Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista colombiana de Gastroenterología
versão impressa ISSN 0120-9957
Rev Col Gastroenterol vol.34 no.4 Bogotá out./dez. 2019
https://doi.org/10.22516/25007440.362
Original articles
Endoscopic hemostasis in intensive care unit patients with upper digestive tract bleeding
1Residente del último año de Medicina Interna, Universidad Nacional de Colombia. Bogotá, Colombia
2Profesor titular de Medicina Interna, Coordinador de Gastroenterología, Universidad Nacional de Colombia, Hospital Universitario Nacional de Colombia, Bogotá, Colombia
3Gastroenterólogo, Clínica Fundadores, Docente ad Honorem de Gastroenterología, Universidad Nacional de Colombia. Bogotá, Colombia
Introduction:
Patients hospitalized in an intensive care unit (ICU) are at risk of upper gastrointestinal bleeding. Esophagogastroduodenoscopy (EGD) is the test of choice for these patients. EGD is diagnostic and therapeutic. Many endoscopically identified lesions do not require endoscopic treatment. In Colombia there are no studies on the prevalence of different upper gastrointestinal bleeding lesions in ICU patients, nor on the use of therapeutic EGD in these patients.
Materials and methods:
This is a cross-sectional study conducted at the Clínica Fundadores in Bogotá Colombia between January 2003 and February 2017. Adult ICU patients who underwent EGD due to upper gastrointestinal bleeding were included.
Results:
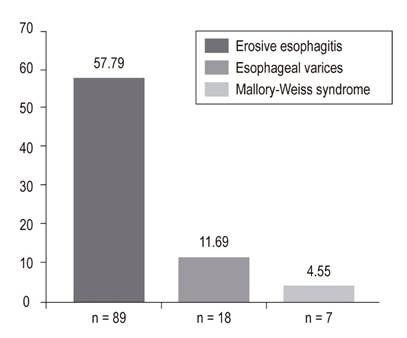
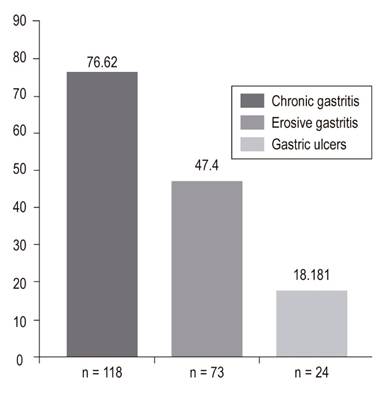
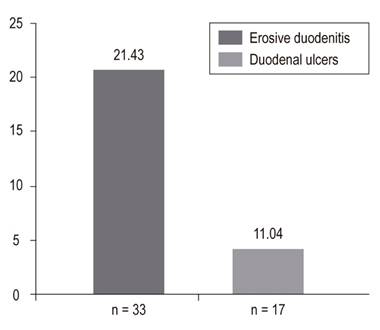
In the final analysis, 156 patients who underwent EGD were included. Of these, 76.62% (118) had chronic gastritis, 57.79% (89) had erosive esophagitis grades A to D, 47.4% (73) had erosive gastritis, 21.43% (33) had erosive duodenitis, 18.18% (28) had gastric ulcer, 11.69% (18) had esophageal varices, 11.04% (17) had duodenal ulcers, and 4.55% (8) Mallory Weiss tears. Only 15% of patients, including those with esophageal varices, required endoscopic management.
Conclusions:
In this study, 15% of patients with upper gastrointestinal bleeding required endoscopic treatment. Prospective work should be done to establish risk factors to predict the need for therapeutic EGD in patients with upper gastrointestinal bleeding. Patients do not have these predictors should be treated empirically with PPI to avoid unnecessary expenses of diagnostic EGDs.
Keywords: Intensive care; hemorrhage; endoscopy
Introducción:
los pacientes hospitalizados en la unidad de cuidados intensivos (UCI) tienen riesgo de hemorragia digestiva alta (HVDA). La endoscopia digestiva alta (EVDA) es el examen de elección en esos pacientes y es diagnóstica y terapéutica. Muchas lesiones identificadas endoscópicamente no requieren tratamiento endoscópico. En Colombia no hay estudios sobre la prevalencia de las diferentes lesiones sangrantes digestivas altas en pacientes de la UCI, ni sobre la utilización de EVDA terapéutica en esos pacientes.
Materiales y métodos:
estudio de corte transversal realizado en la Clínica Fundadores de Bogotá, Colombia, entre enero del 2003 a febrero del 2017. Se incluyeron pacientes adultos de la unidad de cuidado intensivo con EVDA indicada por HVDA.
Resultados:
en el análisis final se incluyeron 156 EVDA. Los hallazgos fueron los siguientes: gastritis crónica 76,62% (118), esofagitis erosiva (grado A-grado D) 57,79% (89), gastritis erosiva 47,4% (73), duodenitis erosiva 21,43% (33), úlcera gástrica 18,18% (28), varices esofágicas 11,69% (18), úlcera duodenal 11,04% (17) y desgarro de Mallory-Weiss 4,55% (8). Solo el 15% de los pacientes requirió manejo endoscópico, incluidos los que tenían várices esofágicas.
Conclusión:
en el presente estudio, el 15% de los pacientes con HVDA requirió tratamiento endoscópico. Se deben realizar trabajos prospectivos que permitan establecer factores de riesgo que puedan predecir la necesidad de EVDA terapéutica en pacientes con HVDA. Quien no tenga esos predictores se debe tratar empíricamente con IBP y evitar gastos innecesarios en EVDA diagnósticas.
Palabras clave: Cuidados intensivos; hemorragia; endoscopia
Introduction
Patients hospitalized in the intensive care unit (ICU) have a higher risk of upper gastrointestinal bleeding (UGIB) especially due to stress ulcers. 1,2 Its appearance is associated with adverse outcomes including 2 to 4 times higher mortality rates and 4 to 8 day longer ICU stays. 3,4 The incidence of gastrointestinal bleeding in ICUs ranges from 0.17% to 7.0%. 3,5 An upper gastrointestinal endoscopy (esophagogastroduodenoscopy or EGD) is the test of choice for patients with UGIB, including those in the ICU. EGD can be both diagnostic, macroscopic examination of lesions and taking of biopsies, and therapeutic, various methods of achieving hemostasis. 5,6 Its performance is well demonstrated in patients with UGIB. 7-10 However, EGDs are more controversial when they are performed in patients admitted to the ICU for other reasons. In addition, the discovery of gastritis, esophagitis, or other GI problems may not require any endoscopic or pharmacological treatment. 5,7,11,12
Among the causes of UGIB in ICU patients are esophageal varices, gastric varices, esophagitis, ulcers and stress-induced gastritis. 13 It has been estimated that up to 90% of critically ill patients may present gastroduodenal mucosal damage after three days in an ICU and that damage may progress to ulcers and cause bleeding,. Bleeding from erosive gastritis is another potential danger. 14 Nevertheless, UGIB is clinically important bleeding in only 2% to 3% of these cases, and stress ulcers are identified endoscopically as the source of bleeding in less than 50% of these patients. 4,15,16 These data have raised a discussion about the real need for EGD given its costs and given the real impact of indiscriminate use on this type of patients, especially since only a small percentage progresses towards manifest and clinically important gastrointestinal bleeding. 17
Other less common conditions responsible for UGIB are Mallory-Weiss syndrome and vascular lesions. 13 The principal upper gastrointestinal risk factors in an ICU include mechanical ventilation for more than 48 hours, active coagulopathy, liver disease, and kidney disease. 5,18,19 Other risk factors are shock, liver failure, kidney failure, sepsis, multiple traumas, burns of more than 35% of the body surface, organ transplantation, skull or spinal cord trauma, history of previous ulcerative disease and hypoalbuminemia. 20-24
We found no publications on the prevalence of bleeding lesions of the upper digestive tract in ICU patients in Colombia, nor did we find literature on the frequency of endoscopic hemostasis in these patients. Taking into account the limited information available, we decided to perform this study in the Gastroenterology Unit of the Clínica Fundadores in Bogotá by identifying ICU patients who had developed UGIB and had undergone EGD.
Materials and methods
This is a cross-sectional study based on EGD findings from ICU patients at the Clínica Fundadores who underwent EGD because of UGIB. Adult patients over 18 years of age who were treated during the period from January 1, 2003 to December 31, 2017 were included.
Inclusion Criteria
Patients hospitalized in the ICU for critical illnesses were included if they developed UGIB after 24 hours of hospitalization and underwent an EGD.
Exclusion Criteria
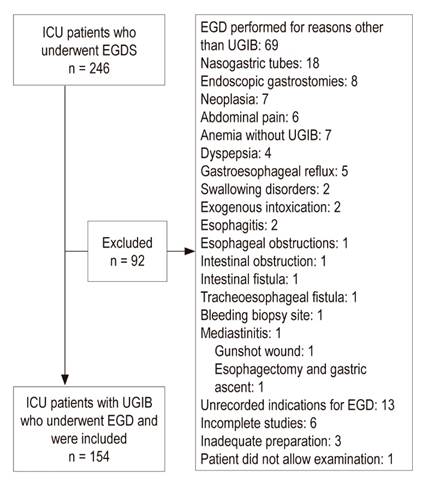
ICU patients who had undergone EGD because of any indication other than UGIB were excluded. Patients with incomplete EGDs, those hospitalized in the intermediate care unit and pregnant women were also excluded. Those who were hospitalized in the ICU because of severe UGIB were not included.
Information was obtained from EGD reports of the gastroenterology unit corresponding to the ICU and a review of the medical histories of the patients identified. Variables of each patient were recorded in a data base built for this study. Because only patients with EGD were included, the number of ICU patients with UGIB for whom no endoscopy was performed during the study period is unknown.
Overall Objective
Our overall objective was to determine the need for endoscopic hemostasis in ICU patients with UGIB.
Statistical Analysis
Qualitative variables are presented in the form of absolute numbers and proportions. Prevalence was defined as (the number of patients who underwent endoscopy/the total population) x 100. Prevalence was stratified by age groups. Averages, measures of dispersion, and statistical distributions are presented for quantitative variables. Distributions were evaluated with the Shapiro-Wilk test. Statistical significance was considered to be p less than 0.05.
Results
During the study period, 246 EGDs were performed on ICU patients. Ninety-two of these were excluded:
Sixty-nine were excluded because they were performed for reasons other than UGIB.
Eighteen were excluded because patients had nasogastric tube.
Eight were excluded because they has had endoscopic gastrostomies.
Seven were excluded due to neoplasia.
Six were excluded due to abdominal pain
Four were excluded due to anemia.
Four were excluded due to dyspepsia.
Three were excluded due to known cirrhosis.
Three were excluded due to esophageal varices.
Two were excluded due to gastroesophageal reflux.
Two were excluded due to swallowing disorders
Two were excluded due to esophagitis
Two were excluded due to exogenous intoxication.
One was excluded due to an esophageal obstruction
One was excluded due to an intestinal obstruction
One was excluded due to an intestinal fistula
One was excluded due to a tracheoesophageal fistula
One was excluded due to a bleeding biopsy site.
One was excluded due to mediastinitis
One was excluded due to a gunshot wound
One was excluded due to an esophagectomy and gastric ascent
Thirteen were excluded due to unrecorded indications for EGD
Six were excluded due to incomplete studies
Three were excluded due to inadequate preparation
One patient did not allow the examination
We included 154 patients who underwent EGDs (Figure 1) including 99 men (64.29%) and 55 women (35.71%). Their median age was 68, and half of them were between 59 and 76 years of age.
EGD findings in the esophagus, stomach and duodenum are shown in Figures 2, 3 and 4.
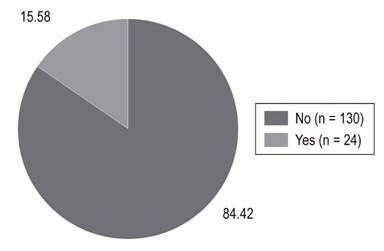
Twenty-four patients (15.58%) were treated endoscopically, but it was not necessary in the remaining 130 patients (84.4%) (Figure 5). Among the diagnoses recorded in the medical records for esophageal varices, Child Pugh C cirrhosis with encephalopathy, mostly secondary to diabetes mellitus and alcohol was the most common. Other findings were presented with ambivalent information due to the large number of pathologies and comorbidities of these critical patients. They included sepsis, shock, heart failure, renal failure, respiratory failure, and coagulopathy.
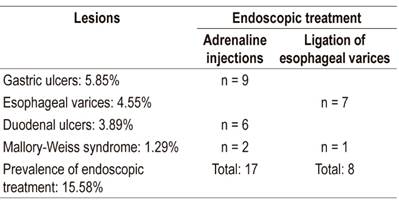
Modalities of therapeutic endoscopy used are shown in Table 1. Hemostasis of bleeding was achieved in all patients.
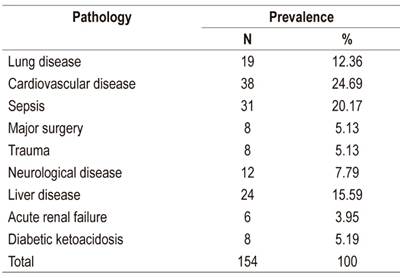
The prevalences of the primary pathologies responsible for ICU admissions are shown in Table 2.
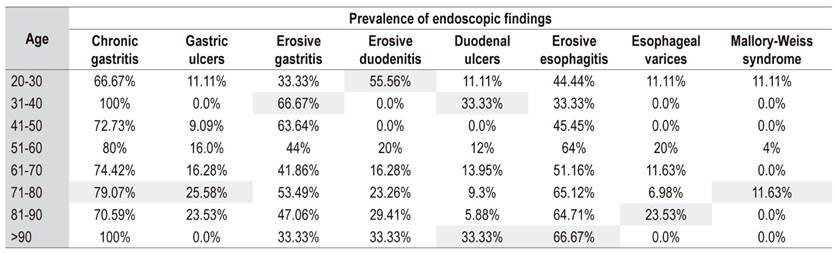
The prevalence of mechanical ventilation was 20% (n = 30), and the prevalence of prophylaxis with proton pump inhibitors (PPI) was 32% (n = 49). The distribution of lesions by age group is shown in Table 3.
Discussion
Fifteen percent of the ICU patients with UGIB in this study required endoscopic treatment. The need for therapeutic EGD is similar to a study of 66 ICU patients from the University of Pennsylvania. That study found that 15% of those patients merited hemostatic endoscopic therapy. 25 Another study by Kim et al. of 66 patients at the University of Seoul found that endoscopic management of bleeding had been needed in 19% of their patients. 26
EGDs are frequently requested for critically ill patients with gastrointestinal bleeding for both diagnostic and therapeutic purposes. However, not all mucosal lesions identified by this means require endoscopic treatment which increases the costs of patient care and the likelihood of complications. 7,27 In general, these patients die due to the severity of the underlying medical condition or due to multiorgan dysfunction rather than due to bleeding. 28
Lee et al. conducted a prospective study of 105 patients with gastrointestinal bleeding who were in a critical care unit and found that the prevalence of erosive disease was 21.9%. 11 In our study, erosive gastritis had a prevalence of 47.4% (n = 73), but none of these lesions required endoscopic treatment. The clinical relevance of these lesions is controversial, since only a small percentage progress towards overt and clinically important gastrointestinal bleeding. 17,22
The most frequent esophageal finding in our study was erosive esophagitis which was found in 59% of the patients, a higher rate than found in previously published studies. 25,29-31 This finding demonstrates once again that the majority of EGDs performed in ICU patients with UGIB will find lesions that do not merit therapeutic endoscopy. Consequently, there will be no impact on the treatment of these patients. 7 Mallory-Weiss syndrome was found in 8 patients (4.55%), none of whom required endoscopic therapy. Mallory-Weiss syndrome is related to a sudden increase in intragastric or intra-abdominal pressure which is transmitted to the esophagogastric junction. 32 Risk factors include severe nausea, vomiting, closed abdominal hypo-trauma, cardiopulmonary resuscitation, coughing, shouting, barotrauma and seizures. In our series, this syndrome occurred less frequently than reported in other publications. The multiple predisposing factors could explain this difference. 10,33 However, it would be important to minimize related factors since Mallory-Weiss syndrome can deepen and produce transmural rupture which leads to Boeerhäve syndrome. 34.
Gastric ulcers were found in 18.18% (n = 28) of the patients in our study. In 2005, Skok et al. conducted a prospective cohort study of 486 patients in Slovenia which found gastric ulcers in 84 patients (17.3%). 35 However, only 5.8% (9 patients) in our study required endoscopic treatment for gastric ulcers. A retrospective observational study of 88 French patients between 2007 and 2012 evaluated the clinical impact of EGD in critically ill patients with suspected bleeding. It found that only 3.5% of patients required endoscopic management for gastric ulcers. 7 These results are important, since EGDs are relatively expensive and are not risk-free. Both costs and risks increase as the procedure becomes generalized. 35
Our study found that non-varicose causes occurred twice as frequently as varicose causes (11.03% vs. 4.55%) which is similar to other studies. 36 There were 17 patients with duodenal ulcers (11.04%) which is half of what has been reported in the literature. 29 We do not know the reason for this discrepancy, but it could be related to the prevalence of Helicobacter pylori, the frequency of use of prophylactic PPIs and the type of critical pathology of patients, especially mechanical ventilation, active coagulopathies, liver disease and renal disease. In our study, many patients had these predisposing pathologies, and 32% of the patients used prophylactic PPIs. Prophylactic PPIs have been shown to decrease the rate of clinically significant bleeding below the rate of patients taking placebos (2.5% vs. 4.2%). PPIs are recommended in these circumstances. 2,37
Many doctors are still afraid to use PPIs prophylactically because of the theoretical risks of possible adverse effects such as pneumonia, myocardial ischemia, and C. difficile. 15 Nevertheless, the evidence that supports these fears is very weak, and so far only association and non-causality have been established. 38 In our study, the rate of erosive duodenitis was higher than that reported in the literature (21.43% vs. 6%), but we identified no active bleeding related to this pathology in any of our patients. 29,34
This study’s limitations include its retrospective nature and changing diagnoses due to multiple concomitant pathologies. Also, it was not easy to determine the risk factors that predispose to UGIB which would merit endoscopic treatment and thus avoid the 85% of EGDs which are unnecessary.
In conclusion, 15% of our ICU patients with UGIB needed endoscopic therapy. Prospective studies, preferably multicenter, are needed to identify risk factors that can predict the need for therapeutic EGDs in patients with UGIB. Patients who do not have these predictors should be treated empirically with PPIs which will avoid unnecessary expenses of diagnostic EGDs. To date, the published literature has identified ICU patients at risk of UGIB but has not identified those whose bleeding may require therapeutic EGD.
Acknowledgements
We thank Liliana Oino, the Biomedical Engineer who is the administrative coordinator of the Gastroenterology Unit of Clínica Fundadores and the National University of Colombia - Clínica Fundadores Teaching-Assistance Agreement, for her interest, enthusiasm and continuous assistance in all phases of this study. She facilitated access to all information on endoscopic procedures and identification of medical records of patients. We would also like to thank Dr. Jairo Antonio Pérez Cely, an Intensive care specialist at the National University Hospital of Colombia, for his critical reading of the manuscript and his suggestions for improving it
REFERENCES
1. Marik P, Vasu T, Hirani A, Pachinburavan M. Stress ulcer prophylaxis in the new millennium: a systematic review and meta-analysis. Crit Care Med. 2010;38(11):2222-8. doi: https://doi.org/10.1097/CCM.0b013e3181f17adf. [ Links ]
2. Krag M, Perner A, Wetterslev J, Wise M, Borthwick M, Bendel S, et al. Stress ulcer prophylaxis in the intensive care unit: an international survey of 97 units in 11 countries. Acta Anaesthesiol Scand. 2015;59(5):576-85. doi: https://doi.org/10.1111/aas.12508. [ Links ]
3. Krag M, Perner A, Wetterslev J, Wise M, Borthwick M, Bendel S, et al. Prevalence and outcome of gastrointestinal bleeding and use of acid suppressants in acutely ill adult intensive care patients. Intensive Care Med. 2015;41(5):833-45. doi: https://doi.org/10.1007/s00134-015-3725-1. [ Links ]
4. Cook D, Griffith L, Walter S, Guyatt G, Meade M, Heyland DK, et al. The attributable mortality and length of intensive care unit stay of clinically important gastrointestinal bleeding in critically ill patients. Crit Care. 2001;5(6):368. doi: https://doi.org/10.1186/cc1071. [ Links ]
5. Cook D, Fuller H, Guyatt G, Marshall J, Leasa D, Hall R, et al. Risk factors for gastrointestinal bleeding in critically ill patients. N Engl J Med. 1994;330(6):377-81. doi: https://doi.org/10.1056/NEJM199402103300601. [ Links ]
6. Cipolletta L, Cipolletta F, Granata A, Ligresti D, Barresi L, Tarantino I, et al. What is the best endoscopic strategy in acute non-variceal gastrointestinal bleeding? Curr Treat Optios Gastro. 2018;16(4):363-75. doi: https://doi.org/10.1007/s11938-018-0192-0. [ Links ]
7. Jean-Baptiste S, Messika J, Hajage D, Gaudry S, Barbieri J, Duboc H, et al. Clinical impact of upper gastrointestinal endoscopy in critically ill patients with suspected bleeding. Ann Intensive Care. 2018;8(1):75. doi: https://doi.org/10.1186/s13613-018-0423-5. [ Links ]
8. Barkun A, Bardou M, Kuipers E, Sung J, Hunt R, Martel M, et al. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Inter Med. 2010;152(2):101-13. doi: https://doi.org/10.7326/0003-4819-152-2-201001190-00009. [ Links ]
9. Lau J, Barkun A, Fan DM, Kuipers E, Yang YS, Chan F. Challenges in the management of acute peptic ulcer bleeding. Lancet. 2013;381(9882):2033-43. doi: https://doi.org/10.1016/S0140-6736(13)60596-6. [ Links ]
10. Chak A, Cooper G, Lloyd L, Kolz C, Barnhart B, Wong R. Effectiveness of endoscopy in patients admitted to the intensive care unit with upper GI hemorrhage. Gastrointest Endoscop. 2001;53(1):6-13. doi: https://doi.org/10.1067/mge.2001.108965. [ Links ]
11. Lee YC, Wang HP, Wu MS, Yang CS, Chang YT, Lin JT. Urgent bedside endoscopy for clinically significant upper gastrointestinal hemorrhage after admission to the intensive care unit. Intensive Care Med. 2003;29(10):1723-8. doi: https://doi.org/10.1007/s00134-003-1921-x. [ Links ]
12. Tam W, Bertholini D. Tension pneumoperitoneum, pneumomediastinum, subcutaneous emphysema and cardiorespiratory collapse following gastroscopy. Anaesth Intensive Care. 2007;35(2):307-9. [ Links ]
13. Conrad SA. Acute upper gastrointestinal bleeding in critically ill patients: causes and treatment modalities. Crit Care Med . 2002;30(6):S365-S8. doi: https://doi.org/10.1097/00003246-200206001-00006. [ Links ]
14. Eddleston J, Pearson R, Holland J, Tooth J, Vohra A, Doran BH. Prospective endoscopic study of stress erosions and ulcers in critically ill adult patients treated with either sucralfate or placebo. Critic Care Med. 1994;22(12):1949-54. doi: https://doi.org/10.1097/00003246-199422120-00010. [ Links ]
15. Marker S, Krag M, Møller M. What’s new with stress ulcer prophylaxis in the ICU? Intensive Care Med. 2017;43(8):1132-4. doi: https://doi.org/10.1007/s00134-017-4733-0. [ Links ]
16. Granholm A, Lange T, Anthon C, Marker S, Krag M, Meyhoff T, et al. Timing of onset of gastrointestinal bleeding in the ICU: protocol for a preplanned observational study. Acta Anaesth Scand. 2018. doi: https://doi.org/10.1111/aas.13144. [ Links ]
17. Plummer M, Blaser A, Deane A. Stress ulceration: prevalence, pathology and association with adverse outcomes. Crit Care. 2014;18(2):213. doi: https://doi.org/10.1186/cc13780. [ Links ]
18. Steinberg K. Stress-related mucosal disease in the critically ill patient: risk factors and strategies to prevent stress-related bleeding in the intensive care unit. Crit Care Med . 2002;30(6):S362-S4. doi: https://doi.org/10.1097/00003246-200206001-00005. [ Links ]
19. Siddiqui F, Ahmed M, Abbasi S, Avula A, Siddiqui A, Philipose J, et al. Gastrointestinal bleeding in patients with acute respiratory distress syndrome: a national database analysis. J Clin Med Res. 2019;11(1):42-8. doi: https://doi.org/10.14740/jocmr3660. [ Links ]
20. Alvarado J. Profilaxis de sangrado digestivo en la Unidad de Cuidados Intensivos. Univ Med. 2002;43(1):33-5. [ Links ]
21. Schuster D, Rowley H, Feinstein S, McGue M, Zuckerman G. Prospective evaluation of the risk of upper gastrointestinal bleeding after admission to a medical intensive care unit. Am J med. 1984;76(4):623-30. doi: https://doi.org/10.1016/0002-9343(84)90286-9. [ Links ]
22. Huang HB, Jiang W, Wang CY, Qin HY, Du B. Stress ulcer prophylaxis in intensive care unit patients receiving enteral nutrition: a systematic review and meta-analysis. Crit Care . 2018;22(1):20. doi: https://doi.org/10.1186/s13054-017-1937-1. [ Links ]
23. Beejay U, Wolfe M. Acute gastrointestinal bleeding in the intensive care unit: the gastroenterologist’s perspective. Gastroenterol Clin North Am. 2000;29(2):309-36. doi: https://doi.org/10.1016/S0889-8553(05)70118-7. [ Links ]
24. Morales C, Sierra S, Hernández A, Arango A, López G. Hemorragia digestiva alta: factores de riesgo para mortalidad en dos centros urbanos de América Latina. Rev Esp Enferme Dig. 2011;103(1):20-4. doi: https://doi.org/10.4321/S1130-01082011000100004. [ Links ]
25. Lewis J, Shin E, Metz D. Characterization of gastrointestinal bleeding in severely ill hospitalized patients. Crit Care Med . 2000;28(1):46-50. doi: https://doi.org/10.1097/00003246-200001000-00007. [ Links ]
26. Kim J, Kim J, Chun J, Lee C, Im J, Kim J. Early versus late bedside endoscopy for gastrointestinal bleeding in critically ill patients. Korean J Intern med. 2018;33(2):304-12. doi: 10.3904/kjim.2016.182. [ Links ]
27. Hayden S, Albert T, Watkins T, Swenson E. Anemia in critical illness: insights into etiology, consequences, and management. American J Respir Crit Care Med . 2012;185(10):1049-57. doi: https://doi.org/10.1164/rccm.201110-1915CI. [ Links ]
28. Sesler J. Stress-related mucosal disease in the intensive care unit. AACN Advanced Crit Care . 2007;18(2):119-28. doi: https://doi.org/10.4037/15597768-2007-2004. [ Links ]
29. Silverstein F, Gilbert D, Tedesco F, Buenger N, Persing J. The national ASGE survey on upper gastrointestinal bleeding: II. Clinical prognostic factors. Gastrointest Endoscop. 1981;27(2):80-93. doi: https://doi.org/10.1016/S0016-5107(81)73156-0. [ Links ]
30. van Leerdam M. Epidemiology of acute upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2008;22(2):209-24. doi: https://doi.org/10.1016/j.bpg.2007.10.011. [ Links ]
31. Ovenden C, Plummer M, Selvanderan S, Donaldson T, Nguyen N, Weinel L, et al. Occult upper gastrointestinal mucosal abnormalities in critically ill patients. Acta Anaesthesiol Scand . 2017;61(2):216-23. doi: https://doi.org/10.1111/aas.12844. [ Links ]
32. Rich K. Overview of Mallory-Weiss syndrome. J Vasc Nurs. 2018;36(2):91-3. doi: https://doi.org/10.1016/j.jvn.2018.04.001. [ Links ]
33. Guelrud M. Mallory-Weiss syndrome. UptoDate. 2017. Último acceso: 15 de junio de 2018. Disponible en: Disponible en: https://www.uptodate.com/contents/mallory-weiss-syndrome [ Links ]
34. Cucci M, Capputo F, Fraternali G, Roncallo A, Ventura F. Transition of a Mallory-Weiss syndrome to a Boerhaave syndrome confirmed by anamnestic, necroscopic, and autopsy data. A case report. Medicine (Baltimore). 2018;97(49):e13191. doi: https://doi.org/10.1097/MD.0000000000013191. [ Links ]
35. Skok P, Sinkovič A. Upper gastrointestinal haemorrhage: predictive factors of in-hospital mortality in patients treated in the medical intensive care unit. J Int Med Res. 2011;39(3):1016-27. doi: https://doi.org/10.1177/147323001103900337. [ Links ]
36. Gralnek I, Dumonceau J, Kuipers E, Lanas A, Sanders D, Kurien M, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47(10):a1-a46. doi: https://doi.org/10.1055/s-0034-1393172. [ Links ]
37. Krag M, Marker S, Perner A, Wetterslev J, Wise M, Schefold J, et al. Pantoprazole in patients at risk for gastrointestinal bleeding in the ICU. N Engl J Med. 2018;379(23):2199-208. [ Links ]
38. Rehman A, Iscimen R, Yilmaz M, Khan H, Belsher J, Gomez JF, et al. Prophylactic endotracheal intubation in critically ill patients undergoing endoscopy for upper GI hemorrhage. Gastrointest Endoscop. 2009;69(7):e55-e9. doi: https://doi.org/10.1016/j.gie.2009.03.002. [ Links ]
Received: January 28, 2019; Accepted: March 27, 2019











 texto em
texto em