Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.34 no.4 Bogotá oct./dic. 2019
https://doi.org/10.22516/25007440.367
Review articles
Liver diseases and pregnancy
1Hepatólogo clínico y de trasplantes. Director Unidad Funcional de Trasplantes y Enfermedades Digestivas de los Hospitales de San Vicente Fundación. Magister en Economía de la Salud. Medellín-Rionegro, Antioquia, Colombia
2Hepatóloga clínica y de trasplantes, Hospitales de San Vicente Fundación, Medellín-Rionegro, Antioquia, Colombia
3Médico general, estudiante de maestría en Epidemiología, Hospital San Vicente Fundación, Rionegro, Antioquia, Colombia
4Especialista en Ginecología y Obstetricia, Jefe departamento de Obstetricia y Ginecología, Universidad de Antioquia. Medellín, Antioquia, Colombia
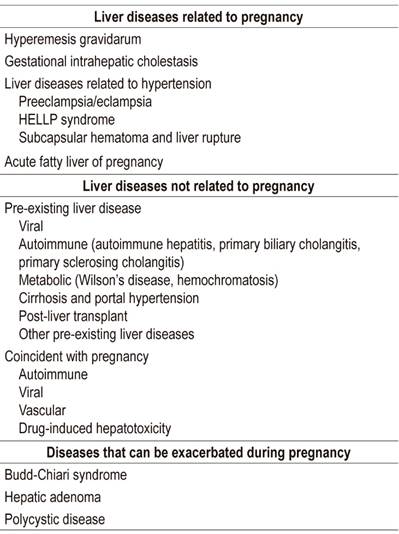
Liver diseases develop in 3% to 5% of all gestations. Among the causes are: 1. Physiological changes of pregnancy. 2. Pre-existing liver diseases and conditions. The most common are cholestatic diseases such as primary biliary cholangitis and primary sclerosing cholangitis. Others include autoimmune hepatitis, Wilson’s disease, chronic viral hepatitis, cirrhosis of any etiology and histories of liver transplantation. 3. Liver disease acquired during pregnancy, especially viral hepatitis, drug-induced toxicity and hepatolithiasis. 4. Pregnancy-related liver diseases including hyperemesis gravidarum, intrahepatic cholestasis of pregnancy, preeclampsia, HELLP syndrome and fatty liver of pregnancy.
Severity ranges from absence of symptoms to acute liver failure and even death. Severe cases have significant morbidity and mortality for both mother and fetus. These cases require rapid evaluation, accurate diagnosis and appropriate management by a multidisciplinary team including high-risk obstetrics, hepatology, gastroenterology and interventional radiology. Availability of liver transplantation is also important for obtaining good outcomes.
Keywords: Pregnancy; hyperemesis gravidarum; gestational cholestasis; HELLP; preeclampsia; hepatitis B virus; cirrhosis; liver transplantation
La prevalencia de las enfermedades hepáticas en el embarazo no es despreciable, ya que estas se presentan en 3%-5% de todas las gestaciones. Entre las múltiples causas se encuentran cambios fisiológicos del embarazo; enfermedad hepática preexistente, siendo las más comunes las enfermedades colestásicas (colangitis biliar primaria y colangitis esclerosante primaria), hepatitis autoinmune, enfermedad de Wilson, hepatitis virales crónicas, cirrosis establecida de cualquier etiología y paciente con historia de trasplante hepático; enfermedad hepática adquirida durante el embarazo, siendo las principales las hepatitis virales, la toxicidad inducida por medicamentos y la hepatolitiasis; hepatopatía relacionada con el embarazo, en la cual se encuentran 5 entidades principales: hiperémesis gravídica, colestasis intrahepática del embarazo, preeclampsia, síndrome HELLP e hígado graso del embarazo.
La severidad de estas entidades tiene una amplia gama de presentaciones, desde la paciente que es completamente asintomática, hasta la falla hepática aguda e incluso la muerte. La gravedad del cuadro se asocia con una morbilidad y mortalidad significativas tanto para la madre como para el feto, lo cual hace que una evaluación rápida, diagnóstico certero y manejo apropiado por un equipo multidisciplinario (incluida obstetricia de alto riesgo, hepatología, gastroenterología y radiología intervencionista), en un servicio que tenga la posibilidad de ofrecer trasplante hepático, sean fundamentales para obtener buenos desenlaces.
Palabras clave: Embarazo; hiperémesis gravídica; colestasis gestacional; HELLP; preeclampsia; virus de la hepatitis B; cirrosis; trasplante hepático
Introduction
All women, regardless of age, are at risk of suffering some type of acute or chronic liver disease. Optimal management is of vital importance when these pathologies affect women in pregnancy both because of adverse effects themselves and also because of maternal and fetal outcomes. Acute or chronic liver diseases in women, pregnant and not, require changes in gynecological care, contraception, planning of pregnancy, exploration of cervical cancer, papilloma vaccine and postmenopausal hormone replacement therapy. .
Women with liver transplants require gynecological care adapted to their immunosuppressed state for both the well-being of the mother and the viability of the fetus.
Normal physiological changes during pregnancy
Many physiological and hormonal changes normally occur in pregnant women’s bodies during pregnancy. Some may resemble those that occur in patients with liver disease. Maternal heart rate, cardiac output, and circulatory volume all increase while peripheral vascular resistance decreases. These changes can also occur patients with decompensated chronic liver disease. Physical examination may show palmar erythema and arachnid nevi in up to 70% of pregnant women. Liver blood flow remains constant during pregnancy, and the liver is not palpable because it is displaced a little upwards in the thoracic cavity due to the growth of the uterus. Gallbladder motility decreases, resulting in increased risk of gallstone development. 1
During pregnancy, biochemical and hematological indices should be interpreted in light of the normal ranges for a pregnant woman. Alkaline phosphatase increases in the third trimester, as it is produced by the placenta and by development of fetal bone. Alpha-fetoprotein increases in pregnancy and is produced by the fetal liver. Urea levels, hemoglobin levels, and prothrombin times remain unchanged or are slightly reduced due to hemodilution. Elevated transaminases, bilirubin, and prothrombin time are abnormal and indicate disease states which require appropriate studies.
Pregnancy is a procoagulant state in which coagulation factors I, II, V, VII, X, and XII as well as fibrinogen are increased. Small esophageal varices can be seen in up to 50% of pregnant women in the second and third trimesters. They are due to compression of the inferior vena cava by the uterus and consequent reduction of venous return. A liver biopsy is rarely indicated, but if one is performed during pregnancy, the risks are very similar to those for women who are not pregnant. 2,3 Liver dysfunction during pregnancy may be due to liver disease associated with pregnancy, exacerbation of pre-existing liver disease, or conditions unrelated to pregnancy (Table 1).
Hyperemesis gravidarum
Hyperemesis gravidarum (HG), persistent vomiting unrelated to other causes, occurs in 0.3% to 3% of pregnancies. 5,6 It can lead to dehydration, ketosis, and loss of more than 5% of preconception weight. 5 It usually begins very early in gestation and resolves at week 20. 2,7 Diagnosis is made by exclusion, and its exact cause is not clear. Numerous theories based on genetic, psychiatric, psychological, cultural and hormonal factors that have been put forward. Others are based on gastric motility alterations, changes in the autonomic nervous system, and Helicobacter pylori infections. 5,6,8,9
The first trimester peak of human chorionic gonadotropin (HCG) is correlated with the severity of hyperemesis gravidarum, and hyperthyroidism occurs in 60% of patients with HG. 1,2,8,9 HCG activates thyroid stimulating hormone (TSH) receptors leading to thyroid suppression and increased thyroxine levels. 2,5
Clearly identified risk factors include molar and twin pregnancies, trophoblastic disease, previous history of HG, previous history of fetal abnormalities, increased body mass index, psychiatric diseases, and diabetes. 2,7,8 Alterations of liver biochemistry occur in 50% to 60% of hospitalized patients with HG and are characterized by slight elevations of transaminases. Jaundice and synthetic dysfunction are rare. 2,7 In addition, patients may present renal dysfunction and electrolyte disorders such as hyponatremia, hypokalemia, and hypochloremic alkalosis. 2,6 In severe cases, dehydration can lead to orthostatic hypotension, tachycardia, and lethargy. Vomiting can lead to bleeding from esophageal lacerations and vitamin deficiency which has produced neurological disorders in very rare cases. 5,6 The relationship of HG with fetal morbidity has not been clearly demonstrated in the literature, but some studies have reported higher rates of low birth weight and preterm deliveries. 6,9
Management of HG is based on support measures until symptoms resolve with progression of gestational age. 2,6,10 Intravenous rehydration, correction of electrolyte disorders, and thiamine replacement are indicated for prevention of Wernicke’s encephalopathy, especially in patients who receive dextrose solutions. 6,9 Vitamin B6 for control of nausea and vomiting is considered the first-line therapy while antiemetics are considered second line. 2,5 Systemic steroids and ondansetron can be considered for refractory patients, but their safety profiles must be taken into account. 2,5 Patients who cannot maintain weight and do not respond to antiemetics require advanced enteral nutrition and may even require total parenteral nutrition. 5,6,9
Biochemical abnormalities usually improve with resolution of vomiting and do not leave permanent liver sequelae. If liver tests are not normalized with resolution of vomiting, other causes should be suspected. 8
Intrahepatic cholestasis of pregnancy
Intrahepatic cholestasis of pregnancy (ICP) affects 1 in 140 pregnancies in the UK and is more common among women with family or personal histories of ICP, itching related to oral contraceptives, gallstones, or multiple pregnancies. Some studies suggest a high prevalence in patients with hepatitis C virus infections and non-alcoholic fatty liver disease. 4,11,12 It usually occurs in the 3rd trimester of pregnancy, but cases have been reported as early as the eighth week of pregnancy. ICP is characterized by itching, abnormal liver function tests, and increased serum bile acid levels. The symptoms are choluria, acholia, anorexia, fatigue, epigastric pain and steatorrhea due to fat malabsorption. Transaminases are usually elevated. 4,8,11,12
Itching is predominantly nocturnal and usually affects palms and soles. It can become severe but characteristically improves within 48 hours of delivery. Elevation of bilirubin is rare, but when it occurs, it increases 2 to 4 weeks after the onset of itching and can occur before or after bile acid levels increase. ALT has been found to be a more sensitive marker of ICP than AST because ALT levels increase between two and ten times more than do those of AST. Clinical jaundice occurs in 10% to 15% of pregnant women with ICP and is generally mild, with bilirubin levels not exceeding 100 μmol/L or 5.85 mg/dL. Hyperbilirubinemia usually occurs at the expense of direct bilirubin. Vitamin K malabsorption increases the risk of postpartum hemorrhaging. 4
ICP is benign for the mother, but when bile acid levels are higher than 40 mmol/L the risk of adverse outcomes including preterm deliveries, fetal distress, meconium-stained amniotic fluid, long-term neonatal ICU care and stillbirths increases. 12,13
ICP’s pathogenesis is unknown but is related to abnormal bile transport through the canalicular membrane. Multidrug resistance protein 3 (MDR3) is the major phospholipid transporter, and mutation of the gene which expresses it leads to a loss in function and increased levels of serum bile acids. The MDR3 mutation is located on chromosome 7q21.1 and has been identified in 15% of the cases of ICP. Abnormal placental transport of bile acids from fetal to maternal circulation increases maternal bile acids while the immature fetal transport system can contribute to increased bile acid levels in the fetus itself. 4
When a diagnosis of ICP is ruled out, other causes of cholestasis should be sought. 13 Serum bile acids are the most sensitive and specific test for diagnosis and monitoring of this condition. Usually, a liver biopsy is not necessary for diagnosis, but if one is performed, findings predominate in zone 3. They include centrilobular cholestasis without inflammation and bile plugs in hepatocytes and canaliculi. 4
ICP is treated with 10-15 mg/kg of ursodeoxycholic acid (UDCA). This is the only drug that has shown to benefit maternal symptoms and liver biochemistry and to have possible beneficial effects on perinatal outcomes. Some studies have shown that it increases the expression of placental bile acid transporters which improves transfer. It is anti-apoptotic and increases the excretion of pruritogens such as progesterone sulfate. Studies suggest that its use has a positive impact on premature deliveries, reduces admission of newborns to the ICU, reduces placental damage and reduces fetal arrhythmias. 2,4,12,14 Other medications, including cholestyramine, S-adenosine L-methionine, and dexamethasone are less effective than UDCA at reducing itching and improving liver function. Cholestyramine has even been found to exacerbate vitamin K deficiency. 8,12,14
Rifampicin may be useful as an adjunct treatment in women with severe or refractory disease, and it improves symptoms and biochemistry in a third of the patients who did not respond to UDCA. Nevertheless, it should only be used if transaminases are not too high. 2,12 An association between stillbirths in ICP and the 38th week of pregnancy has been found, so many centers have chosen to end these pregnancies before that moment.
Symptoms and liver profile changes usually disappear after delivery, but sometimes they continue. Bile acid and liver enzyme levels should be tested 6 to 8 weeks after delivery. If enzyme levels have not improved, other causes of cholestasis should be sought. There is a high risk of recurrence of ICP in subsequent pregnancies, and some women even experience cholestasis with menstrual cycles or with the use of oral contraceptives and should avoid estrogen-containing medications. 12,14 There is evidence that those women who developed ICP have high risks of developing hepatobiliary diseases or metabolic syndrome at some point in their lifetimes. 4,12
Hellp syndrome
Preeclampsia is the most common cause of liver function abnormalities during pregnancy. It is characterized by hypertension and proteinuria after 20 weeks of pregnancy. Pain in the right upper quadrant of the abdomen is suggestive of hepatic involvement and requires close monitoring. 13
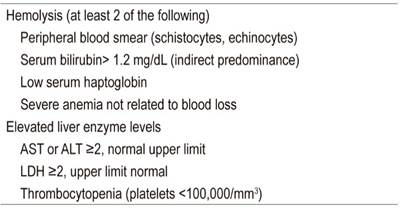
HELLP syndrome is characterized by microangiopathic hemolytic anemia (MAHA), elevated liver enzymes, and decreased platelet levels. This syndrome occurs in 0.2% to 0.8% of pregnancies and 70% to 80% of these cases coexist with pre-eclampsia. Most cases occur in the third trimester of pregnancy. Perinatal mortality secondary to premature births and maternal complications occurs in 6% to 70% of cases. Risk factors include advanced age of the mother, nulliparity and multiparity. 4,10
HELLP is believed to be due to impaired platelet activation, increased proinflammatory cytokines, and segmental vasospasms with vascular endothelial damage. 4 Most patients gain weight and develop abdominal pain in the right upper quadrant, nausea, vomiting, malaise, headaches, visual changes, and peripheral edema. Hypertension and proteinuria occur in 80% of cases. Jaundice only occurs in 5% of patients. Symptoms associated with thrombocytopenia such as bleeding from the mucosa, hematuria, petechiae, and bruising are also unusual. Less common are renal failure with increased uric acid, diabetes insipidus, and antiphospholipid syndrome (APS). 4,11,15
Due to hemolysis, patients have elevated indirect bilirubin levels and increased lactic dehydrogenase (LDH) levels. ALT and AST levels are moderately high. In initial stages, the prothrombin time (PT) and the partial thromboplastin time (PTT) are normal, but in later stages, disseminated intravascular coagulation (DIC) with high levels of fibrin degradation products, D-dimer and thrombin-antithrombin complex can occur. 4 Diagnostic criteria for HELLP syndrome have been standardized by the Task Force of the American College of Obstetrics and Gynecology on hypertension in pregnancy and are shown in Table 2. 15
It is estimated that 1 in every 1,000 pregnant women develops HELLP. Of these, 2% to 3% develop liver complications including liver failure requiring liver transplantation. A ruptured liver is rare but is a life threatening complication. It is usually preceded by hepatic hemorrhaging that progresses from the parenchyma to become a subscapular hematoma contained in the right liver lobe. Uric acid levels of over 464 μmol/L (7.8 mg/dL) are associated with increased maternal and fetal morbidity and mortality. 4,8
Once HELLP develops, the only treatment is delivery of the baby. If the gestational age is between 24 and 34 weeks, steroids are required for lung maturation. Delivery take place 24 hours after application of steroids. Continuous maternal follow-up after delivery is required because worsening thrombocytopenia and increased LDH may occur up to 48 hours postpartum. If gestational age is over 34 weeks, of if there is evidence of fetal distress or maternal complications such as severe organ compromise, disseminated intravascular coagulation, kidney failure, pulmonary edema, intrahepatic bleeding, stroke, or placental abruption, the pregnancy should be terminated as rapidly as possible. 4,8,15 Indications for transplant include persistent bleeding from bruising, ruptured liver, or liver failure. It has been found that 88% of these patients survive for 5 years after transplantation. 8
Hematoma, infarction and ruptured liver
Hemorrhaging and a ruptured liver can complicate preeclampsia, eclampsia, or HELLP syndrome and have been associated with a 50% mortality rate. Patients present abdominal pain, pyrexia and severe hypovolemic shock with cardiovascular collapse. There is a marked increase in transaminases and anemia. Computed tomography and magnetic resonance imaging are the diagnostic methods of choice. 2
Hematomas that are contained can be managed conservatively with aggressive coagulation support, prophylactic antibiotics, and transfusions. Any evidence of hemodynamic instability should be treated with angiography with embolization of the hepatic artery, liver packing, ligation of the hepatic artery and resection.
Necrotic infarcts can occur as complications of preeclampsia. Often, patients have unexplained increases in transaminases, fever, anemia, and leukocytosis associated with signs of liver failure. In most cases, the liver recovers, but when areas of extensive infarction occur, multiple organ failure and death due to a ruptured liver may ensue. 2 Rupture of the hepatic capsule generates intraperitoneal bleeding. Mortality is highest after a liver ruptures. Treatment is rehydration and either surgery or angiographic embolization, as appropriate. 2,4,11
Acute fatty liver of pregnancy
Acute fatty liver of pregnancy (AFLP) is a rare occurring pathology with a reported incidence of 1 in 7,000 to 15,000 pregnancies. A prospective population study conducted in the UK with a cohort of 1.1 million pregnancies has estimated an incidence of 1:20,000 births. 16,17 AFLP can be life-threatening and is considered an obstetric emergency. It can lead to acute liver failure and, if diagnosis is delayed, to death of the fetus and the mother. Although maternal mortality rates have improved from 92% before 1970 to less than 10% reported in 2008, they are still very high. 2
Typically, this pathology occurs in the third trimester between week 30 and 38, but sometimes it is not recognized until after delivery. However, there are reports of cases as early as the 26th week of gestation. 18,19 AFLP is an infiltrative disease with evidence of microvesicular steatosis upon biopsy. It is a mitochondrial liver disease which is associated with a homozygous fetal mutation (1528G> C) in the gene coding for long-chain 3-hydroxyacyl-CoA dehydrogenase which produces severe decrease or total loss of enzymatic activity. This mutation results in an accumulation of long-chain fatty acids in the placenta which pass into maternal circulation and lead to development of acute liver damage. 20
AFPL risk factors include histories of previous episodes, multiple gestation, male fetal sex, coexistence of other liver diseases during gestation (HELLP, pre-eclampsia) and a body mass index under 20 kg/m2. 16,17 Initial symptoms of AFPL are generally nonspecific with 1-2 weeks of nausea, vomiting, abdominal pain, malaise, and anorexia. Signs and symptoms of acute liver failure such as encephalopathy, jaundice, and coagulopathy may appear with rapid development of moderate to severe hypoglycemia. Approximately 50% of these patients present preeclampsia, although hypertension is generally not severe. 21
The definitive diagnostic method for AFLP is a liver biopsy. In patients with coagulopathy, it should be performed transjugularly given the high risk of bleeding. In many cases a liver biopsy is not necessary to make the diagnosis. The characteristic histological finding is microvascular fat infiltration, which affects the pericentral zone and respects the periportal zone. When a biopsy is performed at an early stage of the disease, the hepatocytes appear ballooned in the absence of large amounts of fat and giant mitochondria. At later stages, hepatocyte destruction can cause loss of the liver parenchyma and cellular atrophy. 18 There is no adequate correlation between the degree of alterations found in laboratory tests and the severity of histological compromise. 22
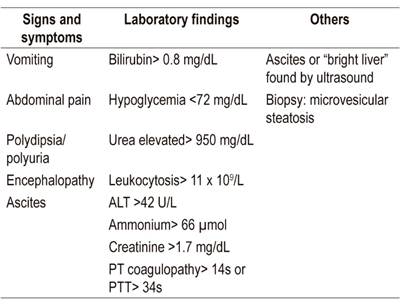
Ultrasound and CT scan findings are inconsistent for detection of fatty infiltration, so diagnosis is usually based on clinical and laboratory findings. The Swansea criteria (Table 3) for diagnosis of AFLP are a proposal that includes symptoms, laboratory findings, and imaging. This model was developed by Ch’ng et al. at the Swansea Midwifery Unit in South West Wales, UK in 2002. It was validated in a cohort study in 2008. When at least six of 15 criteria are present, AFLP is likely. 17,23 These criteria have demonstrated a sensitivity of 100% (95% CI: 77% to 100%), a specificity of 57% (95% CI: 20% to 88%), a positive predictive value of 85%, and a negative predictive value of 100% for diffuse and perivenular microvesicular steatosis. 24
Early diagnosis of AFLP is essential. Initial management is basically supportive to stabilize the mother while avoiding the use of hepatotoxic drugs. In cases with suspected infections, broad-spectrum antibiotics should be administered. Regardless of the severity of AFPL, termination of pregnancy as quickly as possible is the basic treatment. If there is no obstetric contraindication, delivery may be vaginal, although a small study published in 2010 showed a lower maternal mortality rate in the caesarean group than in the vaginal delivery group (16.2% vs. 48.1%). 25,26
AFPL is usually reversible with complete liver function recovery and no sequelae. In case of persistent deterioration and development of liver failure, liver transplantation should be considered. 14,16,25 A retrospective study of 54 patients with liver disease associated with pregnancy found 18 patients with diagnoses of AFPL who had been referred to a liver transplant center. The study documented that lactate > 2.8 mg/dL had a sensitivity of 73% and a specificity of 75% as a predictor of death or need for liver transplantation. When encephalopathy was added, the sensitivity and specificity increased to 90% and 86%, respectively. 27
Autoimmune hepatitis
Autoimmune hepatitis (AIH) is a chronic inflammatory process of the liver of unknown cause that is characterized by hypergammaglobulinemia, circulating autoantibodies, interface hepatitis found by biopsy and favorable response to immunosuppression. 28-30 The clinical spectrum is wide and includes asymptomatic patients; patients with nonspecific symptoms such as fatigue, nausea, abdominal pain and arthralgia; patients with severe acute hepatitis; and even patients with established cirrhosis. 30,31 Some patients also have other autoimmune pathologies. 28,30
The 1993 diagnostic criteria for autoimmune hepatitis of the International Group on Autoimmune Hepatitis (IAIHG), a group of experts, were revised in 1999, 30,31 but due to their complexity Hennes et al. developed a simplified and much more practical scoring system in 2008. It is based on variables that are independent predictors of autoimmune hepatitis and has sensitivity and specificity over 80 % (Table 4). 29
Autoimmune hepatitis manifests itself in the same manner in pregnant women as in others and is therefore diagnosed the same way. 8 Autoimmune hepatitis usually occurs in women of childbearing age. Successful pregnancies are possible in this group of patients, but increased morbidity and mortality rates have been reported for both the fetus and the mother. They have been associated with poor control of inflammatory activity from one year prior to conception through pregnancy. 2,8,32-38 These complications are more frequent and can be fatal in patients with decompensated cirrhosis of the liver. 38,39
Table 4 Simplified autoimmune hepatitis scoring system (29)
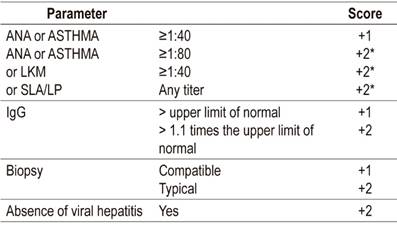
* The maximum score for autoantibodies is +2.
> 6 points: probable.
> 7 points: definitive.
AIH is most frequently activated in the first three months following delivery. Its incidence ranges from 11% to 81%. It probably occurs due to immune reconstitution after delivery and decreasing blood estrogens. 2,8,30,31,33,35,37,38 Improvement including spontaneous remission of inflammatory activity has been described during pregnancy. This is probably related to the predominantly immunotolerant state during pregnancy. 30,32,38,39 Despite this, the disease can be activated at any point during pregnancy and has an incidence of 7% to 21%. 7,20,30,33 This activation can usually be controlled with increased immunosuppression, but for some patients, activation of the disease can lead to liver decompensation, the need for liver transplantation, and even death of the patient and fetus. 2,33,38
Immunosuppression with steroids and drugs such as azathioprine is the mainstay of treatment for AIH. 30,36,40 However, in pregnancy, optimal management has not been well established. What is clear is that these patients require stable immunosuppression throughout the pregnancy, with greater vigilance in the postpartum period. 30,32,33,38,40,41
Due to the teratogenic effects reported in animals, azathioprine is considered a category D drug in pregnant women. However, multiple retrospective studies have shown that it is safe during pregnancy and lactation. Consequently, the teratogenic potential of azathioprine is outweighed by the beneficial effects of disease control since remission decreases the risk of maternal and fetal complications. (2,8 30-33,35,37-40) For patients who have been undergoing treatment of AIH with azathioprine before pregnancy, administration of the drug should be continued at the dosage necessary to control the disease during pregnancy. 2,38 If biochemical hepatitis occurs, it should be treated conventionally with steroids. 2,8,30
Mycophenolate mofetil has been associated with increased teratogenicity and should be discontinued before pregnancy and should not be used during pregnancy. 30,39,40 Calcineurin inhibitors can be safely used during pregnancy according to data obtained from transplanted pregnant women. 30,40 To decrease risks of complications, these patients should plan their pregnancies and have inflammatory activity under control for at least one year prior to becoming pregnant. They should receive treatment throughout pregnancy and deliver their babies in highly complex medical centers with close obstetric and hepatological monitoring during pregnancy and the postpartum period. 2,30,32,33,38-40 The delivery route should be defined by the obstetrician. 33
Hepatitis b infections
It has been calculated that up to a third of the world’s population may have serological evidence of past or present hepatitis B infections. Between 350 and 400 million people are chronic carriers of the surface antigen (HBsAg) and 240 million have chronic hepatitis. 42-44 Every year more than 50 million new cases are reported. Most of them are transmitted vertically. 42,45 Data from the United States indicate a prevalence of 0.7 to 0.9 of HBV in pregnant women which translates into 25,000 newborns at risk of infection annually. 46
The risk of an HBV infection becoming chronic (HBsAg persists beyond 6 months) is related to age at infection. It is 5% in adults, 50% in small children, and up to 90% in infants. 43 The highest vertical transmission rates (70% -90%) have been associated with maternal HBsAg, e antigen (HBeAg), and absence of post-exposure prophylaxis in infants. 47
The behavior of HBV infections in pregnancy has no important differences with HBV in the general population. Ninety-five percent of acute infections in pregnant women spontaneously resolve, and the risk of liver failure is about 1%. 48 If an infection occurs early in pregnancy, it can cause a miscarriage, but it usually resolves without consequences for the mother or fetus. In this scenario, the possibility of vertical transmission is only 10%, but when an infection is acquired in the final stage of pregnancy, vertical transmission is much more likely and can occur in up to 60% of cases. 49 There are several factors that increase the possibility of perinatal transmission of HBV. 50-52
It has been shown that immunoprophylaxis can fail when maternal viral loads are high, especially when they are greater than 6 Log10 copies/mL. 51-53
HBeAg is a marker of replication and infectivity which is usually associated with high viral loads
Lack of immunoprophylaxis is related to possibility of perinatal transmission of over 90% when the mother is HBeAg positive, and 15% when she is HBeAg negative. If adequate immunoprophylaxis is offered, the perinatal transmission rate falls to 2%. The vast majority of these cases occur when viral loads are greater than 200,000 IU/mL (106 copies/mL). 49-54
Prevention of vertical transmission is based on a combination of vaccination and hepatitis B immunoglobulin (HBIG). This must be provided within the first 12 hours after birth to every child born to an HBsAg positive mother regardless of whether she has received antiviral treatment. 55,57 This combination has a great impact on viral transmission rates and has caused perinatal transmission to go from 90% (in cases where immunoprophylaxis is not administered) to minus 10% in this combination. 49
Antiviral treatment is indicated in all pregnant women who are HBsAg positive and have a viral load over 200,000 IU/mL (over 106 copies/mL). In these patients, the use of immunoprophylaxis alone has a possibility of failure up to 30%, so they are considered a high-risk group for perinatal transmission. 49,57,58 In patients with hepatitis B e antigen (HBeAg)-negative chronic hepatitis B (previously called asymptomatic carrier status) and with viral loads of 200,000 IU/mL or more, antiviral prophylaxis is not indicated. 59,60
The goals of treatment in pregnancy are to maintain stable liver function in the mother and to prevent neonatal infection. Levels of aminotransferases should be regularly evaluated during pregnancy, and antiviral medication should be started between the 28th and 32nd week of pregnancy since organogenesis is complete but there is still enough time to reduce HBV DNA levels significantly. 60 Medication should be continued up to 12 weeks after delivery due to the possibility of exacerbation of HBV. This occurs most frequently in patients who are HBeAg positive whose risk is 2.56 times higher when patients who are HBeAg negative. 59-62
Three antiviral drugs whose fetal safety profile is adequate are available: lamivudine, telbivudine, and tenofovir 63-66. Lamivudine has been falling into disuse due to resistance rates which can be as high as 70% over 5 years. This drug limits future maternal treatment options since use for even a short period promotes resistant viral variants in 20% of cases. 67
Tenofovir is the antiviral agent of choice during pregnancy. It should be administered orally in doses of 300 mg/day. It is the most potent nucleoside analog, has the lowest resistance rates, and extensive safety data are available for use during pregnancy. 49,64,68,69 Patients with chronic HBV infection treated with tenofovir should continue with this medication. If they have been treated with entecavir, treatment should be switched to tenofovir. 59
The indication for delivery is obstetric. There is no contraindication for vaginal birth since no studies have found that caesarean sections decrease the possibility of perinatal transmission. 70,71 There is also no contraindication for breastfeeding for untreated women or for mothers who receive an antiviral agent such as tenofovir. The risk of exposure to this drug in utero is greater than that through breast milk and its use, and tenofovir is recommended during pregnancy. 59,72-75 What is clear is that breastfeeding should be avoided when the mother’s nipples are cracked or bleeding because of the risk of mixing serous exudates with breast milk which could potentially lead to transmission of HBV to the infant. 76
Hepatitis c infections
Hepatitis C virus (HCV) infection constitute one of the leading causes of chronic liver disease worldwide. 77 The natural history of this entity varies greatly from minimal histological changes to cirrhosis, with or without hepatocellular carcinoma. Worldwide, there are approximately 71 million people who have chronic HCV infections. It is estimated that 1% to 8% of pregnant women have HCV. 78
Transmission of the infection from mother to child can occur during three different periods:
Intrauterine transmission is defined by HCV RNA in the serum of the newborn within the first 3 days of life. It accounts for 30% to 40% of cases. Among the proposed mechanisms are the passage of viral particles from mother to fetus, maternal-fetal flow of infected mononuclear cells, and infection of trophoblasts.
Peripartum transmission is defined by a finding of HCV RNA in the serum of a newborn within the first 28 days after birth. This is the most important period in vertical transmission (60% of cases). It correlates with exposure to maternal blood. For this period, the main risk factors are invasive obstetric procedures (for example, amniocentesis and fetal monitoring), as well as lacerations of the vaginal or perineal mucosa during vaginal delivery and prolonged rupture of the membranes.
Postpartum transmission is rare and is attributed to breastfeeding. However, despite the fact that HCV RNA is detectable in human colostrum, breastfeeding is not considered a risk factor for mother-to-child transmission. 79
A 2014 metaanalysis reported vertical transmission rates of 5.8% in viremic women and 10.8% for those who were coinfected with HIV. 80 Transmission occurs almost exclusively from mothers who are HCV RNA-positive, and risk correlates with the mother’s viral load titers. Transmission is four times higher for patients with 6 log copies/mL than for those who have lower viral loads (14.3% vs. 3.9%). 81
There is no universal consensus on screening for HCV during pregnancy. The American College of Obstetricians and Gynecologists (ACOG) and the Centers for Disease Control and Prevention (CDC) recommend screening only for women with risk factors for HCV infections (Table 5). (79) Nevertheless, a retrospective study by Selvapatt et al. that evaluated 35,355 women who had undergone prenatal HCV screening found that a total of 136 mothers (0.38%) tested positive for HCV antibodies. Of these, forty-four (0.12%) had been recently diagnosed with chronic hepatitis C, thirty-four had previous diagnoses, and 58 had negative HCV viral loads. Three cases of vertical transmission (6.8%) were documented in the children of these newly diagnosed mothers. This and other studies have shown that universal screening in pregnancy should be implemented. 82,83
There is no evidence that termination of pregnancy by caesarean section decreases transmission of HCV, and breastfeeding is not contraindicated except in cases of cracked or bleeding nipples. These recommendations are the same as those for HBV. 85
The use of interferon and ribavirin is contraindicated in pregnancy due to teratogenic effects. 86 There are no adequate human data for the use of second-generation direct-acting antivirals during pregnancy, but data obtained from animal studies demonstrate that they do not confer risk to the fetus. Due to the lack of human studies, no direct-acting antiviral therapy has yet been approved for treating HCV infections during pregnancy. 87
Cirrhosis and pregnancy
The prevalence of cirrhosis in women of childbearing age is low because hepatocellular damage leads to metabolic and hormonal changes such as anovulation, amenorrhea, and infertility. 8,10,11,32 Although pregnancy is rare in patients with liver disease, it implies a complicated clinical condition when it does occur. 8 Outcomes of these pregnancies are related to the severity of liver disease. A preconception MELD score of over 10 points is associated with a decompensation risk of 10% while a MELD score of less than six points is not associated with liver complications during pregnancy. 2,7
The most frequent causes of cirrhosis in pregnant women are autoimmune factors and hepatotropic viral infections. 10 Physiological changes during pregnancy and fetal needs worsen portal hypertension and increase both maternal and fetal risks. 2,7,10 Bleeding varices are the most frequent and catastrophic complications of portal hypertension during pregnancy. The greatest risks of bleeding occurs during the second trimester during which the highest peak of portal hypertension is reached and during delivery due to Valsalva maneuvers. 7,8
Increased maternal blood volume and cardiac output associated with decreased peripheral vascular resistance secondary to the effects of progesterone and the development of the placental vascular bed lead to a hyperdynamic state with increased flow to the collaterals. This significantly increases the risk of bleeding varices. 10 External compression of the inferior vena cava by the gravid uterus further increases portal pressure. 8 Pregnant cirrhotic women have nearly 30% probability of variceal bleeding, and this increases to 70% in patients with pre-existing varicose veins. 8,10,32 Mortality associated with bleeding is 18% to 50%. 2,10 Bleeding varices increase the incidences of miscarriage and preterm delivery. Given the high risk of variceal bleeding and its complications, the American College of Gastroenterology recommends screening for esophageal varices in pregnant patients in the second trimester after completion of organogenesis, but before delivery, at a time of increased risk of bleeding.
Despite limited data regarding prophylaxis and management of acute variceal bleeding in pregnant patients, primary prophylaxis can be performed with endoscopic ligation or with β-blockers just as in the general population. 10 Secondary prophylaxis requires both endoscopic band ligation and the use of β blockers. These drugs are considered safe in pregnancy despite being category C, but they carry risks of fetal bradycardia, intrauterine growth restriction and neonatal hypoglycemia. 2,7,10 Treatment of acute variceal bleeding is similar to that for the general population. The mother should be resuscitated and stabilized, then endoscopic ligation of varicose veins is the main therapeutic measure. Endoscopy is safe during pregnancy although it carries a small risk of fetal hypoxia secondary to sedation and patient position. Octreotide is a category B medication in pregnancy. Despite a lack of good studies in pregnant women, it appears to be safe. Terlipressin is category D since it produces uterine ischemia: it is not recommended for pregnant women. 2 Third-generation cephalosporins are the recommended prophylactic antibiotics since quinolones are contraindicated. 10 Emergency Transjugular intrahepatic portosystemic shunting (TIPS) has been successfully performed in pregnant patients. 2,32
Ascites and hepatic encephalopathy occur in 24% of pregnant patients with cirrhosis and can appear at any stage of pregnancy. 88 Treatment of ascites is based on salt restriction and the use of diuretics. When spontaneous bacterial peritonitis is found, it the usually treated with third-generation cephalosporins. 10 Hepatic encephalopathy is usually associated with a precipitating event that should be actively sought. Lactulose is a category B drug, while rifaximin is category C. 32
Ultrasound is considered to be the safest imaging method for visualizing the liver parenchyma and even the bile duct in a pregnant patient. In case a more detailed image is required, MRI without contrast is also safe in the second and third trimesters of pregnancy. The limitations of uncontrasted images for evaluating liver lesions, especially hepatocellular carcinoma, must always be taken into account. 8,11 Data comparing vaginal and cesarean deliveries in these patients are too scare to recommend one route or the other, so the individual obstetrician must decide. 2,89
Pregnant patients with cirrhosis are at greater risk of postpartum hemorrhaging. It occurs in 7% to 10% of cases in relation to coagulopathy secondary to liver dysfunction and thrombocytopenia due to hypersplenism. It is treated with transfusions and agents that promote uterine contraction. 10 The probabilities of fetal complications, spontaneous abortions, preterm deliveries, stillbirths and perinatal deaths all increase. 2,7,89
Due to the high risks of complications, comprehensive management of these patients requires an interdisciplinary group in a highly complex medical center. The group should include gynecologists, neonatologists, hepatologists, endoscopists, and even intensive care specialists. Ideally, these patients’ liver disease should be compensated, their esophageal varices managed, and medications contraindicated during pregnancy should be discontinued. Periodic examinations should be done throughout the pregnancy in order to decrease the risk of complications for both the mother and the fetus.
Transplants and pregnancy
Perinatal outcomes in women who have had liver transplants are good, especially if they wait at least 12 months after surgery or an acute episode of rejection to become pregnant since this lessens the need for immunosuppressants and lessens the risks of opportunistic infections. The live birth rate is reported to be between 70% and 90%. There are high risks of hypertensive disorders of pregnancy, pre-eclampsia, preterm deliveries, and low birth weights. 11,12
Administration of immunosuppressants should continue as part of pre-pregnancy care, and high-dose folic acid supplements (5 mg/day) should be taken. 12 Overall, the impacts of steroids, azathioprine, and calcineurin inhibitors (tacrolimus and cyclosporine) on maternal-fetal abnormalities are small. Neonatal leukopenia has been seen with azathioprine, but this normalizes after the first year of life. Cyclosporine is associated with low birth weights and premature births. Calcineurin inhibitors can cause hyperkalemia, preterm delivery, and renal dysfunction. 4,11,90,91
In contrast, mycophenolate, sirolimus, and everolimus are all associated with high rates of fetal abnormalities. Mycophenolate not only increases the rate of miscarriages and fetal toxicity, but is related to malformations of many organs and parts of the body including ears, extremities, kidneys, hearts, esophagi and faces (cleft palate and cleft lip). Sirolimus and everolimus have not been sufficiently studied, but their antiproliferative effects can harm a fetus, so they are contraindicated in pregnancy. If possible, a woman receiving this type of immunosuppressant should be switched to an alternative regimen at least 6 months before conception. 4,11,90,91
Graft survival appears to be unaffected by pregnancy although there are reports of rejection in 2% to 17% of cases during pregnancy and in 3% to 12% of cases in the postpartum period. As in women who are not pregnant, episodes of rejection should be managed by investigating the cause of rejection and adjusting administration of immunosuppressants. 2,12
Prenatal medical examinations should be done every four weeks and should always include screening for normal pregnancy infections. Vaccinations should be updated. Examinations should also include periodic evaluations for cytomegalovirus which can not only cause various malformations in fetal development, but can also compromise graft survival. Once 24 weeks is reached, monitoring of fetal well-being every two weeks is recommended. This should include Doppler of the umbilical and uterine arteries. Women who have had transplants and become pregnant should maintain hemoglobin levels between 10-12 g/dL. Iron and erythropoietin stimulating agents, which are not contraindicated in pregnancy, are recommended for this purpose. 90,91
A review of the largest published studies of pregnancy in liver transplant patients shows that the incidences of maternal complications during pregnancy are 33.9% for hypertension, 26.5% for infections, 21.5% for preeclampsia, 8.4% for rejection and 6.7% for diabetes. 90 Cesarean deliveries are preferred primarily out of concern over obstetric complications. 92
Women who are on steroid-only management are allowed to breastfeed, but breastfeeding is not advised for the vast majority who take more than one immunosuppressant since some effects of immunosuppression on newborns are unknown. 4,91
Hormonal contraceptives taken by liver transplant patients have no effects on liver function, incidence of rejection, complications, patients’ cardiovascular health and no interactions that would require discontinuation of medications. However, calcineurin inhibitor toxicity associated with inhibition of cytochrome P-450 by oral contraceptives may develop. Consequently, women who have liver transplants who use hormonal contraceptives require strict control of cyclosporine and tacrolimus levels. Intrauterine devices are the most widely used contraceptive method in these patients, since they are safe for graft viability and do not increase the risk of intrauterine infections. 90-92
Referencias
1. Lai M, Wolf J. The liver in pregnancy. Handbook of Liver Disease. Elsevier. 2018. p. 308-23. Disponible en: https://doi.org/10.1016/B978-0-323-47874-8.00023-7. [ Links ]
2. Westbrook R, Dusheiko G, Williamson C. Pregnancy and liver disease. J Hepatol. 2016;64(4):933-45. doi: https://doi.org/10.1016/j.jhep.2015.11.030. [ Links ]
3. Shekhar S, Diddi G. Liver disease in pregnancy. Taiwan J Obstet Gynecol. 2015;54(5):475-82. doi: https://doi.org/10.1016/j.tjog.2015.01.004. [ Links ]
4. Than N, Neuberger J. Liver abnormalities in pregnancy. Best Pract Res Clin Gastroenterol. 2013;27(4):565-75. doi: https://doi.org/10.1016/j.bpg.2013.06.015. [ Links ]
5. Clinical management guidelines for obstetrician-gynecologists. Number 153, September 2015: (replaces practice bulletin number 52, April 2004). Obstet Gynecol. 2003;126(12):12-24. [ Links ]
6. London V, Grube S, Sherer D, Abulafia O. Hyperemesis gravidarum: a review of recent literature. Pharmacology. 2017;100(3-4):161-71. doi: https://doi.org/10.1159/000477853. [ Links ]
7. Westbrook R, Yeoman A, O’Grady J, Harrison P, Devlin J, Heneghan M. Model for end-stage liver disease score predicts outcome in cirrhotic patients during pregnancy. Clin Gastroenterol Hepatol. 2011;9(8):694-9. doi: https://doi.org/10.1016/j.cgh.2011.03.036. [ Links ]
8. Deepak J, Andra J, Quaglia A, Westbrook R, Heneghan M. Liver disease in pregnancy. Lancet. 2010;375:594-605. doi: https://doi.org/10.1016/S0140-6736(09)61495-1. [ Links ]
9. Management of hyperemesis gravidarum. Drug Ther Bull. 2013;51(11):126-9. doi: https://doi.org/10.1136/dtb.2013.11.0215. [ Links ]
10. Aggarwal N, Negi N, Aggarwal A, Bodh V, Dhiman R. Pregnancy with portal hypertension. J Clin Exp Hepatol. 2014;4(2):163-71. doi: https://doi.org/10.1016/j.jceh.2014.05.014. [ Links ]
11. Tran T, Ahn J, Reau N. ACG clinical guideline: liver disease and pregnancy. Am J Gastroenterol. 2016;111(2):176-94. doi: https://doi.org/10.1038/ajg.2015.430. [ Links ]
12. Geenes V, Williamson C. Gastrointestinal and liver disease in pregnancy. Obstet Gynaecol Reprod Med. 2017;27(3):91-8. doi: https://doi.org/10.1016/j.ogrm.2017.01.005. [ Links ]
13. Frise C, Williamson C. Liver disease in pregnancy. Medicine (Baltimore). 2015;43(11):636-8. doi: https://doi.org/10.1016/j.mpmed.2015.08.010. [ Links ]
14. Geenes V, Williamson C. Liver disease in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2015;29(5):612-24. doi: https://doi.org/10.1016/j.bpobgyn.2015.04.003. [ Links ]
15. Ditisheim A, Sibai B. Diagnosis and management of HELLP syndrome complicated by liver hematoma. Clin Obstet Gynecol. 2017;60(1):190-7. doi: https://doi.org/10.1097/GRF.0000000000000253. [ Links ]
16. Liu J, Ghaziani T, Wolf L. Acute fatty liver disease of pregnancy: updates in pathogenesis, diagnosis, and management. Am J Gastroenterol. 2017;112(6):838-46. doi: https://doi.org/10.1038/ajg.2017.54. [ Links ]
17. Knight M, Nelson-Piercy C, Kurinczuk J, Spark P, Brocklehurst P, UK Obstetric Surveillance System. A prospective national study of acute fatty liver of pregnancy in the UK. Gut. 2008;57(7):951-6. doi: https://doi.org/10.1136/gut.2008.148676. [ Links ]
18. Papafragkakis H, Singhal S, Anand S. Acute fatty liver of pregnancy. South Med J. 2013;106(10):588-93. doi: https://doi.org/10.1097/SMJ.0000000000000007. [ Links ]
19. Buytaert I, Elewaut G, van Kets H. Early occurrence of acute fatty liver in pregnancy. Am J Gastroenterol. 1996;91(3):603-4. [ Links ]
20. Natarajan S, Ibdah J. Role of 3-hydroxy fatty acid-induced hepatic lipotoxicity in acute fatty liver of pregnancy. Int J Mol Sci. 2018;19(1):1-17. doi: https://doi.org/10.3390/ijms19010322. [ Links ]
21. Lee N, Brady C. Liver disease in pregnancy. World J Gastroenterol. 2009;15(8):897-906. doi: https://doi.org/10.3748/wjg.15.897. [ Links ]
22. Steingrub J. Pregnancy-associated severe liver dysfunction. Crit Care Clin. 2004;20(4):763-76. doi: https://doi.org/10.1016/j.ccc.2004.05.006. [ Links ]
23. Ch’ng C, Morgan M, Hainsworth I, Kingham J. Prospective study of liver dysfunction in pregnancy in Southwest Wales. Gut. 2002;51(6):876-80. doi: https://doi.org/10.1136/gut.51.6.876. [ Links ]
24. Maléth J, Venglovecz V, Rázga Z, Tiszlavicz L, Rakonczay Z, Hegyi P. Non-conjugated chenodeoxycholate induces severe mitochondrial damage and inhibits bicarbonate transport in pancreatic duct cells. Gut. 2011;60(1):136-8. doi: https://doi.org/10.1136/gut.2009.192153. [ Links ]
25. Remiszewski P, Pawlak J, Skwarek A, Grzelak I, Patkowski W, Grodzicki M, et al. Orthotopic liver transplantation for acute liver failure resulting from «acute fatty liver of pregnancy». Ann Transplant. 2003;8(3):8-11. [ Links ]
26. Wei Q, Zhang L, Liu X. Clinical diagnosis and treatment of acute fatty liver of pregnancy: a literature review and 11 new cases. J Obstet Gynaecol Res. 2010;36(4):751-6. doi: https://doi.org/10.1111/j.1447-0756.2010.01242.x. [ Links ]
27. Westbrook R, Yeoman A, Joshi D, Heaton N, Quaglia A, OGrady JG, et al. Outcomes of severe pregnancy-related liver disease: refining the role of transplantation. Am J Transplant. 2010;10(11):2520-6. doi: https://doi.org/10.1111/j.1600-6143.2010.03301.x. [ Links ]
28. Gatselis N, Zachou K, Koukoulis G, Dalekos G. Autoimmune hepatitis, one disease with many faces: etiopathogenetic, clinico-laboratory and histological characteristics. World J Gastroenterol . 2015;21(1):60. doi: https://doi.org/10.3748/wjg.v21.i1.60. [ Links ]
29. Hennes E, Zeniya M, Czaja A, Parés A, Dalekos G, Krawitt EL, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48(1):169-76. doi: https://doi.org/10.1002/hep.22322. [ Links ]
30. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: autoimmune hepatitis. J Hepatol . 2015;63(4):971-1004. doi: https://doi.org/10.1016/j.jhep.2015.06.030. [ Links ]
31. Manns M, Czaja A, Gorham J, Krawitt E, Mieli-Vergani G, Vergani D, et al. Diagnosis and management of autoimmune hepatitis. Hepatology . 2010;51(6):2193-213. doi: https://doi.org/10.1002/hep.23584. [ Links ]
32. Almashhrawi A, Khulood A, Rubayat R, Ghassan H, Jamal I. Liver diseases in pregnancy: diseases not unique to pregnancy. World J Gastroenterol . 2013;19(43):7630. doi: https://doi.org/10.3748/wjg.v19.i43.7630. [ Links ]
33. Braga A, Vasconcelos C, Braga J. Pregnancy with autoimmune hepatitis. Bed Bench. 2016;9(3):220-4. [ Links ]
34. Schmeltzer P, Russo M. Clinical narrative: autoimmune hepatitis. Am J Gastroenterol. 2018;113(7):951-8. doi: https://doi.org/10.1038/s41395-018-0058-z. [ Links ]
35. Schramm C, Herkel J, Beuers U, Kanzler S, Galle P, Lohse A. Pregnancy in autoimmune hepatitis: outcome and risk factors. Am J Gastroenterol. 2006;101(3):556-60. doi: 10.1111/j.1572-0241.2006.00479.x. [ Links ]
36. Orgul G, Ozkan E, Celik H, Beksac M. Autoimmune hepatitis and pregnancy: report of two cases with different maternal outcomes. Clin Exp Hepatol. 2017;4:212-4. doi: https://doi.org/10.5114/ceh.2017.71445. [ Links ]
37. Heneghan M, Norris S, O´Grady J, Harrison P, McFalane I. Management and outcome of pregnancy in autoimmune hepatitis. Gut . 2001;48(1):97-102. doi: https://doi.org/10.1136/gut.48.1.97. [ Links ]
38. Westbrook R, Yeoman A, Kriese S, Heneghan M. Outcomes of pregnancy in women with autoimmune hepatitis. J Autoimmun. 2012;38(2-3):J239-44. doi: https://doi.org/10.1016/j.jaut.2011.12.002. [ Links ]
39. Bremer L, Schramm C, Tiegs G. Immunology of hepatic diseases during pregnancy. Semin Immunopathol. 2016;38(6):669-85. doi: https://doi.org/10.1007/s00281-016-0573-1. [ Links ]
40. Sebode M, Schramm C. AIH: which alternative for difficult-to-treat patients? Dig Dis. 2015;33(2):83-7. doi: https://doi.org/10.1159/000440752. [ Links ]
41. Lammert C, Loy V, Oshima K, Gawrieh S. Management of difficult cases of autoimmune hepatitis. Curr Gastroenterol Rep. 2016;18(2). doi: http://link.springer.com/10.1007/s11894-015-0484-7. [ Links ]
42. Terrault N, Bzowej N, Chang KM, Hwang J, Jonas M, Murad M, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatol Baltim Md. 2016;63(1):261-83. doi: https://doi.org/10.1002/hep.28156. [ Links ]
43. Association E. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol . 2012;57(1):167-85. doi: https://doi.org/10.1016/j.jhep.2012.02.010. [ Links ]
44. Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97-107. doi: https://doi.org/10.1046/j.1365-2893.2003.00487.x. [ Links ]
45. Tran T. Hepatitis B in pregnancy. Clin Infect Dis. 2016;62(4):S314-7. doi: https://doi.org/10.1093/cid/ciw092. [ Links ]
46. Dionne-Odom J, Tita A, Silverman N. Hepatitis B in pregnancy screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214(6-14). [ Links ]
47. Patton H, Tran T. Management of hepatitis B during pregnancy. Nat Rev Gastroenterol Hepatol. 2014;11(7):402-9. doi: https://doi.org/10.1038/nrgastro.2014.30. [ Links ]
48. Rac M, Sheffield J. Prevention and management of viral hepatitis in pregnancy. Obstet Gynecol Clin N Am. 2014;41:573-92. doi: https://doi.org/10.1016/j.ogc.2014.08.004. [ Links ]
49. Castillo E, Murphy K, van Schalkwyk J. Hepatitis B and pregnancy. J Obstet Gynaecol Can JOGC J. 2017;39(3):181-90. doi: https://doi.org/10.1016/j.jogc.2016.11.001. [ Links ]
50. Liu CP, Zeng YL, Zhou M, Chen LL, Hu R, Wang L, et al. Factors associated with mother-to-child transmission of hepatitis B virus despite immunoprophylaxis. Intern Med Tokyo Jpn. 2015;54(7):711-6. doi: https://doi.org/10.2169/internalmedicine.54.3514. [ Links ]
51. Wen WH, Chang MH, Zhao LL, Ni YH, Hsu HY, Wu JF, et al. Mother-to-infant transmission of hepatitis B virus infection: significance of maternal viral load and strategies for intervention. J Hepatol . 2013;59(1):24-30. doi: https://doi.org/10.1016/j.jhep.2013.02.015. [ Links ]
52. Lu LL, Chen BX, Wang J, Wang D, Ji Y, Yi HG, et al. Maternal transmission risk and antibody levels against hepatitis B virus e antigen in pregnant women. Int J Infect Dis Off Publ Int Soc Infect Dis. 2014;28:41-4. doi: https://doi.org/10.1016/j.ijid.2014.07.028. [ Links ]
53. Zou H, Chen Y, Duan Z, Zhang H, Pan C. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to HBsAg-positive mothers. J Viral Hepat . 2012;19(2):18-25. doi: https://doi.org/10.1111/j.1365-2893.2011.01492.x. [ Links ]
54. Ott J, Stevens G, Wiersma S. The risk of perinatal hepatitis B virus transmission: hepatitis B e antigen (HBeAg) prevalence estimates for all world regions. BMC Infect Dis. 2012;12:131. doi: https://doi.org/10.1186/1471-2334-12-131. [ Links ]
55. Lee C, Gong Y, Brok J, Boxall E, Gluud C. Hepatitis B immunisation for newborn infants of hepatitis B surface antigen-positive mothers. Cochrane Database Syst Rev. 2006;(2):CD004790. doi: https://doi.org/10.1002/14651858.CD004790.pub2. [ Links ]
56. Beasley R, Hwang L, Lee G, Lan C, Roan C, Huang F, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet Lond Engl. 1983;2(8359):1099-102. doi: https://doi.org/10.1016/S0140-6736(83)90624-4. [ Links ]
57. Pan C, Duan ZP, Bhamidimarri K, Zou HB, Liang XF, Li J, et al. An algorithm for risk assessment and intervention of mother to child transmission of hepatitis B virus. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2012;10(5):452-9. doi: https://doi.org/10.1016/j.cgh.2011.10.041. [ Links ]
58. Brown R, McMahon B, Lok A, Wong J, Ahmed A, Mouchli M, et al. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: a systematic review and meta-analysis. Hepatol Baltim Md . 2016;63(1):319-33. doi: https://doi.org/10.1002/hep.28302. [ Links ]
59. European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol . 2017;67(2):370-98. doi: 10.1016/j.jhep.2017.03.021. [ Links ]
60. Shao Z, Al Tibi M, Wakim-Fleming J. Update on viral hepatitis in pregnancy. Cleve Clin J Med. 2017;84(3):202-6. doi: https://doi.org/10.3949/ccjm.84a.15139. [ Links ]
61. Chang C, Aziz N, Poongkunran M, Javaid A, Trinh H, Lau D, et al. Serum alanine aminotransferase and hepatitis B DNA flares in pregnant and postpartum women with chronic hepatitis B. Am J Gastroenterol . 2016;111(10):1410-5. doi: https://doi.org/10.1038/ajg.2016.296. [ Links ]
62. Giles M, Visvanathan K, Lewin S, Bowden S, Locarnini S, Spelman T, et al. Clinical and virological predictors of hepatic flares in pregnant women with chronic hepatitis B. Gut . 2015;64(11):1810-5. doi: https://doi.org/10.1136/gutjnl-2014-308211. [ Links ]
63. Wu Q, Huang H, Sun X, Pan M, He Y, Tan S, et al. Telbivudine prevents vertical transmission of hepatitis B virus from women with high viral loads: a prospective long-term study. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc . 2015;13(6):1170-6. doi: https://doi.org/10.1016/j.cgh.2014.08.043. [ Links ]
64. Pan C, Duan Z, Dai E, Zhang S, Han G, Wang Y, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374(24):2324-34. doi: https://doi.org/10.1056/NEJMoa1508660. [ Links ]
65. Deng M, Zhou X, Gao S, Yang SG, Wang B, Chen HZ, et al. The effects of telbivudine in late pregnancy to prevent intrauterine transmission of the hepatitis B virus: a systematic review and meta-analysis. Virol J. 2012;9:185. doi: https://doi.org/10.1186/1743-422X-9-185. [ Links ]
66. Sarkar M, Terrault N. Ending vertical transmission of hepatitis B: the third trimester intervention. Hepatol Baltim Md . 2014;60(2):448-51. doi: https://doi.org/10.1002/hep.27145. [ Links ]
67. Ayres A, Yuen L, Jackson K, Manoharan S, Glass A, Maley M, et al. Short duration of lamivudine for the prevention of hepatitis B virus transmission in pregnancy: lack of potency and selection of resistance mutations. J Viral Hepat . 2014;21(11):809-17. doi: https://doi.org/10.1111/jvh.12212. [ Links ]
68. Chen JZ, Liao ZW, Huang FL, Su RK, Wang WB, Cheng XY, et al. Efficacy and safety of tenofovir disoproxil fumarate in preventing vertical transmission of hepatitis B in pregnancies with high viral load. Sci Rep. 2017;7(1):4132. doi: https://doi.org/10.1038/s41598-017-04479-x. [ Links ]
69. Jourdain G, Ngo-Giang-Huong N, Cressey T, Hua L, Harrison L, Tierney C, et al. Prevention of mother-to-child transmission of hepatitis B virus: a phase III, placebo-controlled, double-blind, randomized clinical trial to assess the efficacy and safety of a short course of tenofovir disoproxil fumarate in women with hepatitis B virus e-antigen. BMC Infect Dis . 2016;16:393. doi: https://doi.org/10.1186/s12879-016-1734-5. [ Links ]
70. Hu Y, Chen J, Wen J, Xu C, Zhang S, Xu B, et al. Effect of elective cesarean section on the risk of mother-to-child transmission of hepatitis B virus. BMC Pregnancy Childbirth. 2013;13:119. doi: https://doi.org/10.1186/1471-2393-13-119. [ Links ]
71. Yang J, Zeng X, Men Y, Zhao L. Elective caesarean section versus vaginal delivery for preventing mother to child transmission of hepatitis B virus-A systematic review. Virol J . 2008;5:100. doi: https://doi.org/10.1186/1743-422X-5-100. [ Links ]
72. Hill J, Sheffield J, Kim M, Alexander J, Sercely B, Wendel G. Risk of hepatitis B transmission in breast-fed infants of chronic hepatitis B carriers. Obstet Gynecol. 2002;99(6):1049-52. doi: https://doi.org/10.1097/00006250-200206000-00018. [ Links ]
73. Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827-41. doi: https://doi.org/10.1542/peds.2011-3552. [ Links ]
74. Benaboud S, Pruvost A, Coffie P, Ekouévi D, Urien S, Arrivé E, et al. Concentrations of tenofovir and emtricitabine in breast milk of HIV-1-infected women in Abidjan, Cote d’Ivoire, in the ANRS 12109 TEmAA Study, Step 2. Antimicrob Agents Chemother. 2011;55(3):1315-7. doi: https://doi.org/10.1128/AAC.00514-10. [ Links ]
75. Brown R, Verna E, Pereira M, Tilson H, Aguilar C, Leu CS, et al. Hepatitis B virus and human immunodeficiency virus drugs in pregnancy: findings from the Antiretroviral Pregnancy Registry. J Hepatol . 2012;57(5):953-9. doi: https://doi.org/10.1016/j.jhep.2012.06.031. [ Links ]
76. Ayoub W, Cohen E. Hepatitis B management in the pregnant patient: an update. J Clin Transl Hepatol. 2016;4(3):241-7. doi: https://doi.org/10.14218/JCTH.2016.00014. [ Links ]
77. European association for the study of the liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol . 2018;69(2):461-511. doi: 10.1016/j.jhep.2018.03.026. [ Links ]
78. Page C, Hughes B, Rhee E, Kuller J. Hepatitis C in pregnancy: review of current knowledge and updated recommendations for management. Obstet Gynecol Surv. 2017;72(6):347-55. doi: https://doi.org/10.1097/OGX.0000000000000442. [ Links ]
79. Pott H, Theodoro M, de Almeida Vespoli J, Senise J, Castelo A. Mother-to-child transmission of hepatitis C virus. Eur J Obstet Gynecol Reprod Biol. 2018;224:125-30. doi: https://doi.org/10.1016/j.ejogrb.2018.03.034. [ Links ]
80. Benova L, Mohamoud Y, Calvert C, Abu-Raddad L. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis Off Publ Infect Dis Soc Am. 2014;59(6):765-73. doi: https://doi.org/10.1093/cid/ciu447. [ Links ]
81. Delotte J, Barjoan E, Berrébi A, Laffont C, Benos P, Pradier C, et al. Obstetric management does not influence vertical transmission of HCV infection: results of the ALHICE group study. J Matern-Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet. 2014;27(7):664-70. doi: https://doi.org/10.3109/14767058.2013.829813. [ Links ]
82. Selvapatt N, Ward T, Bailey H, Bennett H, Thorne C, See LM, et al. Is antenatal screening for hepatitis C virus cost-effective? A decade’s experience at a London centre. J Hepatol . 2015;63(4):797-804. doi: https://doi.org/10.1016/j.jhep.2015.05.015. [ Links ]
83. Diab-Elschahawi M, Dosch V, Honsig C, Jatzko B, Segagni L, Assadian O, et al. Evaluation of a universal vs a targeted hepatitis C virus screening strategy among pregnant women at the Vienna University Hospital. Am J Infect Control. 2013;41(5):459-60. doi: https://doi.org/10.1016/j.ajic.2012.06.003. [ Links ]
84. Bernstein H, Dunkelberg J, Leslie K. Hepatitis C in pregnancy in the era of direct-acting antiviral treatment: potential benefits of universal screening and antepartum therapy. ClinObstet Gynecol . 2018;61(1):146-56. doi: https://doi.org/10.1097/GRF.0000000000000345. [ Links ]
85. Cottrell E, Chou R, Wasson N, Rahman B, Guise JM. Reducing risk for mother-to-infant transmission of hepatitis C virus: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;158(2):109-13. doi: https://doi.org/10.7326/0003-4819-158-2-201301150-00575. [ Links ]
86. Valladares G, Chacaltana A, Sjogren MH. The management of HCV-infected pregnant women. Ann Hepatol. 2010;9:92-7. doi: https://doi.org/10.1016/S1665-2681(19)31731-4. [ Links ]
87. Barritt A, Jhaveri R. Treatment of hepatitis C during pregnancy-weighing the risks and benefits in contrast to HIV. Curr HIV/AIDS Rep. 2018;15(2):155-61. doi: https://doi.org/10.1007/s11904-018-0386-z. [ Links ]
88. Benjaminov F, Heathcote J. Liver disease in pregnancy. Am J Gastroenterol . 2004;99(12):2479-88. doi: 10.1111/j.1572-0241.2004.30231.x. [ Links ]
89. Giard JM, Terrault N. Women with cirrhosis. Gastroenterol Clin North Am. 2016;45(2):345-58. doi: https://doi.org/10.1016/j.gtc.2016.02.010. [ Links ]
90. Ramirez C, Doria C. Pregnancy after liver transplantation. Best Pract Res Clin Obstet Gynaecol . 2014;28(8):1137-45. doi: https://doi.org/10.1016/j.bpobgyn.2014.07.022. [ Links ]
91. Blume C, Pischke S, von Versen-Höynck F, Günter H, Gross M. Pregnancies in liver and kidney transplant recipients: a review of the current literature and recommendation. Best Pract Res Clin Obstet Gynaecol . 2014;28(8):1123-36. doi: https://doi.org/10.1016/j.bpobgyn.2014.07.021. [ Links ]
92. Akarsu M, Unek T, Avcu A, Ozbilgin M, Egeli T, Astarcioglu I. Evaluation of pregnancy outcomes after liver transplantation. Transplant Proc. 2016;48(10):3373-7. doi: https://doi.org/10.1016/j.transproceed.2016.09.033. [ Links ]
Received: February 18, 2018; Accepted: May 13, 2019











 texto en
texto en