Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista colombiana de Gastroenterología
versão impressa ISSN 0120-9957
Rev Col Gastroenterol vol.34 no.4 Bogotá out./dez. 2019
https://doi.org/10.22516/25007440.346
Original articles
Clinical and histopathological characterization of children with autoimmune hepatitis at a referral center in Bogotá, Colombia
1Asistente de Investigación, departamento de Patología y Laboratorios, Fundación Santa Fe de Bogotá. Estudiante, especialización en Epidemiología, Universidad del Rosario-CES, Bogotá, Colombia
2Patólogo institucional, departamento de Patología y Laboratorios, Fundación Santa Fe de Bogotá; líder Grupo de Investigación Enfermedades Complejas, Fundación Santa Fe de Bogotá, Universidad de los Andes, Bogotá, Colombia
3Gastroenterólogo Pediatra, sección de Gastroenterología, Hepatología y Nutrición Pediátrica, Fundación Santa Fe de Bogotá: líder Grupo de Investigación PediAFe, Fundación Santa Fe de Bogotá, Colombia
4Patóloga institucional, departamento de Patología y Laboratorios, Fundación Santa Fe de Bogotá, Colombia. Grupo de Investigación Enfermedades Complejas, Fundación Santa Fe de Bogotá, Universidad de los Andes, Bogotá, Colombia
Autoimmune hepatitis (AIH) is a progressive inflammatory liver disease. It is uncommon in children and adolescents, and is a diagnostic challenge for clinicians and pathologists. We describe the clinical, biochemical and histopathological characteristics of 21 pediatric patients with AIH diagnosed in the last 14 years. Liver biopsies were reassessed to analyze histopathological findings in detail. Of the 21 cases evaluated, 12 (57.1%) were girls and young women, the median age was 14 years old, and 17 (80.9%) had type 1 AIH. The most frequent clinical signs were jaundice (66.7%), choluria (44.4%), evidence of portal hypertension with esophageal varices (47.1%), and splenomegaly (41.2%). Histories of other autoimmune diseases were found in 11.8% of these patients. Elevated levels of aminotransferases were found in 89.5% of the patients, hyperbilirubinemia was found in 88.9%, and 60.0% of the cases had low levels of serum albumin. Reassessed biopsies showed portal lymphoplasmocytic infiltrate (94.4%), interface hepatitis (77.8%) and rosette formation (50.0%). Hyaline inclusions were found in Kupffer cells in 42.9% of the biopsies. About 33.5% of the cases showed cirrhosis at the initial biopsy. Despite immunosuppressive treatment, four patients required liver transplantation and two are on the waiting list. AIH in children can manifest with jaundice, choluria, signs of portal hypertension, elevated aminotransferases, hyperbilirubinemia and circulating antibodies. Hyaline inclusions in Kupffer cells may be a useful finding in the histopathological diagnosis of AIH in children.
Keywords: Autoimmune hepatitis;, child; biopsy; pathology
La hepatitis autoinmune (HAI) es una enfermedad hepática inflamatoria progresiva poco frecuente en niños y adolescentes, la cual es un reto diagnóstico para clínicos y patólogos. Describimos las características clínicas, bioquímicas e histopatológicas de 21 pacientes pediátricos con HAI diagnosticados en los últimos 14 años. Las biopsias hepáticas se reevaluaron para analizar detalladamente los hallazgos histopatológicos. De los 21 casos evaluados, 12 (57,1%) fueron mujeres, la mediana de edad fue 14 años, y 17 (80,9%) tenían HAI tipo 1. Los signos clínicos más frecuentes fueron ictericia (66,7%) y coluria (44,4%); también hubo evidencia de hipertensión portal con várices esofágicas (47,1%) y esplenomegalia (41,2%). El 11,8% de los pacientes tenía antecedentes de otras enfermedades autoinmunes. El 89,5%, 88,9% y 60,0% de los casos tenía elevación de aminotransferasas, hiperbilirrubinemia y bajos niveles de albúmina sérica, respectivamente. Las biopsias reevaluadas mostraron infiltrado linfoplasmocitario portal (94,4%), hepatitis de interfase (77,8%) y formación de rosetas (50,0%). En el 42,9% de las biopsias se hallaron inclusiones hialinas en las células de Kupffer. Cerca del 33,5% de los casos mostró cirrosis en la biopsia inicial. A pesar del tratamiento inmunosupresor, 4 pacientes requirieron trasplante hepático y 2 están en lista de espera. La HAI en niños puede manifestarse con ictericia y coluria, signos de hipertensión portal, aminotransferasas elevadas, hiperbilirrubinemia y anticuerpos circulantes. Las inclusiones hialinas en las células de Kupffer pueden ser un hallazgo útil en el diagnóstico histopatológico de la HAI en niños.
Palabras clave: Hepatitis autoinmune; niño; biopsia; patología
Introduction
Autoimmune hepatitis (AIH) is a progressive inflammatory liver disease characterized by high levels of aminotransferases and immunoglobulin G (IgG), circulating antibodies, and a favorable response to immunosuppressive therapy. 1-3 Although AIH has a wide clinical spectrum, in children and adolescents it often presents acutely and has a more aggressive course than in middle-aged and elderly patients. It can rapidly progress to cirrhosis and liver failure if diagnosis is delayed and treatment is not timely. 4
Two types of AIH have been recognized: anti-smooth muscle antibody (antiSMA) and antinuclear antibodies (antiANA) define AIH type 1 whereas liver kidney microsomal (LKM) type 1 antibodies and antigenic antibodies to liver cytosol (anti-LC1) define AIH type 2. 5-7. Before the 1990s, the epidemiology of AIH in children was imprecise,and there was no standard diagnostic method. Since the the International Autoimmune Hepatitis Group’s scoring system was created, some countries have begun to estimate the incidence and prevalence of this disease. 8 An article by Boberg has noted that incidence of type 1 AIH among adults and children among Caucasian populations in Europe and North America ranges from 0.1 to 1.9 cases per 100,000 people per year while the prevalence of AIH was 8.0 per 100,000 inhabitants of Iceland between 1977 and 1979 and 16.9 per 100,000 inhabitants of Oslo, Norway in 1995. 9 In addition, AIH type 1 is said to represent two thirds of all cases and usually occurs during puberty while AIH type 2 tends to appear during childhood. 10
Histological evidence of inflammatory liver damage consistent with AIH is a prerequisite for its diagnosis. 8,11 The presence of interface hepatitis with portal and periportal lymphoplasmocytic infiltrate, formation of hepatocyte rosettes, and emperipolesis with lymphocytes inside the cytoplasm of injured hepatocytes are the characteristic findings from a liver biopsy. 1-3 Recently, Tucker et al. reported that periodic acid-Schiff (PAS) staining showed the presence of hyaline drops in the cytoplasm of Kupffer cells which is a new histological finding for diagnosis of AIH. 12 Nevertheless, these findings are not pathognomonic and must be considered in the context of the clinical, biochemical and serological findings of each patient. 13
The objective of this study is to present the clinical characteristics and histopathological findings of pediatric patients with AIH diagnosed in our institution during the last 14 years.
Materials and methods
This is a cross-sectional study performed to identify patients under the age of 18 who were diagnosed with AIH at the Fundación Santa Fe de Bogotá from January 2004 to December 2017. Diagnostic criteria for AIH were those established in the regulations of the International Autoimmune Hepatitis Group published in 1993 and revised in 1999. 14,15 Clinical and biochemical variables were collected retrospectively from the clinical history registration system available at our institution. Variables collected included each patient’s age, sex, form of clinical presentation, clinical manifestations, signs of portal hypertension, family history of autoimmune diseases, association with immunological diseases, liver function tests, bilirubin levels, serum albumin, international normalized ratio (INR), IgG, circulating antibodies and any need for liver transplantation. Similarly, liver biopsies available in the pathology department were reevaluated to make detailed histopathological analyses emphasizing the recently described finding of hyaline drops in Kupffer cells. 12
The data was analyzed with STATA® version 12.0 (StataCorp LP, College Station, Texas, USA). Continuous variables were described with measures of central tendency and dispersion according to the distribution of the data, and qualitative variables were described in terms of absolute and relative frequencies. This study was reviewed and endorsed by the Corporate Research Ethics Committee of our institution (reference number CCEI-2813-2015).
Results
In the study period, 280 liver biopsies of pediatric patients were analyzed in the Pathology Department. Of these, 22 had histopathological and clinical findings suggestive of, or conclusive for, AIH. Of these 22 patients, 12 (57.1%) were women and 9 (42.9%) were men with a female: male ratio of 1.3:1.0. The median age was 14 years and the age range was from 2 to 17 years. Seventeen (80.9%) had AIH type 1, one (4.8%) had AIH type 2, and three (14.3%) had no record of antibodies. Clinical data, laboratory results and histopathological findings are summarized in Table 1. The most frequent symptoms were jaundice (66.7%), choluria (44.4%) and abdominal pain (27.8%). The least frequent symptoms were nausea, vomiting, headaches, neurological deterioration, and epistaxis. Symptoms were similar to those of acute hepatitis in 72.2% of cases. Physical examinations found that 41.2% (7/17) had evidence of portal hypertension with splenomegaly and about half had esophageal varices found by endoscopy.
Table 1 Clinical, Biochemical, and Histopathological Findings for Children with Autoimmune Hepatitis
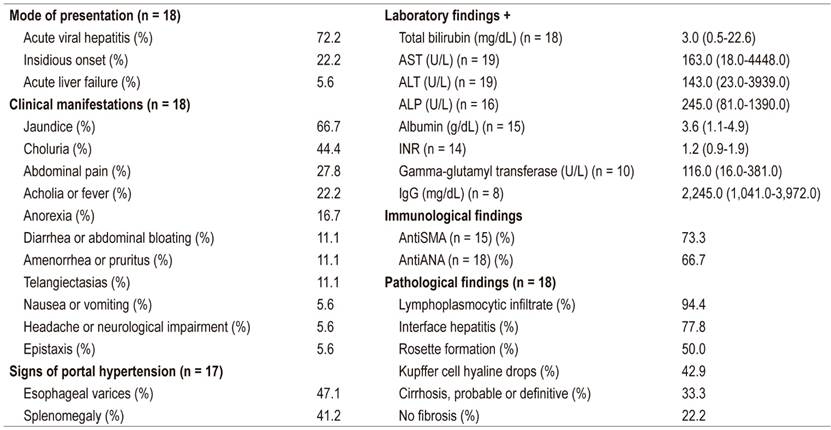
+ Data presented as median (range); ALT: alanine aminotransferase; antiANA: antinuclear antibodies; antiSMA: anti- smooth muscle antibodies; AST: aspartate aminotransferase; ALP: alkaline phosphatase; IgG: immunoglobulin G; INR: international normalized ratio.
The clinical records of two of the seventeen patients (11.8%) included other autoimmune diseases. One had been diagnosed with macrophage activation syndrome before AIH was diagnosed while another case had systemic lupus erythematosus and membranoproliferative glomerulonephritis. Only one patient (5.9%) had a family history of autoimmune disease. High levels of aminotransferases were found 89.5% of the cases, and 88.9% had hyperbilirubinemia. Alkaline phosphatase levels were elevated in 33.3% of the cases, and the INRs were high in 35.7. Sixty percent had hypoalbuminemia while 44.4% had hypergammaglobulinemia.
Liver biopsy samples were taken from 85.7% of the patients at the beginning of the disease (Figure 1). Reevaluation of biopsies confirmed the diagnosis of AIH based on the presence of interface hepatitis (77.8%), lymphoplasmacytic infiltrate in portal spaces (94.4%) and rosette formation in liver cells (50.0%). It should be noted that the liver biopsies of 6 of 18 children (33.3%) showed cirrhosis. As of this writing, despite treatment with corticosteroids and azathioprine, four patients have undergone liver transplantation and two are on the waiting list. The presence of hyaline droplets in the cytoplasm of Kupffer cells was verified in 42.9% of cases.
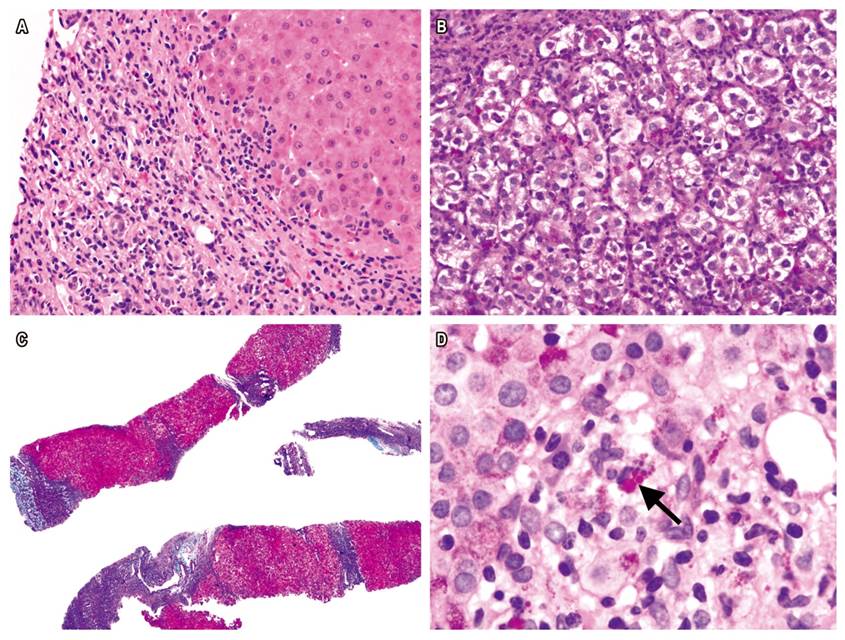
Figure 1 A. H&E stain of liver biopsy from AIH patient shows interface hepatitis and portal lymphoplasmacytic infiltrate (400x). B. Rosette formation (400x). C. Masson’s trichrome shows regenerative nodules surrounded by fibrotic septa (40x). D. PAS-diastase stain shows hyaline drops in Kupffer cells (1000x).
Discussion
AIH is a rare cause of terminal liver disease in children. Failures of immune system regulation, environmental triggers and host genetic susceptibility are the mechanisms of this disease’s pathogenesis. 8 Although AIH was first described in a group of young women in 1950 by Swedish professor Jan Waldenström, it can also affect men and boys who account for 25% to 30% of all AIH patients. It can occur at any age and in all ethnic groups. 2,5,16 Of the 21 pediatric patients with AIH described in this study, 42.9% were male and 71.4% were adolescents (over the age of 12 years). About 12% of these patients had other autoimmune diseases although only one patient had a family history of autoimmune disease. This figure is slightly lower than the figures reported in the literature which show that about 20% of children who test positive for anti-SMA/ANA and anti-LKM1 antibodies have associated autoimmune diseases before or after the diagnosis of AIH, and 40% of patients have first-degree relatives who have autoimmune diseases. 17 The reason for these slight discrepancies may be related to limitations of retrospective data collection related to the fact that our institution is a referral center. Some of the liver biopsies processed in the pathology department are referrals from other institutions and access to these patients’ clinical information may be limited. Nevertheless, every effort was made to obtain clinical information for all cases by consulting the primary source whenever possible and by checking the clinical history records of the pediatric gastroenterology outpatient clinic.
Three patterns of clinical presentations of AIH among children have been described in the literature:
Presentation of about 40% of cases is similar to that of acute hepatitis with nonspecific symptoms, such as fatigue, nausea, emesis, anorexia, and abdominal pain followed by jaundice, choluria, and acholia.
Onset is insidious in 25% to 40% of patients. It is characterized by progressive fatigue, recurrent jaundice, headache, anorexia, amenorrhea, and weight loss which can last for several months or even years before diagnosis.
About 10% of pediatric cases have no histories of jaundice. Diagnosis of AIH is made due to portal hypertension, splenomegaly, hematemesis due to esophageal varices, and/or chronic diarrhea. 18
In our series, 72.2% of patients initial symptoms were similar to acute hepatitis and 22.2% had insidious onsets. Nevertheless, it is striking that 41.2% had splenomegaly and 47.1% esophageal varices, signs of portal hypertension and indications of late diagnoses. 19 Although patients with AIH rarely develop fulminant liver failure, one of our cases was admitted with severe liver damage, and 33.3% had initial liver biopsy findings suggestive of cirrhosis. 5,16 It has been reported that among AIH patients in the United States, Hispanics have the highest prevalence of cirrhosis (55%) followed by white Americans (30%) and Asians (29%). 20 Similarly, South American patients are commonly young children with severe liver inflammation. 21 We assume that genetic predisposition, risk factors, and socioeconomic reasons including limited access to health care services and delayed diagnosis can explain these differences. 2
Only 4.8% of our cases were diagnosed with AIH type 2, and only one of our patients had negative antibodies at the time of initial diagnosis. Still, antibody titers may vary during the course of the disease, and individuals who are seronegative at diagnosis may later express these antibodies. 2 For adults, titers are considered significant when they present a dilution of 1:40 or higher by indirect immunofluorescence. For children, dilutions of 1:20 or more for ANA and SMA or 1:10 or higher for antiLKM1 support a diagnosis of AIH when other clinical and laboratory findings also suggest the disease. 2-4 Antibodies are correlated with disease activity in pediatric AIH patients and can be used to monitor response to treatment. 22 Of our patients 89.5% had elevated levels of aminotransferases, 88.9% hyperbilirubinemia, 60.0% had hypoalbuminemia, and 35.7% had coagulopathy. These last two signs are associated with cirrhosis and late diagnosis. It must be taken into account that up to 25% of patients, especially children, the elderly and acute cases, have IgG levels within normal limits. 11
Liver biopsy results are essential for confirmation of an AIH diagnosis and for assessing severity of liver damage because of the great variability of clinical manifestations and because serum antibodies and elevated IgG levels are very nonspecific. 5,8 Interface hepatitis is a typical finding, but it is not exclusive to AIH. It is characterized by portal and periportal lymphocytic or lymphoplasmacytic infiltration with edema of hepatocytes. In cases of acute AIH and during relapses, panlobular hepatitis with bridging necrosis can occur while in fulminant cases massive necrosis often occurs. Other findings include hepatic regeneration with rosette formation and emperipolesis. 2,5,8 In 2008, the International Autoimmune Hepatitis Group developed a system of simplified criteria for AIH diagnosis. Interface hepatitis with lymphoplasmacytic infiltrate, rosettes, and emperipolesis are necessary to categorize a case as typical, and all three findings must be present for a 2-point histological score. 23,24
Several studies indicate that the simplified criteria have excellent sensitivity and specificity for pediatric patients except those cases with fulminant liver failure. 25 A novel histological finding recently described by Tucker et al. is the presence of hyaline drops in the cytoplasm of Kupffer cells. This provides information that distinguishes AIH from other forms of chronic hepatitis. 12 In our cases, the most frequent histopathological findings were portal lymphoplasmacytic infiltrate and interface hepatitis. Hyaline drops were present in 42.9% of the liver biopsies.
Certain histological changes and their severity serve as markers for disease phenotypes. 1 A study by Miao et al. has shown that emperipolesis is associated with the most severe necroinflammatory and fibrotic changes in AIH. 26 It should be noted that up to 24% of patients have bile duct alterations including pleomorphic and destructive lymphocytic cholangitis. These cases should be investigated for possible association with primary sclerosing cholangitis or primary biliary cholangitis/cirrhosis (AIH overlap syndromes). 1 The initial biopsy provides information on inflammatory activity and the stage of fibrosis which helps guide treatment decisions. Similarly, follow-up biopsies may be necessary to assess response to treatment since residual inflammatory activity predicts relapses after interruption of immunosuppression. 3 Finally, biopsy allows differentiation between AIH and other autoimmune liver diseases such as primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune cholangitis. 6
In conclusion, AIH should be considered in any pediatric or adolescent patient with acute or chronic liver compromise, especially if they have elevated levels of aminotransferases and/or hyperbilirubinemia which are suggestive of portal hypertension and other autoimmune diseases associated with the presence of serum antibodies and hypergammaglobulinemia. The presence of signs of portal hypertension, cirrhosis, hypoalbuminemia, or coagulopathy indicate a late clinical diagnosis. A liver biopsy is important for both initial diagnosis and for long-term follow-up of AIH. The presence of hyaline drops in the cytoplasm of Kupffer cells is a useful histological finding for diagnosis of AIH in children.
As previously mentioned, the relatively small number of pediatric and adolescent patients with AIH and retrospective data collection are limitations of this study, but our results are comparable to those described in elsewhere in the literature.
Referencias
1. Wang Q, Yang F, Miao Q, Krawitt E, Gershwin M, Ma X. The clinical phenotypes of autoimmune hepatitis: a comprehensive review. J Autoimmun. 2016;66:98-107. doi: https://doi.org/10.1016/j.jaut.2015.10.006. [ Links ]
2. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: autoimmune hepatitis. J Hepatol. 2015;63:971-1004. doi: https://doi.org/10.1016/j.jhep.2015.06.030. [ Links ]
3. Manns M, Lohse A, Vergani D. Autoimmune hepatitis-update 2015. J Hepatol. 2015;62:S100-11. doi: https://doi.org/10.1016/j.jhep.2015.03.005. [ Links ]
4. Mieli-Vergani G, Vergani D. Autoimmune liver diseases in children-what is different from adulthood? Best Pract Res Clin Gastroenterol. 2011;25:783-95. doi: https://doi.org/10.1016/j.bpg.2011.10.007. [ Links ]
5. Liberal R, Grant C, Mieli-Vergani G, Vergani D. Autoimmune hepatitis: a comprehensive review. J Autoimmun. 2013;41:126-39. doi: https://doi.org/10.1016/j.jaut.2012.11.002. [ Links ]
6. Heneghan M, Yeoman A, Verma S, Smith A, Longhi MS. Autoimmune hepatitis. Lancet. 2013;382:1433-44. doi: https://doi.org/10.1016/S0140-6736(12)62163-1. [ Links ]
7. Carbone M, Neuberger J. Autoimmune liver disease, autoimmunity and liver transplantation. J Hepatol. 2014;60:210-23. doi: https://doi.org/10.1016/j.jhep.2013.09.020. [ Links ]
8. Pathak S, Kamat D. Autoimmune hepatitis in children. Pediatr Ann. 2018;47:e81-6. doi: https://doi.org/10.3928/19382359-20180126-01. [ Links ]
9. Boberg K. Prevalence and epidemiology of autoimmune hepatitis. Clin Liver Dis. 2002;6:635-47. doi: https://doi.org/10.1016/S1089-3261(02)00021-1. [ Links ]
10. Vitfell-Pedersen J, Jørgensen M, Müller K, Heilmann C. Autoimmune hepatitis in children in Eastern Denmark. J Pediatr Gastroenterol Nutr. 2012;55:376-9. doi: https://doi.org/10.1097/MPG.0b013e3182602b20. [ Links ]
11. Gatselis N, Zachou K, Koukoulis G, Dalekos G. Autoimmune hepatitis, one disease with many faces: etiopathogenetic, clinico-laboratory and histological characteristics. World J Gastroenterol. 2015;21:60-83. doi: https://doi.org/10.3748/wjg.v21.i1.60. [ Links ]
12. Tucker S, Jonas M, Perez-Atayde A. Hyaline droplets in Kupffer cells: a novel diagnostic clue for autoimmune hepatitis. Am J Surg Pathol. 2015;39:772-8. doi: https://doi.org/10.1097/PAS.0000000000000395. [ Links ]
13. Sahebjam F, Vierling J. Autoimmune hepatitis. Front Med. 2015;9:187-219. doi: https://doi.org/10.1007/s11684-015-0386-y. [ Links ]
14. Johnson P, McFarlane I. Meeting report: International Autoimmune Hepatitis Group. Hepatology. 1993;18:998-1005. doi: https://doi.org/10.1002/hep.1840180435. [ Links ]
15. Álvarez F, Berg P, Bianchi F, Bianchi L, Burroughs A, Cancado E, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol . 1999;31:929-38. doi: https://doi.org/10.1016/S0168-8278(99)80297-9. [ Links ]
16. Liberal R, Grant C, Longhi M, Mieli-Vergani G, Vergani D. Diagnostic criteria of autoimmune hepatitis. Autoimmun Rev. 2014;13:435-40. doi: https://doi.org/10.1016/j.autrev.2013.11.009. [ Links ]
17. Gregorio G, Portmann B, Reid F, Donaldson P, Doherty D, McCartney M, et al. Autoimmune hepatitis in childhood: a 20-year experience. Hepatology. 1997;25:541-7. doi: https://doi.org/10.1002/hep.510250308. [ Links ]
18. Floreani A, Liberal R, Vergani D, Mieli-Vergani G. Autoimmune hepatitis: contrasts and comparisons in children and adults-a comprehensive review. J Autoimmun . 2013;46:7-16. doi: https://doi.org/10.1016/j.jaut.2013.08.004. [ Links ]
19. Krawitt E. Autoimmune hepatitis. N Engl J Med. 2006;354:54-66. doi: https://doi.org/10.1056/NEJMra050408. [ Links ]
20. Wong R, Gish R, Frederick T, Bzowej N, Frenette C. The impact of race/ethnicity on the clinical epidemiology of autoimmune hepatitis. J Clin Gastroenterol. 2012;46:155-61. doi: https://doi.org/10.1097/MCG.0b013e318228b781. [ Links ]
21. Czaja A. Autoimmune hepatitis in special patient populations. Best Pract Res Clin Gastroenterol. 2011;25:689-700. doi: https://doi.org/10.1016/j.bpg.2011.09.011. [ Links ]
22. Gregorio G, McFarlane B, Bracken P, Vergani D, Mieli-Vergani G. Organ and non-organ specific autoantibody titres and IgG levels as markers of disease activity: a longitudinal study in childhood autoimmune liver disease. Autoimmunity. 2002;35:515-9. doi: https://doi.org/10.1080/0891693021000056721. [ Links ]
23. de Boer Y, van Nieuwkerk C, Witte B, Mulder C, Bouma G, Bloemena E. Assessment of the histopathological key features in autoimmune hepatitis. Histopathology. 2015;66:351-62. doi: https://doi.org/10.1111/his.12558. [ Links ]
24. Balitzer D, Shafizadeh N, Peters M, Ferrell L, Alshak N, Kakar S. Autoimmune hepatitis: review of histologic features included in the simplified criteria proposed by the international autoimmune hepatitis group and proposal for new histologic criteria. Mod Pathol. 2017;30:773-83. doi: https://doi.org/10.1038/modpathol.2016.267. [ Links ]
25. Mileti E, Rosenthal P, Peters M. Validation and modification of simplified diagnostic criteria for autoimmune hepatitis in children. Clin Gastroenterol Hepatol. 2012;10:417-21. doi: https://doi.org/10.1016/j.cgh.2011.11.030. [ Links ]
26. Miao Q, Bian Z, Tang R, Zhang H, Wang Q, Huang S, et al. Emperipolesis mediated by CD8 T cells is a characteristic histopathologic feature of autoimmune hepatitis. Clin Rev Allergy Immunol. 2015;48:226-35. doi: https://doi.org/10.1007/s12016-014-8432-0. [ Links ]
Received: January 29, 2018; Accepted: April 30, 2019











 texto em
texto em 


