Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.35 no.1 Bogotá Jan./Mar. 2020
https://doi.org/10.22516/25007440.370
Original articles
Diagnostic concordance of abdominal CT scans, Endoscopic Ultrasonography and Fine Needle Puncture Aspiration for solid pancreatic lesions suggestive of malignant neoplasms
1Profesor Asociado de Medicina, Pontificia Universidad Javeriana, Gastroenterólogo, Hospital Universitario San Ignacio, Jefe Unidad de Endoscopia, Clínica de Marly, Bogotá, Colombia
2Profesor Asistente de Medicina, Pontificia Universidad Javeriana, Gastroenterólogo, Jefe Unidad de Gastroenterología, Hospital Universitario San Ignacio, Bogotá, Colombia
3Profesor asociado, Departamento de Medicina Interna, Pontificia Universidad Javeriana, Hospital Universitario San Ignacio, Bogotá, Colombia
4Gastroenteróloga, Hospital San Rafael, Bogotá, Colombia
5 Estudiante Facultad de Medicina, Pontificia Universidad Javeriana, Bogotá, Colombia
Introduction and objective:
Diagnostic tests for solid pancreatic lesions frequently produce discordant results which lead to confusion and delays of therapeutic decisions. Concordance among abdominal computed tomography with contrast, endoscopic ultrasound (EUS) pancreatobiliary and EUS guided fine needle aspiration had not previously been evaluated in Colombia.
Materials and methods:
We evaluated a series of adult patients with solid pancreatic masses suspected of malignancy treated at the San Ignacio University Hospital in Bogotá, Colombia. At least two of the following tests were performed: CT scans, EUS, and EUS guided fine needle aspiration. Results were defined as compatible with neoplasia, not compatible with neoplasia or inconclusive. Concordance of results was then evaluated.
Results:
Fifty-seven patients were included. A high percentage EUS results compatible with neoplasia were discordant with CT scan results and with EUS guided fine needle aspiration results (33.3% and 52.5%, respectively). Agreement between imaging and EUS guided fine needle aspiration results was minimal (Kappa 0.02; 95% CI:-0.04 to 0.08). The probability of detecting vascular compromises was significantly higher for EUS (42.1% vs. 23.8%, p: 0.06), but lymph node compromises were detected more frequently by imaging (CT or MRI) (23.8% vs. 7.1%, p: 0.01).
Conclusions:
The results of this study suggest poor agreement between these diagnostic methods implying a need for improvements such as elastography and contrast media, new needle modalities for sampling, and/or the an on-site cytopathologist.
Keywords: Epidemiology; Pancreatic malignancies; Biliopancreatic ultrasound; PAAF; Concordance studies
Introducción y objetivo:
la realización de pruebas para el diagnóstico de lesiones sólidas de páncreas conduce frecuentemente a resultados discordantes, lo que genera confusión y retraso en las decisiones terapéuticas. La concordancia entre los resultados de la tomografía axial computarizada de abdomen con contraste (TAC), la ultrasonografía endoscópica (USE) biliopancreática y la punción por aspiración con aguja fina guiada por ultrasonografía endoscópica (PAAF-USE) no ha sido evaluada en nuestro medio.
Materiales y métodos:
se evaluó una serie de pacientes adultos con masas sólidas del páncreas sospechosas de malignidad, atendidos en el Hospital Universitario San Ignacio (HUSI) de Bogotá (Colombia), en los cuales se realizaron, por lo menos, dos de las siguientes pruebas: TAC, USE o PAAF-USE. Se evaluó la concordancia de los resultados, definidos como compatibles con neoplasia, no compatibles con neoplasia o resultado no conclusivo.
Resultados:
se incluyeron 57 pacientes. Un alto porcentaje de estos, con USE compatible con neoplasia, tuvieron resultados discordantes con la TAC (33,3 %) y con la PAAF-USE (52,5 %). La concordancia entre imágenes y PAAF-USE fue mínima (kappa = 0,02; intervalo de confianza [IC] 95 %, 0,04-0,08). La probabilidad de detectar un compromiso vascular fue significativamente mayor en la USE (42,1 % frente a 23,8 %, p = 0,06) a diferencia del compromiso ganglionar, que fue detectado más frecuentemente por imágenes (TAC/resonancia magnética nuclear [RMN]) (23,8 % frente a 7,1 %, p = 0,01).
Conclusiones:
los resultados de este estudio sugieren un pobre acuerdo entre los diferentes métodos diagnósticos y advierten que es necesario implementar mejoras como la elastografía y medios de contraste, nuevas modalidades de aguja para la toma de muestras o la presencia de un citopatólogo in situ.
Palabras clave: Epidemiología; neoplasias pancreáticas; ultrasonografía biliopancreática; punción por aspiración con aguja fina (PAAF); estudios de concordancia
Introduction
Pancreatic cancer is an aggressive neoplasm whose prognosis is poor prognosis. Its incidence rate is 8-10 cases per 100,000 inhabitants per year, and it is the second most frequent gastrointestinal malignancy and the fourth leading cause of cancer death among adults. Aggravating circumstance make it one of the most difficult malignancies to treat especially since it produces very few symptoms in initial stages and diagnosis is usually late leaving little chance of cure. 1
Despite many advances in the last decade, there are few treatment options in advanced stages, and 5-year survival rates are less than 5%. 2 Nevertheless, surgery can extend survival time of a significant proportion of patients if tumors are diagnosed at an early stage. 3
Detection of pancreatic cancers when they are small undoubtedly offers better prognoses than those detected when they are large, so early detection is a necessary strategy for reducing high mortality rates. Imaging techniques such as computed tomography (CT) scans, magnetic resonance imaging (MRI) and endoscopic ultrasound (EUS) are the best tools for diagnosing these lesions. Today, they are considered essential for proper study of solid lesions of the pancreas to define whether they are malignant or benign.
The high image resolution of EUS is particularly useful for detecting small pancreatic lesions that other modalities can miss, especially solid lesions that measure less than 2 cm. Consequently, EUS is currently considered a cornerstone that should be performed in practically every patient with obstructive jaundice of probable neoplastic etiology for whom (CT) and/or MRI do not identify a pancreatic lesion that explains the etiology. Furthermore, EUS guided fine needle aspiration puncture (EUS-FNA) can provide samples of these difficult to identify lesions allowing histological diagnosis. 4
Nevertheless, evaluation of the operational characteristics of tests available for diagnosis of pancreatic masses is a challenge because there is no definitive reference standard. Normally, researchers have a standard reference outcome that takes into account tissue samples from complete removals of tumors, clinical evolution of patients who have undergone surgery, and patients whose tumors progressed until they caused mortality.
Currently, there are several diagnostic methods for characterizing solid neoplastic lesions of the pancreas, but none can be considered the unique reference standard. The initial test of choice is usually an abdominal CT scan or MRI. Both are reported to have a sensitivity and specificity for pancreatic cancer between 95% and 96%. 5-8
Multiplanar CT reconstruction is important for tumor staging since it provides selective views of important arterial and venous structures which allows accurate visualization of the relationship of the primary tumor to the superior mesenteric artery (AMS), superior mesenteric vein (VMS), and celiac trunk thus aiding assessment of vascular invasion and resectability.
A CT scan can distinguish compression, invasion, narrowing, or occlusion of the portal vein and VMS at the confluence and allow a surgeon to determine whether venous reconstruction is technically feasible. Still, the precision of CT scans for evaluation of vascular invasion is not strong, and recent studies show its sensitivity to be only 60% even though its specificity is 94%. 9 CT scans also play important roles for indicating whether or not a tumor is resectable. If a tumor surrounds more than 180 degrees of a vessel, and the VMS or portal vein is occluded, there are no surgical options for reconstruction. Recent studies have shown that CT scans’ sensitivity for unresectable disease is between 52% and 91% while their specificity ranges from 92% to 100%.
MRI has been shown to be just as sensitive and specific for diagnosis and staging of pancreatic cancer as CT scans. Their sensitivity is 88%, and their specificity is 89%. MRI is not widely used as the primary imaging modality in most centers because of cost and availability limits and the fact that it offers no diagnostic advantages over CT scans.
Imaging results frequently indicate performance of biliopancreatic EUS to improve characterization of the images and to sample the lesion. EUS is performed under sedation and involves endoscopic examination of the upper gastrointestinal tract using a radial or linear echoendoscope.
The echoendoscope transducer is placed in the duodenum or stomach in direct proximity to the pancreas. It produces detailed high-resolution images of surrounding vessels, lymph nodes, and the left lobe of the liver. In addition, it facilitates performance of fine needle aspiration (FNA) to obtain a sample for cytopathological diagnosis.
As mentioned, EUS is particularly ideal for lesions measuring less than two cm and for obtaining histological confirmation when there is clinical suspicion of pancreatic cancer which other modalities have failed to identify (Figures 1A and 1B). EUS is considered the most sensitive imaging technique for characterization of pancreatic tumors. The literature reports that it detects 89% to 100% of adenocarcinomas of the pancreas. 5-7
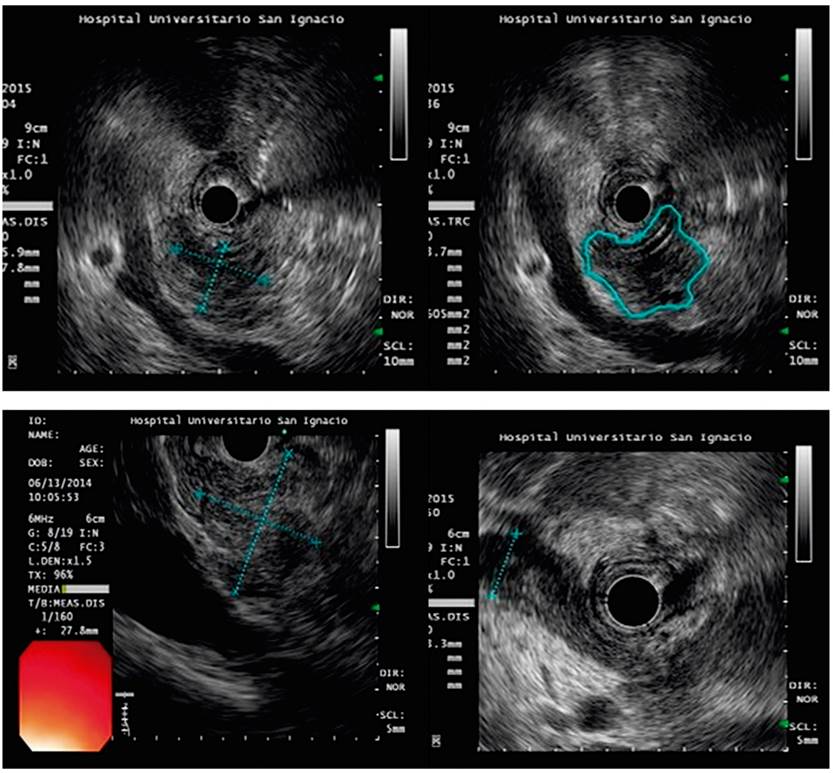
Figure 1 EUS images for diagnosis of solid lesions suggestive of pancreatic malignancies. A. Neoplastic, hypoechoic lesion on the head of the pancreas. B. Irregular hypoechoic image suspected of neoplasm. FNA sample tested negative for malignancy. Not every solid mass of the pancreas is a malignancy. FNA sample showing adenocarcinoma of the pancreas.
Despite the great sensitivity of EUS, it is difficult to differentiate adenocarcinoma of the pancreas from other types of solid lesions using only endosonographic characteristics. 7,10 Use of elastography and contrasts has allowed characterization of patterns that improve the visual sensitivity of the images. They also help with orientation for taking a biopsy to obtain tissue for histological confirmation of lesions which is essential for deciding on therapeutic measures.
EUS-FNA is a safe and useful technique for obtaining material for cytological study. In some cases, it can be used to take tissue extraction biopsies which allow histopathological study. 10,11 EUS-FNA has become the standard method for obtaining tissue samples from pancreatic lesions and fortunately has low risk of complications and tumor seeding. 12
Metaanalysis data suggest that the sensitivity of EUS-FNA for diagnosing pancreatic cancer is 85% to 92% while its specificity is 96% to 98%. However, obtaining representative and sufficient samples for diagnosis poses multiple challenges for EUS-FNA. The use of an adequate technique, the experience of the operator, the number of samples, and the presence of an on-site cytopathologist (almost never available in medical centers) are required. In addition, interpretation of histopathological findings can be difficult even for trained pathologists.
As we can see, multiple limitations condition performance of EUS and EUS-FNA. In some medical centers, specificity only reaches 88.6%. This is especially true when in-situ cytopathological study is not available. 13
Radiological methods are also not infallible, and their performance is very poor performance when the lesion is smaller than two cm. This occurs because small lesions may not be apparent, and this has an impact on concordance figures between radiological examinations, EUS, and EUS-FNA.
In summary, evaluation of pancreatic masses is especially difficult and constitutes a diagnostic challenge especially considering that not every solid lesion of the pancreas is an adenocarcinoma. It is also necessary to understand that differential diagnosis includes neoplasms of clinical relevance that suppose a different approach to treatment.
These conditions include carcinoid tumors, lymphomas, and metastases. This adds to the importance of ruling out benign neoplasm-like lesions such as inflammatory lesions due to chronic pancreatitis, pancreatic pseudotumors, and autoimmune pancreatitis with focal compromise. Diagnosis of one of these lesions totally changes the therapeutic approach since they are managed without surgery. Correct diagnosis can avoid morbidity and unnecessary surgery.
In clinical practice, we very often observe that several diagnostic tests are necessary before a conclusion can be made. This reflects the difficulty of establishing a definitive diagnosis of a solid pancreatic lesion. Multiple diagnostic tests in these patients frequently leads to different and even discordant results. This can generate confusion about therapeutic behaviors and distort the patient’s perception of the disease. In addition, it can contribute to worsening the prognosis through therapeutic delay further deepened by the difficulties of our health care system.
Thus, the objective of this study was to evaluate concordance among abdominal CT scans, MRI, EUS, and EUS-FNA to determine whether their results are clinically equivalent for diagnosis of solid pancreatic lesions suggestive of adenocarcinoma. The study was conducted in a group of patients managed at the Hospital Universitario San Ignacio (HUSI).
Materials and methods
This study of diagnostic tests was based on identification of patients with solid pancreatic lesions who were treated at HUSI between January 2014 and September 2017. Older individuals with solid lesions suggestive of malignancy were included when results of at least two of the following tests were available: a CT scan, MRI, biliopancreatic EUS and EUS-FNA.
Patients with low-quality images that were not suitable for institutional reading were excluded. The study had the approval of the institution’s Ethics and Research Committee. Data included patients’ demographic characteristics, medical histories, indications for use of a diagnostic method and were obtained from the the MEDICAP database and the electronic medical record of the HUSI Gastroenterology Unit.
To guide performance of a CT scan, MRI or EUS, information about characteristics, location and size of the lesion was obtained together with information about presence of lymph nodes and vascular or metastatic compromises, choledochal or pancreatic duct dilation, peripancreatic fat infiltration, and conclusions of the interpretation.
CT scans and MRI were performed by members of the radiology service, while EUS was performed by a gastroenterologist with formal training in this technique. An expert cytopathologist evaluated EUS-FNA samples. Honeycomb patterns, irregularities of the nuclear membrane, prominent nucleolus in the absence of inflammation and clearance of perinuclear chromatin were recorded.
Continuous variables were expressed as means. Standard deviation was used as the measure of dispersion for variables with normal distributions while medians and interquartile ranges (IQR) were used for other variables. Categorical variables were expressed as percentages.
The consistency approximation approach was used to establish agreement between and among conclusions based on images, EUS and analysis of EUS-FNA samples. The approach assumes that none of the tests are the reference standard. 14 This approach allows evaluation of whether results from different tests are equivalent to each other but does not assume that the results of one or another are correct and therefore does not allow determination of which test is best.
The conclusions of each test were categorized as compatible with malignancy, not compatible with malignancy, or inconclusive. Concordance of findings of lymph nodes and vascular compromises was analyzed for CT scans, MRI and EUS. Whether or not any compromise was present was recorded.
An unweighted kappa statistic was used for evaluation, and an alpha (α) significance level of 0.05 was set. Calculations were done with Stata. 15
Results
We identified 137 patients with pancreatic masses suspected of malignancy. Of these, 57 met the inclusion criteria. Demographic characteristics of the population are presented in Table 1. The median age was 64 years (IQR = 42 to 83) while the most frequent clinical indication for studies were jaundice (37%) and abdominal pain (35%). The most common comorbidity was chronic pancreatitis (64%). All patients included in the study had undergone biliopancreatic EUS, forty-two (74%) had diagnostic radiological images available, and forty (70%) had pathology reports with samples suitable for reading. It should be noted that 25 patients (44%) had all three diagnostic tests available simultaneously (Figure 2).
Table 1 Demographic characteristics of study patients
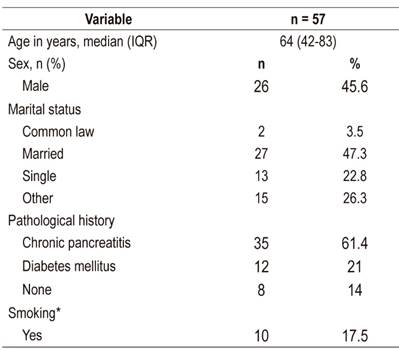
IQR: interquartile range; *smoking defined as active tobacco use at diagnosis.
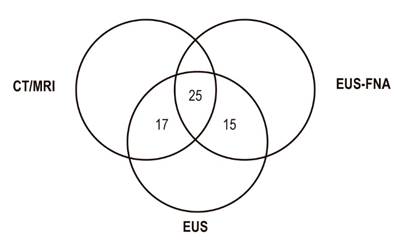
Figure 2 Availability of diagnostic tests for patients included. CT: computerized axial tomography; MRI: magnetic resonance imaging; EUS- FNA: endoscopic ultrasound guided fine needle aspiration, EUS: endoscopic ultrasound
According to EUS findings, most lesions (59%) were larger than 3 cm in size and were mostly located in the head of the pancreas (93%). A significant percentage of the patients had common bile duct and pancreatic duct compromises according to both EUS and radiological imaging (38% and 45%, respectively).
EUS identified masses in 10 cases that were not detected by radiological images. Most of these lesions were located in the head of the pancreas. The probability of finding a vascular compromise was significantly higher for EUS (42.1% vs. 23.8%, p = 0.06), but imaging (CT/MRI) detected a higher proportion of patients with compromised lymph nodes (23.8% vs. 7%, p = 0.01). The characteristics of the lesions found by CT scans and biliopancreatic EUS are presented below (Table 2).
Table 2 Characteristics of lesions found by imaging and EUS
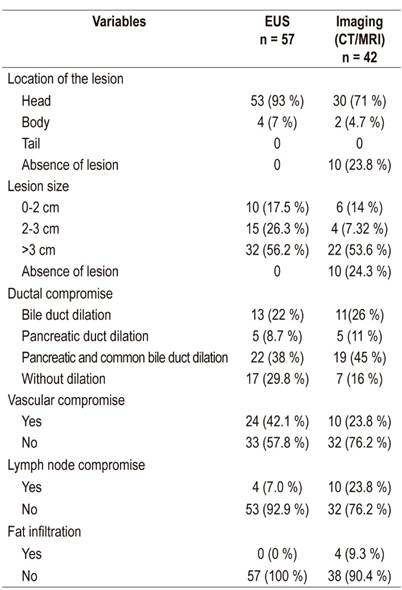
CT: computerized axial tomography; MRI: magnetic resonance imaging
Of the 40 samples suitable for reading, only 47.5% (n = 19) were compatible with a neoplastic lesion. The characteristic most frequently found in EUS-FNA readings was chromatin clearance (57.5%). The histological findings of the samples taken by EUS-FNA are described below (Table 3).
Table 3 Histological findings from samples taken by EUS-FNA.
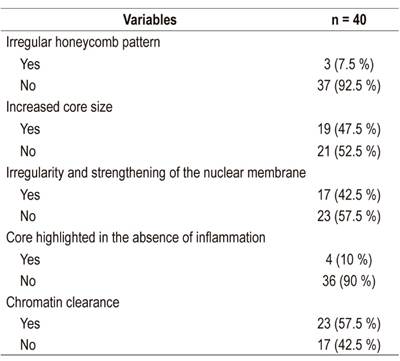
FNA: fine needle aspiration; EUS: endoscopic ultrasound
According to EUS results, all patients (n = 57) were compatible with neoplasms of the pancreas, unlike imaging and FNA results in which only 66.7% and 47.5% were compatible, respectively. The conclusions of the different diagnostic tests are presented below. They are categorized as compatible with neoplasm of the pancreas, not compatible with neoplasm or inconclusive (Table 4).
Table 4 Classification of lesions according to imaging. EUS and FNA results
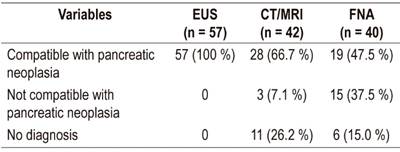
FNA: fine needle aspiration puncture; EUS: endoscopic ultrasound; MRI: magnetic resonance imaging
The kappa coefficient of agreement between EUS and imaging for classification of lesions according to the three categories described could not be evaluated since all pancreatic lesions were interpreted as compatible with neoplasia by EUS. Of the 42 patients for whom both CT and MRI images were available, 28 were be compatible with malignancy, three were not compatible with malignancy, and 11 had inconclusive results.
Similarly, 40 patients simultaneously had EUS and EUS-FNA. The tests were interpreted in EUS as pancreatic lesions compatible with neoplasia. Of these, nineteen (47.5%) had FNA sample test findings compatible with neoplasia, while 15 were not compatible with malignancy, and 6 were inconclusive (Table 4).
Table 5 shows the evaluation of concordance between radiological image results and EUS-FNA biopsy results for 25 patients for whom the results of the two diagnostic tests were available. The kappa coefficient was 0.02 (95% confidence interval [CI]: −0.04 to 0.08). Fourteen cases had discordant results.
Table 5 Agreement between imaging and FNA biopsy results for diagnosis of pancreatic masses
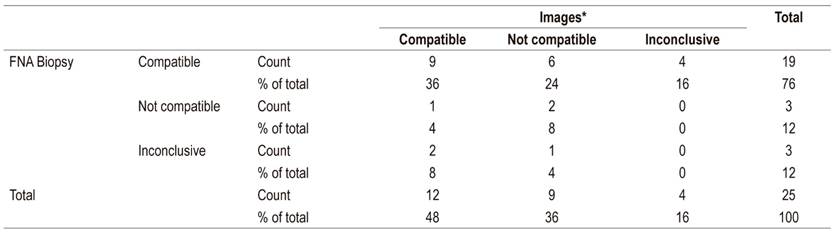
FNA: fine needle aspiration; *Images understood as abdominal CT scans or MRI
Concordance between images and EUS was moderate for vascular compromises (kappa = 0.4240; 95% CI: 0.3570-0.4920) while it was low for lymph node compromises (kappa = 0.2199). ; 95% CI: 0.1599-0.2799).
Discussion
The results of this study suggest poor agreement between the different methods used to diagnose lesions of the pancreas suggestive of malignancy. Despite the fact that all the patients included in our study presented lesions suggestive of neoplasia when observed by EUS, FNA only documented neoplasms in 47.5% while imaging only documented neoplasms in 66.7%. Similarly, agreement between FNA sample findings and diagnostic images was minimal.
It is important to highlight that the majority of our patients had lesions that measured more than two cm at the moment of diagnosis and that these lesions already had a metastatic involvement over 3 cm plus dilation of the pancreatic duct and common bile duct. Other authors have reported similar findings which suggests that the majority of cases are detected in advanced stages. 14
This is the first study that has evaluated concordance between and among the three most currently accepted methods for evaluation of solid pancreatic lesions in Colombian institutions. For this study, we classified the biliopancreatic endosonography images as solid lesions and suggestive of pancreatic adenocarcinoma, according to the criteria established for EUS by Gonçalves et al. 15 We classified CT and MR images as lesions and suggestive of pancreatic adenocarcinoma according to the criteria established by Low et al. 16
Contrary to expectations, location findings for a high percentage of cases were not concordant (kappa = 0.03). This may be secondary to the presence of lesions smaller than one cm which are not clearly detectable by CT or MRI as demonstrated by the studies carried out by Sakamoto et al. 3 We highlight that solid lesions were not identified by images in 10 patients did but were located and characterized with EUS in these patients. This shows the high yield of this test for detecting lesions smaller than two cm.
A recent metaanalysis has found that CT scans are less sensitive than EUS for lymph node staging (24% vs. 58%) and vascular invasions (58% vs. 86%). However, their specificities for lymph node staging (88% vs. 85%) and vascular invasion (95% vs. 93%) were comparable in studies in which both imaging techniques were used.
Our study’s results demonstrate that CT scans and MRI can identify infiltration of peripancreatic fat and nodular compromises in the majority of cases. This suggests that even though concordance between imaging (CT/MRI) and EUS is low, they offer complementary information which may justify performing both.
Thus, our findings suggest that a high percentage of patients whose EUS findings are compatible with neoplasia do not present histological results compatible with this diagnosis. This may be due to factors such as sample quantity and quality, absence of a pathologist in the room to verify adequate cell content in the analysis, and the long slope of the learning curve for interpretation of cytology as warned by Holt et al. 14
EUS-FNA is successful, and EUS is the preferred imaging technique for guiding sample collection. However, a large number of factors influence the diagnostic yields of pancreatic lesion cytology. They include needle size, tissue blocks obtained against lamellae, and the use of Rapid On-Site Evaluation (ROSE) for preliminary analysis of a sample. Also, the sample must be guaranteed to be representative.
All of these factors remain unstandardized and basically depend on local preferences and feasibility. It seems clear that lack of homogeneity is likely to be part of the explanation of the great variability of the diagnostic yield of FNA of the pancreas reported in the literature. Diagnostic yield tends to be less than 80%.
It also seems clear that the use of large needles (19 G) that produce larger tissue samples significantly increases the diagnostic performance of FNA. The same is true for rapid on-site evaluation of material by expert cytopathologists. However, the use of this test continues to be frustrating in daily clinical practice in many centers around the world. 17
Despite the fact that our results were below expectations, they are not surprising. They demonstrate the reality of many medical centers at the beginning of the path to excellence in the study of biliopancreatic diseases. We used 19, 22 and 25 G needles for fine needle aspiration in our patients. We used a suction technique with release of negative pressure before removing the needle from the target, and we also slowly removed the stylet (pull) as recommended by the guidelines of the European Society for Gynecological Endoscopy (ESGE) for the study of solid masses of the pancreas. 18
We highlight the importance of the long learning curve for EUS and use of the new needles available for ultrasound guided sampling of the pancreas. We also highlight the need for interdisciplinary study of solid lesions of the pancreas that allows broad communication among gastroenterology, pathology, radiology, and surgery groups.
Of all these issues, perhaps the ones that have had the greatest impact are development of different needles and development of complementary methods for ultrasound images. Development of reverse bevel design needles (ProCore, Cook Medical, Bloomington, Indiana) has led to better histological evaluation (81.1% vs. 69.4%, p = 0.048) with fewer passes required for diagnosis than required with standard FNA needles.
In addition, Franseen tip (Acquire, Boston Scientific Corp, Natick, Massachusetts, USA) and hairpin tip (SharkCore, Medtronic, Minneapolis, Minnesota, USA) needles can provide larger samples than those obtained with a standard needle. Indeed, the performance of both is comparable to the performance observed from tissue histology.
On the other hand, conventional EUS diagnosis of pancreatic lesions is limited by the fact that most solid lesions manifest as hypoechoic masses which makes it difficult to differentiate between benign and malignant masses. Vascularization assessment can improve characterization of digestive lesions. It can be accomplished by using the recently developed contrast-enhanced harmonic endoscopic ultrasound (CH-EUS).
CH-EUS is likely to play a greater role in the differential diagnosis of biliopancreatic lesions, and CH-EUS could become a useful alternative tool for evaluation of pathologies that involve tumor angiogenesis. 19
Meanwhile, the overall accuracy of FNA for diagnosis of pancreatic adenocarcinoma is approximately 85%. The use of contrast agents during EUS to highlight vascularization and necrotic parts of pancreatic masses can improve the orientation of the biopsy. 20
Our findings imply pathology results should not be assumed to be the diagnostic reference method for adenocarcinoma of the pancreas since there may be a significant percentage of false negatives as demonstrated in the world literature. In other words, pathology results can generate false conclusions and contribute to late diagnosis in a significant percentage of cases which allows progression of disease and to the point that patients may not be operable.
Thus, the reference method for the evaluation of diagnostic tests of pancreatic masses should consider not only a pathology report but should also involve epidemiology, clinical data, evolution and progression of patients, and the sum complementary diagnostic methods.
The high probability of losing complementary information when tomography is used alone without ultrasound and biopsy is worth emphasizing. We consider that it is not possible to elaborate a therapeutic strategy based solely on the results of a CT scan or MRI.
Our study’s limitations include the fact that not all patients had all three diagnostic tests available. This reflects what happens in the real life management and diagnosis of these patients. Limitations of the health care system and access to diagnostic methods do not allow adequate follow-up. We decided to include comparisons of the results of each pair of diagnostic tests considering that a greater amount of information improves precision of estimation of agreement between and among them.
Despite limitations, our data suggests that imaging results from CT scans and MRI cannot be interpreted as clinically equivalent to EUS, and EUS-FNA. Except for exceptional cases, treatment should not be based on the results of only one of these tests. Combined testing is necessary to make more reliable assessments of patients with this type of lesion. In addition, other studies are needed to compare diagnostic tests with a reference standard that includes clinical monitoring over time of patients.
Thus, our results suggest that there is a space for new tools such as elastography and contrast media to improve the diagnostic sensitivity of EUS. In addition, the various modalities of aspiration and puncturing to obtain tissues should be incorporate into practice to improve biopsy performance. Together with the need to have an on-site cytopathologist for immediate analysis of samples obtained, these measures can improve our diagnostic yields significantly.
The availability of this resource is profitable since accuracy of 87% is achieved with only 2.1 passes, compared to four passes required when real-time evaluation of samples is not available.
Conclusions
The results of this study show poor agreement between and among the diagnostic methods used for solid pancreatic lesions. Similarly, the data indicate a need to improve diagnosis of these lesions. The data continues to be difficult to interpret and generates therapeutic uncertainty. Nevertheless, the decision to perform surgery should not be delayed if there is a high clinical suspicion of pancreatic cancer, even though the definition of high clinical suspicion of pancreatic cancer is still unclear.
REFERENCES
1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225-49. http://dx.doi.org/10.3322/caac.20006 [ Links ]
2. Kleeff J, Michalski C, Friess H, Büchler MW. Pancreatic cancer: from bench to 5-year survival. Pancreas. 2006;33(2):111-8. https://doi.org/10.1097/01.mpa.0000229010.62538.f2 [ Links ]
3. Helmstaedter L, Riemann JF. Pancreatic cancer--EUS and early diagnosis. Langenbecks Arch Surg. 2008;393(6):923-7. https://doi.org/10.1007/s00423-007-0275-1 [ Links ]
4. Yoshida T, Yamashita Y, Kitano M. Endoscopic Ultrasound for Early Diagnosis of Pancreatic Cancer. Diagnostics (Basel). 2019;9(3). pii: E81. https://doi.org/10.3390/diagnostics9030081 [ Links ]
5. Sakamoto H, Kitano M, Suetomi Y, Maekawa K, Takeyama Y, Kudo M. Utility of contrast-enhanced endoscopic ultrasonography for diagnosis of small pancreatic carcinomas. Ultrasound Med Biol. 2008;34(4):525-32. https://doi.org/10.1016/j.ultrasmedbio.2007.09.018 [ Links ]
6. Eloubeidi MA, Chen VK, Eltoum IA, Jhala D, Chhieng DC, Jhala N, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy of patients with suspected pancreatic cancer: diagnostic accuracy and acute and 30-day complications. Am J Gastroenterol. 2003;98(12):2663-8. https://doi.org/10.1111/j.1572-0241.2003.08666.x [ Links ]
7. Brand B, Pfaff T, Binmoeller KF, Sriram PV, Fritscher-Ravens A, Knöfel WT, et al. Endoscopic ultrasound for differential diagnosis of focal pancreatic lesions, confirmed by surgery. Scand J Gastroenterol. 2000;35(11):1221-8. https://doi.org/10.1080/003655200750056736 [ Links ]
8. Hewitt MJ, McPhail MJ, Possamai L, Dhar A, Vlavianos P, Monahan KJ. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012;75(2):319-31. https://doi.org/10.1016/j.gie.2011.08.049 [ Links ]
9. Zhang L, Sanagapalli S, Stoita A. Challenges in diagnosis of pancreatic cancer. World J Gastroenterol. 2018;24(19):2047-2060. https://doi.org/10.3748/wjg.v24.i19.2047 [ Links ]
10. Itoi T, Tsuchiya T, Itokawa F, Sofuni A, Kurihara T, Tsuji S, et al. Histological diagnosis by EUS-guided fine-needle aspiration biopsy in pancreatic solid masses without on-site cytopathologist: a single-center experience. Dig Endosc. 2011;23 Suppl 1:34-8. https://doi.org/10.1111/j.1443-1661.2011.01142.x [ Links ]
11. Erturk SM, Mortelé KJ, Tuncali K, Saltzman JR, Lao R, Silverman SG. Fine-needle aspiration biopsy of solid pancreatic masses: comparison of CT and endoscopic sonography guidance. AJR Am J Roentgenol. 2006;187(6):1531-5. https://doi.org/10.2214/AJR.05.1657 [ Links ]
12. Stella SF, Van Borsel M, Markose G, Nair SB. Image-Guided Percutaneous Biopsy for Pancreatic Lesions: 10-Year Experience in a Tertiary Cancer Center. Can Assoc Radiol J. 2019;70(2):199-203. https://doi.org/10.1016/j.carj.2018.10.014 [ Links ]
13. Hébert-Magee S, Bae S, Varadarajulu S, Ramesh J, Frost AR, Eloubeidi MA, et al. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: a meta-analysis. Cytopathology. 2013;24(3):159-71. https://doi.org/10.1111/cyt.12071 [ Links ]
14. Holt BA, Varadarajulu S, Hébert-Magee S. High-quality endoscopic ultrasound-guided fine needle aspiration tissue acquisition. Adv Ther. 2014;31(7):696-707. https://doi.org/10.1007/s12325-014-0129-5 [ Links ]
15. Gonçalves B, Soares JB, Bastos P. Endoscopic Ultrasound in the Diagnosis and Staging of Pancreatic Cancer. GE Port J Gastroenterol. 2015;22(4):161-171. https://doi.org/10.1016/j.jpge.2015.04.007 [ Links ]
16. Low G, Panu A, Millo N, Leen E. Multimodality imaging of neoplastic and nonneoplastic solid lesions of the pancreas. Radiographics. 2011;31(4):993-1015. https://doi.org/10.1148/rg.314105731 [ Links ]
17. Ayres LR, Kmiotek EK, Lam E, Telford JJ. A Comparison of Endoscopic Ultrasound-Guided Fine-Needle Aspiration and Fine-Needle Biopsy in the Diagnosis of Solid Pancreatic Lesions. Can J Gastroenterol Hepatol. 2018;2018:1415062. https://doi.org/10.1155/2018/1415062 [ Links ]
18. Polkowski M, Jenssen C, Kaye P, Carrara S, Deprez P, Gines A, et al. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline-March 2017. Endoscopy. 2017;49(10):989-1006. https://doi.org/10.1055/s-0043-119219 [ Links ]
19. Kitano M, Yamashita Y. New Imaging Techniques for Endoscopic Ultrasonography: Contrast-Enhanced Endoscopic Ultrasonography. Gastrointest Endosc Clin N Am. 2017;27(4):569-583. https://doi.org/10.1016/j.giec.2017.06.002 [ Links ]
20. Seicean A, Jinga M. Harmonic contrast-enhanced endoscopic ultrasound fine-needle aspiration: Fact or fiction? Endosc Ultrasound. 2017;6(1):31-36. https://doi.org/10.4103/2303-9027.196917 [ Links ]
Ethical Responsibilities The authors declare that no human or animal experiments were performed for this research
Received: February 25, 2019; Accepted: June 27, 2019











 text in
text in 


