TSH) receptor, as well as cytotoxic T lymphocytes both of which cause tissue damage have also been found. 19,20
Bassi et al. have also found an association between Graves’ disease and H. pylori infections. Their study suggests that previous infection may be a trigger for the onset of this disease in patients with genetic predisposition. In addition, other studies suggest that H. pylori infections may worsen autoimmune thyroiditis in immunogenically susceptible patients. This implies that eradication of the infection in high-risk children may prevent autoimmune thyroiditis. It has also been reported that patients with Graves’ disease may have elevated levels of gastrin which relates autoimmune hyperthyroidism to autoimmune gastritis. 21 Fallahi et al. have evaluated the prevalence of other autoimmune diseases in patients with autoimmune thyroid disease. The authors’ case and control study included 3,069 patients with Hashimoto’s thyroiditis and found that the disease’s most frequent and significant association was with chronic autoimmune gastritis. 22
In addition, patients with DM1 also frequently have autoimmune thyroiditis. It has been postulated that patients with one autoimmune disease have a higher risk of developing another autoimmune disease. Approximately 20% of patients with DM1 have antithyroid antibodies and 5% of them develop autoimmune hypothyroidism. 23 Mervat et al. have found a high prevalence of H. pylori infections in patients with DM1 and that glycosylated hemoglobin levels were higher in patients with H. pylori infections than in uninfected patients. In addition, they found that autoimmune thyroiditis was more common in patients with DM1 who had thyroid antibodies and who were infected with H. pylori than it was in otherwise healthy individuals. 24
Autoimmune gastritis develops in 13% of patients who already have autoimmune thyroid disease whereas 50% of patients who begin with autoimmune gastritis develop autoimmune thyroiditis. 4,25 Consequently, we believe that pertinent examinations to determine the coexistence of the other disease are justified for all patients with any of these pathologies. Timely detection and determination of potential clinical consequences are need for establishing a program of subsequent surveillance.
Referencias
1. Tudhope GR, Wilson GM. Anaemia in hypothyroidism. Incidence, pathogenesis, and response to treatment. Q J Med. 1960;29:513-37. [ Links ]
2. Valuck RJ, Ruacin JM. A case-control study on adverse effects: H2 blocker or proton pump inhibitor use and risk of vitamin B12 deficiency in older adults. J Clin Epidemiol. 2004;57:422-8. doi: https://doi.org/10.1016/j.jclinepi.2003.08.015. [ Links ]
3. Hirschowitz BI, Worthington J, Mohnen J. Vitamin B12 deficiency in hypersecretors during long-term acid suppression with proton pump inhibitors. Aliment Pharmacol Ther. 2008;27:1110-21. doi: https://doi.org/10.1111/j.1365-2036.2008.03658.x. [ Links ]
4. Valdés Socin H, Lutteri L, Cavalier E, Plus M, Geenen V, Louis E, et al. The thyrogastric autoimmune syndrome: its effects on micronutrients and gastric tumorigenesis. Rev Med Liege. 2013;68:579-84. [ Links ]
5. Carmel R. Nutritional anemias and the elderly. Semin Hematol. 2008;45:225-34. doi: https://doi.org/10.1053/j.seminhematol.2008.07.009. [ Links ]
6. Clarke R, Grimley Evans J, Schneede J, Nexo E, Bates C, Fletcher A, et al. Vitamin B12 and folate deficiency in later life. Age Ageing. 2004;33:34-41. doi: https://doi.org/10.1093/ageing/afg109. [ Links ]
7. Ramírez Pereda A, Pacheco BI, Astiazaran-García H, Esparza-Romero J, Alemán-Mateo H. Vitamina B12 y folato en adultos mayores urbanos no institucionalizados. Arch Latioam Nutr. 2006;56:135-40. [ Links ]
8. Hin H, Clarke R, Sherliker P, Atoyebi W, Emmens K, BirksJ, et al. Clinical relevance of low serum vitamin B12 concentrations in older people: the Banbury B12 study. Age Ageing. 2006;35:416-22. doi: https://doi.org/10.1093/ageing/afl033. [ Links ]
9. Venerito M, Radünz M, Reschke K, Reinhold D, Frauenschlager K, Jechorek D, et al. Autoimmune gastritis in autoimmune thyroid disease. Aliment Pharmacol Ther. 2015;41:686-693. doi: https://doi.org/10.1111/apt.13097. [ Links ]
10. Zulfiqar AA, Andres E. Association pernicious anemia and autoimmune polyendocrinopathy: a retrospective study. J Med and Life. 2017;4:250-3. [ Links ]
11. Dholakia KR, Dharmarajan TS, Yadav D, Oiseth S, Norkus EP, Pitchumoni CS. Vitamin B12 deficiency and gastric histopathology in older patients. Word J Gastroenterol. 2005;11:707-10. doi: https://doi.org/10.3748/wjg.v11.i45.7078. [ Links ]
12. Neumann WL, Coss E, Rugge M, Genta RM. Autoimmune atrophic gastritis-pathogenesis, pathology and management. Nature Rev Gastroenterol Hepatol. 2013;10:529-41. doi: https://doi.org/10.1038/nrgastro.2013.101. [ Links ]
13. Martínez-Marín JD, Henao-Riveros SC, Rey Tovar MH. Levels of vitamin B12 in Colombian patients with chronic atrophic gastritis. Rev Col Gastroenterol. 2010;25:260-2. [ Links ]
14. Bergman MP, Faller G, D’Elios MM, Del Prete G, Vandenbroucke-Grauls C, Appelmelk BJ. Gastric autoimmunity. En: Mobley HLT, Mendz GL, Hazell SL (editores). Washington (DC): ASM Press. 2001. [ Links ]
15. Vargas A, Porras T, Henao SC, Martínez JD, Jaspe E. Detección de anticuerpos contra la mucosa gástrica en pacientes con gastritis superficial, gastritis crónica atrófica y úlcera duodenal infectados con Helicobacter pylori. Rev Col Gastroenterol. 2001;16:127-31. [ Links ]
16. Faller G, Ruff S, Reiche N, Hochberger J, Hahn E, Kirchner T. Mucosal production of antigastric autoantibodies in Helicobacter pylori gastritis. Helicobacter. 2000;5:129-34. doi: https://doi.org/10.1046/j.1523-5378.2000.00020.x. [ Links ]
17. Sabatino A, Lenti MV, Giuffrida P, Valoni A, Corazza GB. New insights into immune mechanisms underlying autoimmune diseases of the gastrointestinal tract. Autoimmunity Rev. 2015;14:1161-9. doi: https://doi.org/10.1016/j.autrev.2015.08.004. [ Links ]
18. Sorrentino D, Faller G, DeVita S, Avellini C, Labombarda A, Ferraccioli G, et al. Gastritis Helicobacter pylori associated antigastric autoantibodies: role in Sjögren’s syndrome. Helicobacter. 2004;9(1):46-53. doi: https://doi.org/10.1111/j.1083-4389.2004.00197.x. [ Links ]
19. Sterzl I, Hrda P, Matucha P, Cervska J, Zamrazil V. Anti-Helicobacter pylori, anti-thyroid peroxidase, anti-thyroglobulin and anti-gastric parietal cells antibodies in Czech population. Physiol Res. 2008;57:S135-S41. [ Links ]
20. Rial Rodríguez JM. Damage and immune response in thyroiditis. Pathogenesis of autoimmune thyroid disease. Rev Esp Endocrinol Pediatr. 2014;5(2). [ Links ]
21. Bassi V, Santinelli C, Lengo A, Romano C. Identification of a correlation between Helicobacter pylori infection and Graves’ disease. Helicobacter. 2010;15:558-62. doi: https://doi.org/10.1111/j.1523-5378.2010.00802.x. [ Links ]
22. El-Eshmawy MM, El-Hawary AK, Abdel Gaward SS, El-Baiomy AA. Helicobacter pylori infection might be responsible for the interconnection between type 1 diabetes and autoimmune thyroiditis. Diabetol Metab Syndr. 2011;3:28. doi: https://doi.org/10.1186/1758-5996-3-28. [ Links ]
23. Fallahi P, Ferrari S, Ruffilli I, Elia G, Biricotti R, Vita R, et al. The association of other autoimmune diseases in patients with autoimmune thyroiditis: review of the literature and report of a large series of patients. Autoimmun Rev. 2016;15(12):1125-8. doi: http://dx.doi.org/10.1016/j.autrev.2016.09.009. [ Links ]
24. Lahner E, Centani M, Agnello G, Gargano L, Vanella L, Iannoni C, et al. Occurrence and risk factors for autoimmune thyroid disease in patients with atrophic body gastritis. Am J Med. 2008;121:136-41. doi: https://doi.org/10.1016/j.amjmed.2007.09.025. [ Links ]
25. Cellini M, Santaguida MG, Virili C, Capriello S, Brusca N, Gargano L, et al. Hashimoto’s thyroiditis and autoimmune gastritis. Front Endocrinol. 2017;26:8-92. doi: https://doi.org/10.3389/fendo.2017.00092. [ Links ]
Received: December 13, 2018; Accepted: February 03, 2019











 texto en
texto en