Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.34 no.4 Bogotá oct./dic. 2019
https://doi.org/10.22516/25007440.261
Case report
Digestive tract hemorrhaging in a patient with Brunner’s gland hyperplasia
1Servicio de Gastroenterología y Hepatología, Hospital Universitario Ramón y Cajal, Madrid, España.
2Servicio de Anatomía Patológica, Hospital Universitario Ramón y Cajal, Madrid, España.
Objective:
This study analyzes the epidemiological characteristics, etiological and pathogenic bases, clinical presentation, diagnosis and treatment of Brunner’s gland hyperplasia.
Methods:
We describe a case of Brunner’s gland hyperplasia that was diagnosed incidentally during elective endoscopy and review the available literature.
Results:
This neoplasm consists of glandular proliferation preferentially located in the proximal duodenum. Its diagnosis, normally made by endoscopic biopsy, can be associated with complications that, although infrequent, should not be underestimated.
Conclusions:
Duodenal neoplasms are a small percentage of those that affect the gastrointestinal tract. Because diagnosis is usually made by chance during a scheduled endoscopy, treatment should be based on the symptoms and size of the lesion according to the treatment standards of each medical center.
Keywords: Digestive hemorrhage; Brunner’s glands; Brunner’s gland hyperplasia; Brunner’s gland adenoma
Objetivo:
analizar las características epidemiológicas, bases etiopatogénicas y presentación clínica, así como el diagnóstico y el tratamiento de la hiperplasia de glándulas de Brunner (HGB).
Métodos:
describir un caso de HGB diagnosticado de forma incidental durante una endoscopia electiva y realizar una revisión de la literatura disponible hasta el momento.
Resultados:
esta neoformación consiste en una proliferación glandular localizada preferentemente en el duodeno proximal. Su diagnóstico, normalmente realizado mediante biopsia endoscópica, puede asociarse con complicaciones que, aunque infrecuentes, no deben ser subestimadas.
Conclusiones:
las neoplasias duodenales representan un porcentaje pequeño dentro del total de las que afectan al tracto gastrointestinal. Debido a que el diagnóstico de estas lesiones suele realizarse de forma casual durante una endoscopia programada, el tratamiento deberá basarse en la sintomatología, así como el tamaño de las mismas, de acuerdo con los estándares de tratamiento de cada centro.
Palabras clave: Hemorragia digestiva; glándulas de Brunner; hiperplasia de glándulas de Brunner; adenoma de glándulas de Brunner
Introduction
Brunner’s gland hyperplasia (BGH), also known as Brunner’s gland adenoma or Brunneroma, consists of a proliferation of these submucosal glands of varying sizes and morphology. Usually they are found in the duodenum. The prevalence of BGH is not well established, since most cases are incidentally discovered during an endoscopic examination. Although they are normally asymptomatic, they can produce a variety of gastrointestinal symptoms ranging from nausea and vomiting to gastrointestinal bleeding which is related to their profuse vascularization. Diagnosis is made by endoscopy, echoendoscopy or other imaging techniques. When treatment is necessary, it must be individual, either endoscopic or surgical.
Clinical case
The patient was a 60-year-old man who smoked and who formerly consumed 30 grams of alcohol daily. He had a history of arterial hypertension, stage IV chronic kidney disease secondary to nephroangiosclerosis, and chronic hepatopathy of enolic origin. There were no data on portal hypertension. He had been taking 25 mg/day of spironolactone and 40 mg/day of enalapril.
He was seen in our endoscopy unit for an upper digestive endoscopy for varicose vein screening. During the exploration, two subepithelial nodular lesions that each measured about 10 mm and were covered with normal mucosa were identified in the second duodenal portion distal to the duodenal papilla (Figure 1). Taking of a diagnostic biopsy sample from one nodule led to persistent seepage of blood which required placement of a hemoclip to achieve hemostasis. No other lesions or evidence of portal hypertension were identified.
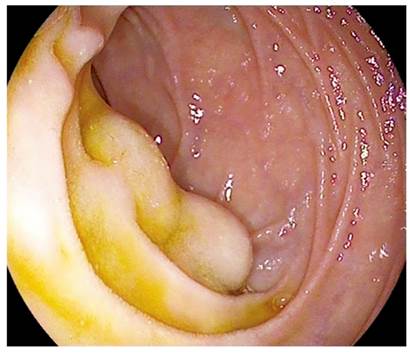
Figure 1 Two subepithelial lesions covered with normal mucosa immediately distal to the duodenal papilla.
Four days later the patient came to the Emergency Department after two days of melena. Upon arrival, he was hemodynamically stable with blood pressure of 110/80 mm Hg and heart rate of 73 beats per minute. He was significantly anemic with hemoglobin of 9.7 g/dL. Previously, it had been 14.3 g/dL. Other parameters were at their usual values. Upper gastrointestinal bleeding was suspected so intravenous treatment with proton pump inhibitors and continuous perfusion was initiated and urgent upper endoscopy was performed. A clot attached to the previously placed hemoclip in the second duodenal portion was identified, but there was no active bleeding. A new hemoclip was placed, which again caused abundant bleeding. Ten mL of adrenaline diluted in 0.9% physiological saline (1:10,000) and 2 mL of Etoxiesclerol® (lauromacrogol 400) were administered at the site, and two hemoclips were placed. This stopped the bleeding. Another blood test found that the patient’s hemoglobin had decreased to 7.5 g/dL, so two packs of red blood cell concentrate were transfused. There were no further complications, and the patient was discharged after 5 days.
The histological study of the lesion found proliferation of submucosal glands, so Brunner’s gland hyperplasia was diagnosed (Figure 2). Computed tomography (CT) showed no extraluminal extension. Given the small size, iatrogenic origin of hemorrhaging, and subsequent absence of symptoms, a waiting attitude was chosen. The patient remains asymptomatic eight months after diagnosis.
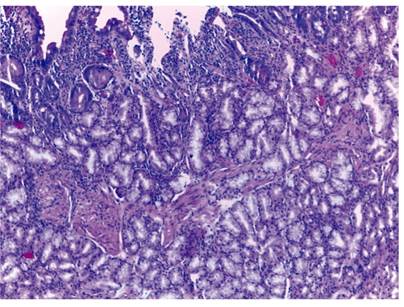
Figure 2 More than half of the mucosa shows correct villus height and Brunner’s gland lobes. These lobes are continued with abundant Brunner’s glands in the submucosa. The cells show typically clear mucous cytoplasm without cytological atypia (H&E x400).
DISCUSSION
Brunner’s glands are acinotubular and are found in the submucosa, most commonly in the proximal duodenum between the pylorus and the duodenal papilla. They are less commonly found in more distal portions of the duodenum and even in the jejunum. Their fundamental function consists of producing and secreting alkaline mucus which helps protect the duodenal mucosa from chime. 1,2 Although there is no established name for proliferation of these glands, Brunner’s gland hyperplasia covers both diffuse hyperplasia (<1 cm) and polypoid lesions (> 1 cm) which are commonly known as Brunneroma. 3 Within this group we can differentiate between hamartoma and adenoma depending on whether or not they have mesenchymal elements. 2
Although the etiopathogenesis of these lesions has not been clearly established, several theories attempt to explain their formation. The hyperchlorhydria hypothesis is most commonly accepted. Overproduction of gastric acid may act as a stimulus for compensatory proliferation of Brunner’s glands, ultimately culminating in an BGH. 4 Other hypotheses place their origin in dysembryoplastic lesions, while others see them as an adaptation to local inflammatory processes such as H. pylori infections or chronic pancreatitis. 1,2
BGH appears predominantly in middle-aged patients without differences of gender distribution. 5 In general, primary tumors of the duodenum are rare and represent less than 1% of gastrointestinal tumors. BGH may have an incidence of 0.008% according to some studies. 5 However, it is difficult to establish its prevalence since most are asymptomatic lesions found incidentally in endoscopy, and only a small proportion are diagnosed clinically. 6-8
In general, larger polyps tend to have symptoms more frequently. The most common are nonspecific symptoms such as nausea, vomiting and chronic abdominal pain. Other less frequent symptoms are digestive hemorrhaging due to profuse vascularization and found as occult blood in feces, recurrent pancreatitis, obstructive jaundice, and even biliary fistulas. 6,9 Regarding potential malignancy, Sakurai et al. observed dysplasia in 2.1% and invasive carcinoma in 0.3% of the 722 cases of GBH they studied. 10
To establish the diagnosis of this entity, upper endoscopy within a biopsy is the first choice. Given the submucosal nature of these glands, histological specimens obtained by conventional biopsy are often negative or inconclusive. This makes deeper biopsy methods such as polypectomy biopsies necessary. They have higher diagnostic yields but are also associated with increased risk of complications such as bleeding and perforation. 9
When histology is inconclusive, echoendoscopy is the technique of choice for characterizing GBH. It allows determination of their origin and study of their vascularization. 1,3 Finally, some authors propose the use of contrast CT scans to establish both the extent and differential diagnosis with other entities such as adenomatous polyps, lipoma, leiomyoma, and gastrointestinal stromal tumors (GIST). These tools make it possible to plan an appropriate therapeutic approach that avoid sexcessively aggressive treatments of these lesions with low potential of malignancy. 2,3
There are no clinical guidelines for treatment or quality studies in this area. It is accepted that these lesions should be resected when they are larger than 2 cm and when they produce symptoms. 2 Treatment can be endoscopic or surgical, and each case must be individualized according to the characteristics of the patient, the lesion, and the experience of the medical center. So far there is no evidence of recurrence after removal by either method. In recent years there has been a strong tendency to use endoscopic treatment due to technical advances that have resulted in lower morbidity and mortality rates. Endoscopic techniques include resection with either a traditional polypectomy loop or an endoloop and submucosal endoscopic dissection. 2 Surgical techniques range from transduodenal polypectomy to pancreaticoduodenectomy.
Conclusion
The diagnosis of duodenal lesions such as BGH is usually made after histological study of biopsies obtained during endoscopy. This procedure has potential complications such as gastrointestinal bleeding due to the abundant vascular irrigation of BGH. When necessary, endoscopic or surgical treatment should be decided on an individual basis.
Referencias
1. Lu L, Li R, Zhang G, Zhao Z, Fu W, Li W. Brunner’s gland adenoma of duodenum: report of two cases. Int J Clin Exp Pathol. 2015;8(6):7565-9. [ Links ]
2. Sorleto M, Timmer-Stranghöner A, Wuttig H, Engelhard O. Brunner’s gland adenoma - A rare cause of gastrointestinal bleeding: case report and systematic review. Case Rep Gastroenterol. 2017;11(1):1-8. doi: https://doi.org/10.1159/000454711. [ Links ]
3. Peloso A, Viganò J, Vanoli A, Dominioni T, Zonta S, Bugada D, et al. Saving from unnecessary pancreaticoduodenectomy. Brunner’s gland hamartoma: case report on a rare duodenal lesion and exhaustive literature review. Ann Med Surg. 2017;17:43-9. doi: https://doi.org/10.1016/j.amsu.2017.03.034. [ Links ]
4. Franzin G, Musola R, Ghidini O, Manfrini C, Fratton A. Nodular hyperplasia of Brunner’s glands. Gastrointest Endosc. 1985;31(6):374-8. doi: https://doi.org/10.1016/S0016-5107(85)72251-1. [ Links ]
5. Levine JA, Burgart LJ, Batts KP, Wang KK. Brunner’s gland hamartomas: clinical presentation and pathological features of 27 cases. Am J Gastroenterol. 1995;90(2):290-4. [ Links ]
6. Van de Walle P, Dillemans B, Vandelanotte M, Proot L. The laparoscopic resection of a benign stromal tumour of the duodenum. Acta Chir Belg. 1997;97(3):127-9. [ Links ]
7. Ohba R, Otaka M, Jin M, Odashima M, Matsuhashi T, Horikawa Y, et al. Large Brunner’s gland hyperplasia treated with modified endoscopic submucosal dissection. Dig Dis Sci. 2006;52(1):170-2. doi: https://doi.org/10.1007/s10620-006-9607-1. [ Links ]
8. Botsford TW, Crowe P, Crocker DW. Tumours of the small intestine. A review of experience with 115 cases including a report of a rare case of malignant hemangio-endothelioma. Am J Surg. 1962;103:358-65. doi: https://doi.org/10.1016/0002-9610(62)90226-X. [ Links ]
9. Jung Y, Chung IK, Lee TH, Cho YS, Jo YG, Park SH, et al. Successful endoscopic resection of large pedunculated Brunner’s gland hamartoma causing gastrointestinal bleeding arising from the pylorus. Case Rep Gastroenterol . 2013;7(2):304-7. doi: https://doi.org/10.1159/000354138. [ Links ]
10. Sakurai T, Sakashita H, Honjo G, Kasyu I, Manabe T. Gastric foveolar metaplasia with dysplastic changes in Brunner gland hyperplasia: possible precursor lesions for Brunner gland adenocarcinoma. Am J Surg Pathol. 2005;29:1442-8. doi: https://doi.org/10.1097/01.pas.0000180449.15827.88. [ Links ]
Received: May 24, 2018; Accepted: July 01, 2018











 texto en
texto en 


