Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.35 no.1 Bogotá ene./mar. 2020
https://doi.org/10.22516/25007440.405
Original articles
Expert recommendations for diagnosis and treatment of cow’s milk protein allergy in the Colombian pediatric population
1Pediatra y nutrióloga pediatra. Facultad de Medicina, Universidad El Bosque, Instituto de Investigación en Nutrición, Genética y Metabolismo, Bogotá, Colombia
2Pediatra alergólogo clínico. Profesor en la Facultad de Medicina, Universidad de Antioquia, Medellín, Colombia
3Gastroenteróloga infantil. Gastroenterología, hepatología y nutrición Pediátrica, Hospital Pablo Tobón Uribe, Medellín, Colombia
4Gastroenteróloga pediatra, Hospital San Ignacio de Bogotá, Colombia
5Pediatra, máster en Nutrición Infantil. Clínica Iberoamericana de Barranquilla, Colombia
6Gastroenterólogo pediatra y nutriólogo. Máster en Epidemiología. Profesor asociado en la Universidad del Rosario, Bogotá, Colombia
7Gastroenterólogo pediatra. Universidad del Bosque. Gastricare S. A. S., Gerente general, Bogotá, Colombia
8Gastroenterólogo pediatra. Sura EPS, Clínica Esimed de Medellín. Pediatra de cuidado intensivo neonatal. Medellín, Colombia
9Gastroenteróloga pediatra, Hospital San Vicente Fundación. Medellín, Colombia
10Gastroenterólogo pediatra. Máster en Ciencias de la Nutrición. Epidemiólogo clínico, Hospital Universitario Fundación Santa Fe de Bogotá; Universidad de los Andes, grupo de investigación: PediAFe. Bogotá, Colombia
Objective:
The objective of this paper is to develop and present recommendations for diagnosis and treatment of Cow’s Milk Protein Allergy (CMPA) which can serve as a reference for pediatric and primary care physicians to consult.
Materials and methods:
This expert position document was developed by a group of doctors who are specialists in several therapeutic areas who have experience in CMPA. The most relevant topics were defined and a review of the available scientific literature was carried out to prepare a proposal for recommendations that was then discussed by the authors.
Results:
A position paper was developed that proposes a practical approach to definition, diagnosis and treatment of CMPA in pediatric patients.
Conclusions:
Early diagnosis and proper management of CMPA can help decrease the burden of this disease and its complications.
Keywords: Cow’s milk protein allergy (CMPA); atopy; breastfeeding; infant formulas; hydrolyzed formulas; atopic dermatitis; infants
Objetivo:
generar recomendaciones sobre el diagnóstico y el tratamiento de la alergia a la proteína de la leche de vaca (APLV), que sirvan de referencia y consulta para los médicos pediatras y de cuidado primario.
Materiales y métodos:
el presente documento de posición de expertos fue desarrollado por un grupo de médicos, especialistas en diferentes áreas terapéuticas y con experiencia en APLV. Se definieron los temas más relevantes y se realizó una revisión de la literatura científica disponible, a fin de elaborar una propuesta de recomendaciones que fue discutida por los autores.
Resultados:
se elaboró un documento de posición que propone un enfoque práctico sobre la definición, el diagnóstico y el tratamiento de la APLV en el paciente pediátrico.
Conclusiones:
el diagnóstico temprano y el manejo adecuado de la APLV pueden contribuir a una disminución de la carga de esta enfermedad y sus complicaciones.
Palabras clave: Alergia a la proteína de la leche de vaca (APLV); atopia; lactancia materna; fórmulas para lactantes; fórmulas hidrolizadas; dermatitis atópica; lactantes
Introduction
Cow’s milk protein allergy (CMPA) is a clinical condition whose origin is immunological origin. It begins at an early age and is the most frequent food allergy from the first months of life through preschool ages. During the first year of life, cow’s milk protein (CMP) is usually the first antigen faced by children fed breast milk or infant formulas. 1,2
CMPA has become an important health problem due to its clinical manifestations and their consequences. It is likely that CMPA is associated with development of other types of allergies such as asthma, atopic dermatitis, and rhinitis. 1,2
The incidence and prevalence of CMPA is difficult to establish, validate, and compare, given factors such as the small number of epidemiological studies, deficiencies or inconsistencies in study designs, and lack of standardization in methods of diagnosis (self-reporting, challenge tests, levels of specific immunoglobulin E [IgE], skin prick tests, and others). Also, the data reported often only relates to rapid reactions and does not include late hypersensitive reactions.
Furthermore, much available data relates to food allergies other than CMPA. Indeed, reports resulting from surveys (self-reporting) may include erroneous diagnoses (intolerance) and simply report children’s dislikes. In addition, manifestations of CMPA are not necessarily gastrointestinal.
According to EuroPrevall, a European study, the incidence of CMPA is 1% to 4%, but it decreases to 1% to 2% in more specific studies. 3 In the United States of America, reviews published in 2007 and 2010 found that CMPA’s prevalence was 3%. These results are not from specific studies such as skin prick tests or CMPA challenges which report prevalences between 0.6% and 1.0%. In general, the prevalence may be approximately 1%. 4,5
Specific reports of the CMPA in Latin America are scarce. According to figures reported by Ávila Castañón et al., the prevalence of milk allergy determined by skin prick tests in patients from the Hospital Infantil de México was 7.7%. In Chile, Martínez reported CMPA prevalences between 2.5 and 7.1% (skin prick test). Reported prevalences depend on the type of study (survey, skin prick test, specific IgE, radioallergosorbent test [RAST]). 6,7
Epidemiological information on CMPA in Colombia is scarce. According to survey results published by Marrugo, the prevalence of CMPA in children aged one to eight years is 2.5%, while that for children between 9 and 16 years old is 11.1%. 8 According to Acevedo, the prevalence of CMPA in children under two years of age is 1.8%. 9 The results of these studies cannot be considered to conclusively represent the Colombian population in general. 8-12
Gastrointestinal manifestations of CMPA are not specific and may be confused with those of gastroesophageal reflux disease (GERD), functional gastrointestinal disorders, lactose intolerance, and food reactions of non-allergic origin. This scenario results in a high probability of over diagnosis or underdiagnosis.
Consequently, early and correct diagnosis of CMPA is a challenge that makes it more difficult for physicians to establish timely and appropriate management of the disease, improve prognoses of patients and minimize CMPA’s burden. In response to these situations, a group of doctors with experience and knowledge regarding CMPA met to develop this position paper. It proposes a practical approach to definition, diagnosis and treatment of CMPA in pediatric patients.
Materials and methods
The recommendations in this document are based on our review of the best quality scientific literature available on CMPA and on the experience of a group of medical specialists in various areas of pediatrics (allergists, gastroenterologists, nutritionists and clinical epidemiologists), from different regions of Colombia. After reviewing the literature, the experts met, recommendations were discussed, and agreement was reached.
Recommendations
Definitions
Atopy is an individual’s genetic predisposition to producing high amounts of IgE before specific antigens. 1,13
Food intolerance is an adverse reaction to a type of food that is not related to an immune mechanism. Lactose intolerance is an example. It is not immunologically mediated but is caused by functional deficiency of the enzyme lactase (β-galactosidase) which results in incomplete absorption of lactose. 1,13
An allergy is a hypersensitivity reaction mediated by specific immunological mechanisms. In most children, this condition is mediated by IgE. Its phenotypic expression is atopy with or without atopic eczema, allergic rhinitis, or asthma. Other children have gastrointestinal manifestations not mediated by IgE (cell mediated). These are frequent symptoms of the disease. 14
Cow’s milk protein allergy (CMPA) is a hypersensitivity reaction mediated by specific and reproducible immunological mechanisms. It causes adverse clinical manifestations. The immune reaction can be IgE-mediated, not IgE-mediated, or mixed. It is an allergy to epitopes of protein origin. 1,13,14
Clinical diagnosis
Risk Factors
Risk factors associated onset of CMPA include a mother who smokes, atopic parents and/or siblings, cesarean birth, milk formula in the first days of life, breastfeeding for less than 3 months, a mother over the age of 30, prematurity, and hyperbilirubinemia (Table 1). 1,15-20
Clinical Manifestations
Clinical manifestations of CMPA can occur at any age. The following factors should be investigated in patients suspected of CMPA. (1,14,21)
Immediate IgE-mediated symptoms
Immediate IgE-mediated symptoms develop within minutes of exposure to an allergen. Their severity ranges from mild to severe, and they can involve the skin (urticaria, angioedema), the gastrointestinal tract (vomiting, enterocolitis), the respiratory system (wheezing, stridor, dyspnea) and the cardiovascular system (Table 2). In young infants, symptoms may be nonspecific including paleness and adynamia. 22-24
Anaphylaxis, an immediate symptom defined as a systemic or generalized severe allergic reaction, constitutes the most critical manifestation of immediate CMPA. Its mortality rate is nearly 10%. 1,25-27
Late symptoms which are not mediated by IgE or mixed
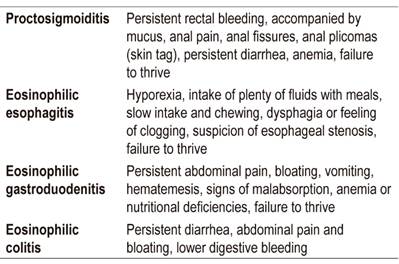
Late symptoms occur several hours or days after ingestion of an allergen (Table 3), predominantly involve the gastrointestinal tract, but are almost always multiple. 2,14,19,28,29
Table 3 Late symptoms (not mediated by IgE or mixed mediation)

Modified from references 2, 14, 19, 28 and 29
The most common late gastrointestinal symptoms include vomiting, irritability (colic), dysphagia, diarrhea, constipation, growth failure, and rectal bleeding.
In addition to diagnoses based on symptoms, clinical diagnoses can also be made through the elimination of milk and its derivatives (or elimination of all foods containing dairy ingredients), through food challenge protocols, or through double-blind tests with controls receiving placebos. The reference treatment for clinical diagnosis is elimination of CMP for two to four weeks followed by reintroduction of CMP in the form of an open challenge or a blind challenge. 18,20
Differential Diagnosis
It is important to consider other entities that could be confused with CMPA. These include lactose intolerance, renal tubular acidosis, pyloric hypertrophy, cystic fibrosis or enterocolitis, and gastroesophageal reflux disease.
Recommendations
It is essential to prepare a clinical history that includes CMPA risk factors to establish an early diagnosis.
Before diagnosing a new allergy or multiple allergies, it should be considered that a patient may have been exposed to hidden derivatives of milk or traces of milk in food.
Except for gastrointestinal allergies, most milk-induced allergic symptoms develop within minutes or hours after ingestion.
Classically, CMPA signs and symptoms present in the skin, the gastrointestinal tract and the respiratory tract.
A differential diagnosis of CMPA must be made.
Changes in gut microbiota are partly responsible for increasing cases of allergic diseases. Ideally, improvement of intestinal microbiota should start at birth with vaginal delivery and be followed by initiation of breast milk in the first hour of life and avoidance of indiscriminate use of antibiotics to reduce the risk of CMPA symptoms. 30,31
Diagnosis: clinical, laboratory and immunological tests
The management of each patient must be individualized and based on use of various diagnostic resources.
Diagnostic Tests
Oral food challenge tests are the reference treatment for diagnosis of CMPA. These tests must be done by either a gastroenterologist or an allergist. Challenge testing is not recommended if the physician does not have the experience and knowledge necessary to ensure patient safety. Oral challenge tests with cow’s milk are in vivo and used to definitively confirm a preliminary suspicion of CMPA.
A test that results in a clinical reaction is defined as a positive or failed challenge, whereas a test without clinical reaction is called a negative or approved challenge. Thus, a positive challenge will indicate the tolerated dose, if any, and will allow planning of partial or complete CMP exclusion diets. 14,18,20
Basic blood tests should include a peripheral blood smear, a ferritin test, and total and differential proteins.
Every patient requires a complete nutritional evaluation to detect nutritional risks of consuming allergens.
Skin tests have high sensitivity and negative predictive value but low specificity. They can confirm sensitization but cannot diagnose whether a patient has CMPA.
Specific IgE level cut-off points of more than five KU/L within one year and more than 15 KU/L in more than one year have been established specifically for cow’s milk with 95% probability when an exposure test is positive. 14
Skin sensitization tests are not recommended for patients with any food allergy, especially those mediated by IgE. 14
Recommendations
Most patients seen in pediatric clinics present non-IgE-mediated processes and do not require immunological diagnostic tests.
The following tests are requested for patients suspected of CMPA whose clinical pictures are compatible with IgE-mediated or mixed reactions and for patients with manifestations of reactions not mediated by IgE who do not respond to treatment: Specific IgE (RAST test or ImmunoCAP) and a skin prick test for cow’s milk. After testing patients can be referred to a pediatric gastroenterologist or allergist.
Evaluation of total serum IgE is not recommended for these patients.
Delayed reactions do not have conventional diagnostic tests so diagnosis is clinical by exclusion and challenge.
A negative response to immunological tests does not rule out a process that is not mediated by IgE.
Digestive endoscopy diagnosis
A patient’s symptoms are the most important factors to consider when considering an endoscopic evaluation of a patient with CMPA regardless of age. Especially important are suspicion of eosinophilic esophagitis, allergic proctocolitis with anemic and persistent manifestations other than bleeding, abdominal bloating and persistent vomiting (which may have a mixed component). 32-34
Indications for Upper and Lower Gastrointestinal Endoscopy in patients with CMPA
Endoscopic tests are not usual for patients diagnosed with CMPA since the diagnosis is clinical. Therefore, the decision to carry out these tests is based on well-defined clinical criteria based on patient characteristics, nutritional status, laboratory tests and evident clinical manifestations. Indications for digestive endoscopy are listed in Table 4. 35,36
Table 4 Indications for digestive endoscopy

FPIES: Food Protein-Induced Enterocolitis Syndrome. Modified from references 35 and 36.
Indications for endoscopy in patients with processes not mediated by IgE are listed in Table 5.
Endoscopic Findings Suggestive of CMPA
Endoscopic findings are nonspecific and on several occasions do not aid in the diagnosis of CMPA-induced proctocolitis. Endoscopic findings suggestive of allergy, mainly when eosinophilic esophagitis is suspected, include linear grooves (33-48%), circular rings (44-55%), alteration of the vascular pattern (41%), whitish papules (27 %), partial stenosis (10-39%) and complete stenosis (9%).
Endoscopy results are normal in 7% to 32% of cases. Even when endoscopic findings are normal, proximal and distal biopsies of the esophagus should be taken from the antrum and corpus of in the stomach, the duodenal bulb, the ileum, colon, cecum and all quadrants to look for eosinophilic gastroenteropathy, celiac disease, Helicobacter pylori and other pathologies. 32-34
Recommendations
Endoscopic procedures should be performed by experienced pediatric gastroenterologists.
Biopsies should be taken from the esophagus, stomach, duodenal bulb, duodenum, ileum, cecum, and the quadrants of the colon as well as an eosinophil count by field. The Marsh classification for celiac disease should be used.
Nutritional management
Basic Concepts
Each patient’s nutrition should be managed individually. This requires a multidisciplinary team that includes a nutritionist who is an expert in management of CMPA.
According to Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMPA), the following issues should be considered:
Strict nutritional surveillance during the process is a requirement.
An elimination diet of at least 6 months but less than one year is recommended.
The elimination diet must be strict, effective and complete. Inhalation of, and skin contact with, products or traces that contain CMP must be avoided.
All elimination diets must be nutritionally safe. It is necessary to avoid macronutrient and micronutrient restrictions. Other types of food including egg and fish should never be restricted if the diagnosis is CMPA. 14
Nutrition Management Objectives
The objective of nutritional management of patients with CMPA is to maintain adequate nutrition to guarantee the child’s growth and development. 37,38
Formulas
Factors that should be considered for choosing a formula for patients with CMPA are shown in Table 6. 37,39
Hypoallergenic Formulas
Hypoallergenic formulas meet the criteria of 90% clinical tolerance (95% confidence interval in children with proven allergy to CMP). Extensively hydrolyzed formula (EHF) and free amino acid-based formulas (AAF) formulas are two types of hypoallergenic formulas (Table 7).
Therapeutic Challenges
The first challenge should be performed six months to one year after diagnosis in patients with mild symptoms.
In cases of anaphylaxis, the challenge should be performed at least 1 year after the episode. Preferably, it should be monitored in a hospital center. This challenge should be prescribed by a competent specialist.
A challenge should not coincide with initiation of complementary feeding in 6 month old children because, if symptoms appear, it will be impossible to determine whether they are due to the complementary feeding or the challenge.
Recommendations in pregnancy and exclusive breastfeeding
There is no evidence that restricting a pregnant woman’s consumption of allergens can prevent the appearance of a CMPA in the baby. Avoid restricting food eaten by the mother during pregnancy and aim for an adequate, varied and balanced diet that maintains recommended weight.
Exclusive breastfeeding is associated with multiple benefits. Restrictions on on mothers’ consumption of CMP are not recommended. Exclusive breastfeeding is ideal up to six months of age, and complementary breastfeeding is ideal until 24 months or until the mother or the child decides.
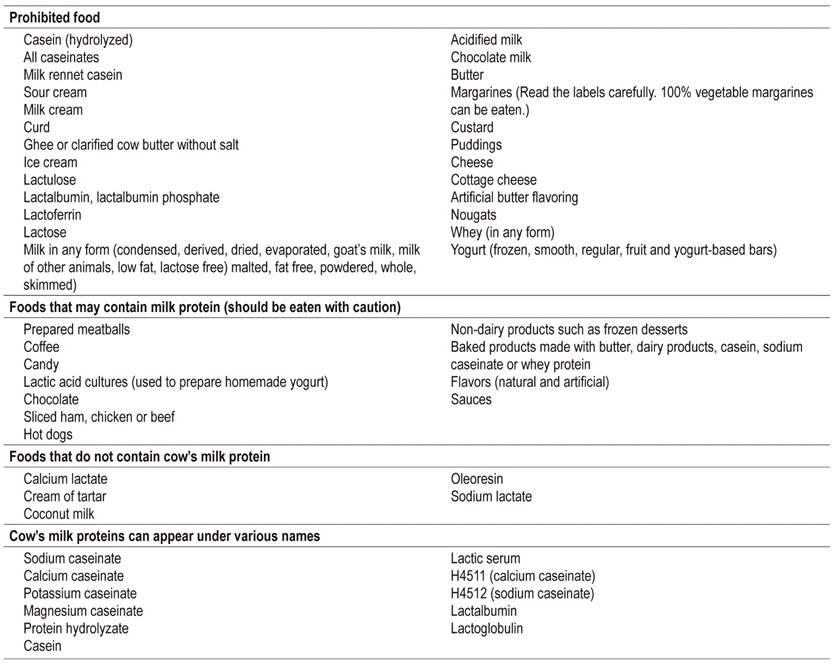
For patients with CMPA, strict elimination of CMP from the mother’s and infant’s diet is required. The mother’s diet should eliminate all sources of milk protein, and calcium supplements should be added to the diet. In addition, parents and caregivers should understand the child’s sensitization pathways and be careful to review labels of all products that may have milk protein components (e.g. cosmetics, soaps, creams, lotions, wet wipes, sauces, and others) (Table 8).
Mothers should be given motivation to continue breastfeeding. Gastrointestinal manifestations improve at 2 weeks and dermatological manifestations improve at 6 weeks after starting CMP restrictions. These manifestations do not constitute a reason to discontinue breastfeeding. If there is no improvement, the mother should be referred to a specialist before breastfeeding is stopped or restricted.
Complementary feeding can be introduced between four and six months of age. There is an increased risk of allergy if solid foods are introduced before four months.
There is no evidence that delaying the introduction of foods considered allergenic (e.g., eggs and strawberries) before the first year reduces the risk of allergy in either the general or atopic population. 41
There is no clear evidence that partially hydrolyzed formulas and EHF prevent any type of allergy or that they are better than breast milk.
Recommendations for children who do not receive breast milk
The recommended formulas for infants with CMPA are EHF and AAF.
Children may require supplementation with micronutrients such as calcium (when a minimum volume of 500 mL of breast milk or formula cannot be guaranteed), iron (when there is heavy bleeding or in patients with iron deficiency) and vitamin D (if as deficiency is demonstrated).
If CMPA persists after the first year, the use of special formulas designed for that age is required.
There is no evidence in Colombia that hypoallergenic formulas (Confort brand) prevent or treat CMPA.
The use of drinks made from almonds, rice, coconut and soybeans and others nuts and grains is not recommended given the high probability of cross-reactions and insufficient nutritional value.
Pharmacological management and immunotherapy
There is no specific pharmacological treatment for CMPA, but epinephrine is the treatment of choice in cases of severe IgE-mediated allergy such as anaphylaxis.
Adrenaline’s half-life is short and multiple doses may be required. In Colombia, it is only used in hospitals, and there are no autoinjectors. When an anaphylactic reaction occurs, it is recommended to immediately go to an emergency department. The recommended undiluted intramuscular dose for hospital management of anaphylaxis with adrenaline is 0.15 mg for patients weighing less than 25 kg A 21 short replaceable needle syringe should be used, and the shot should be administered in the middle lateral thigh. Repeat the dose in 10 to 15 minutes if there is no response. The dose should be 0.3 mg for patients weighing more than 25 kg (anaphylaxis guideline from the European Academy of Allergy and Clinical Immunology (EAACI)). 47,48
In the last decade, other therapies for CMPA including allergen specific immunotherapy, biological therapy, Chinese medicine, probiotics and prebiotics have been investigated.
Immunotherapy
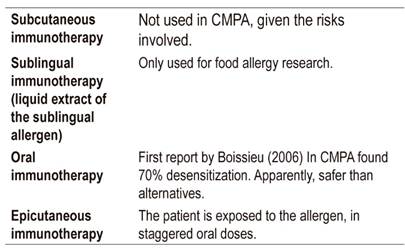
The objective of immunotherapy is to desensitize the patient by increasing the threshold of reactivity to an allergen, thus generating sustained tolerance for months or years without additional treatment. Nevertheless, this goal is not always reached, and it is desirable that there be no severe reaction to accidental exposure (Table 9). 49-51 Treatment implies that the patient continuously receives doses of the allergen. The first immunotherapy studies in food allergy were made for peanut allergies. The overall effectiveness of this treatment is 35%.
In summary, oral immunotherapy is more effective than cutaneous immunotherapy, but adverse events occur more frequently with oral immunotherapy than with cutaneous immunotherapy.
Biological Therapy
Omalizumab, lilizumab, and quilizumab are biologic therapies which are being studied for treatment of CMPA, primarily of the IgE type, and as adjuvants to immunotherapy. 52,53
Chinese Traditional Medicine
Food Allergy Herbal Formulas (FAHF) 1 and 2 consist of a blend of Chinese herbs, the active ingredients of which are berberine and limonine. So far they are experimental treatments and are not currently recommended.
Probiotics and Prebiotics
There is still no conclusive evidence that probiotics and prebiotics are effective treatments of CMPA or that administration during the last trimester of pregnancy and the first months of breastfeeding prevents CMPA. The data suggests that supplementing hydrolyzed formulas with certain strains of probiotics may have benefits in terms of oral tolerance, primarily in atopic eczema. However, additional research is required to define species, dosage, and therapy times. The 2016 World Allergy Organization (WAO) guideline suggests the use of probiotics exclusively in children who are not breastfed. 40,54
Gene Therapy
Research about gene therapy for CMPA is ongoing, but as of yet there are no approved therapies.
REFERENCES
1. Montijo-Barrios E, López-Ugalde MV, Ramírez-Mayans J, Anaya-Flórez MS, Arredondo-García JL, Azevedo-Tenorio I, et al. Guía latinoamericana para el diagnóstico y tratamiento de alergia a las proteínas de la leche de vaca (GL-APLV). Rev Invest Clin. 2014;66 Suppl 2:S9-S72. [ Links ]
2. Koletzko S, Niggemann B, Arato A, Dias JA, Heuschkel R, Husby S, et al. Diagnostic approach and management of cow’s-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr. 2012;55(2):221-9. https://doi.org/10.1097/MPG.0b013e31825c9482 [ Links ]
3. Schoemaker AA, Sprikkelman AB, Grimshaw KE, Roberts G, Grabenhenrich L, Rosenfeld L, et al. Incidence and natural history of challenge-proven cow’s milk allergy in European children--EuroPrevall birth cohort. Allergy. 2015;70(8):963-72. https://doi.org/10.1111/all.12630 [ Links ]
4. Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel report. Nutr Res. 2011;31(1):61-75. https://doi.org/10.1016/j.nutres.2011.01.001 [ Links ]
5. Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, et al. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120(3):638-46. https://doi.org/10.1016/j.jaci.2007.05.026 [ Links ]
6. Ávila MDL, Pérez J, Del Río BE, Rosas MA, Lerma L, Sienra JJL. Hipersensibilidad por prueba cutánea a alimentos en pacientes alérgicos en el Hospital Infantil de México Federico Gómez. Rev Alergia Mex 2002;49(3):74-79. [ Links ]
7. Martínez J, Méndez C, Talesnik E, Campos E, Viviani P, Sánchez D. Pruebas cutáneas de hipersensibilidad inmediata en una población pediátrica seleccionada. Rev Med Chile. 2005;133(2):195-201. https://doi.org/10.4067/S0034-98872005000200007 [ Links ]
8. Marrugo J, Hernández L, Villalba V. Prevalence of self-reported food allerg in Cartagena (Colombia) population. Allergol Immunopathol (Madr). 2008;36(6):320-4. https://doi.org/10.1016/S0301-0546(08)75863-4 [ Links ]
9. Acevedo N, Sánchez J, Zakzuk J, Bornacelly A, Quiróz C, Alvarez Á, et al. Particular characteristics of allergic symptoms in tropical environments: follow up to 24 months in the FRAAT birth cohort study. BMC Pulm Med. 2012;12:13. https://doi.org/10.1186/1471-2466-12-13 [ Links ]
10. Solé D, Mallol J, Wandalsen GF, Aguirre V; Latin American ISAAC Phase 3 Study Group. Prevalence of symptoms of eczema in Latin America: results of the International Study of Asthma and Allergies in Childhood (ISAAC) Phase 3. J Investig Allergol Clin Immunol. 2010;20(4):311-23. [ Links ]
11. Arévalo M, Reyes MA, Victoria L, Villegas A, Badiel M, Herrera S. Asma y rinitis alérgica en pre-escolares en Cali. Colomb Med. 2003;34(1):4-8. [ Links ]
12. Dennis RJ, Caraballo L, García E, Rojas MX, Rondón MA, Pérez A, et al. Prevalence of asthma and other allergic conditions in Colombia 2009-2010: a cross-sectional study. BMC Pulm Med. 2012;12(17);1-9. https://doi.org/10.1186/1471-2466-12-17 [ Links ]
13. Orsi M, Fernández A, Follett FR, Marchisone S, Saieg G, Busoni VB, et al. Alergia a la proteína de la leche de vaca. Propuesta de Guía para el manejo de los niños con alergia a la proteína de la leche de vaca. Arch Argent Pediatr. 2009;107(5):459-470. [ Links ]
14. Fiocchi A, Brozek J, Schünemann H, Bahna SL, von Berg A, Beyer K, et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines. Pediatr Allergy Immunol. 2010;21 Suppl 21:1-125. https://doi.org/10.1111/j.1399-3038.2010.01068.x [ Links ]
15. Björkstén B. Genetic and environmental risk factors for the development of food allergy. Curr Opin Allergy Clin Immunol. 2005;5(3):249-53. https://doi.org/10.1097/01.all.0000168790.82206.17 [ Links ]
16. Eggesbø M, Botten G, Stigum H, Samuelsen SO, Brunekreef B, Magnus P. Cesarean delivery and cow milk allergy/intolerance. Allergy. 2005;60(9):1172-3. https://doi.org/10.1111/j.1398-9995.2005.00857.x [ Links ]
17. Sánchez-Valverde F, Gil F, Martínez D, Fernández B, Aznal E, Oscoz M, et al. The impact of caesarean delivery and type of feeding on cow’s milk allergy in infants and subsequent development of allergic march in childhood. Allergy. 2009;64(6):884-9. https://doi.org/10.1111/j.1398-9995.2008.01931.x [ Links ]
18. Vera JF, Ramírez A. Síntomas digestivos y respuesta clínica en lactantes con alergia a la proteína de leche de vaca. Rev Chil Pediatr. 2013;84:(6):641-649. https://doi.org/10.4067/S0370-41062013000600007 [ Links ]
19. Luyt D, Ball H, Makwana N, Green MR, Bravin K, Nasser SM, et al. BSACI guideline for the diagnosis and management of cow’s milk allergy. Clin Exp Allergy. 2014;44(5):642-72. https://doi.org/10.1111/cea.12302 [ Links ]
20. Zuluaga LC, Ramírez R, Mejía LK, Vera JF. Outcomes of treating infants with cow’s milk protein allergies with an extensively hydrolyzed serum-based formula. Rev Colomb Gastroenterol. 2018;33(2):111-115. https://doi.org/10.22516/25007440.253 [ Links ]
21. Ministerio de Salud de Chile. Guía clínica: alergia a proteína de lecha de vaca. Santiago: Ministerio de Salud; 2012. [ Links ]
22. Pumphrey RS, Gowland MH. Further fatal allergic reactions to food in the United Kingdom, 1999-2006. J Allergy Clin Immunol. 2007;119(4):1018-9. https://doi.org/10.1016/j.jaci.2007.01.021 [ Links ]
23. Simons FE, Ardusso LR, Bilò MB, El-Magal YM, Ledford DK, Ring J, et al. World Allergy Organization anaphylaxis guidelines: summary. J Allergy Clin Immunol. 2011;127(3):587-593. https://doi.org/10.1016/j.jaci.2011.01.038 [ Links ]
24. Skripak JM, Matsui EC, Mudd K, Wood RA. The natural history of IgE-mediated cow’s milk allergy. J Allergy Clin Immunol. 2007;120(5):1172-7. https://doi.org/10.1016/j.jaci.2007.08.023 [ Links ]
25. Muraro A, Roberts G, Clark A, Eigenmann PA, Halken S, Lack G, et al. The management of anaphylaxis in childhood: position paper of the European academy of allergology and clinical immunology. Allergy. 2007;62(8):857-71. https://doi.org/10.1111/j.1398-9995.2007.01421.x [ Links ]
26. Sampson HA, Muñoz-Furlong A, Campbell RL, Adkinson NF, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report-Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391-7. https://doi.org/10.1016/j.jaci.2005.12.1303 [ Links ]
27. Braganza SC, Acworth JP, Mckinnon DR, Peake JE, Brown AF. Paediatric emergency department anaphylaxis: different patterns from adults. Arch Dis Child. 2006;91(2):159-163. https://doi.org/10.1136/adc.2004.069914 [ Links ]
28. Latcham F, Merino F, Lang A, Garvey J, Thomson MA, Walker-Smith JA, et al. A consistent pattern of minor immunodeficiency and subtle enteropathy in children with multiple food allergy. J Pediatr. 2003;143(1):39-47. https://doi.org/10.1016/S0022-3476(03)00193-8 [ Links ]
29. Butt AM, Murch SH, Ng CL, Kitching P, Montgomery S, Philips A, et al. Upregulated eotaxin expression and T cell infiltration in the basal and papillary epithelium in cows’ milk associated reflux oesophagitis. Arch Dis Child. 2002;87(2):124-130. https://doi.org/10.1136/adc.87.2.124 [ Links ]
30. Berni Canani R, Nocerino R, Terrin G, Coruzzo A, Cosenza L, Leone L, et al. Effect of Lactobacillus GG on tolerance acquisition in infants with cow’s milk allergy: a randomized trial. J Allergy Clin Immunol. 2012;129(2):580-2,582.e1-5. https://doi.org/10.1016/j.jaci.2011.10.004 [ Links ]
31. Sicherer SH, Sampson HA. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol. 2018;141(1):41-58. https://doi.org/10.1016/j.jaci.2017.11.003 [ Links ]
32. Kim HP, Vance RB, Shaheen NJ, Dellon ES. The prevalence and diagnostic utility of endoscopic features of eosinophilic esophagitis: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10(9):988-96.e5. https://doi.org/10.1016/j.cgh.2012.04.019 [ Links ]
33. Pasha SF, DiBaise JK, Kim HJ, De Petris G, Crowell MD, Fleischer DE, et al. Patient characteristics, clinical, endoscopic, and histologic findings in adult eosinophilic esophagitis: a case series and systematic review of the medical literature. Dis Esophagus. 2007;20(4):311-9. https://doi.org/10.1111/j.1442-2050.2007.00721.x [ Links ]
34. Liacouras CA, Spergel JM, Ruchelli E, Verma R, Mascarenhas M, Semeao E, et al. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol. 2005;3(12):1198-206. https://doi.org/10.1016/S1542-3565(05)00885-2 [ Links ]
35. Lee KM, Lim HC, Kim JH, Yoon YH, Park HJ, Lee SI. Clinical implications of endoscopically suspected eosinophilic esophagitis. Korean J Gastroenterol. 2010;56(5):285-92. https://doi.org/10.4166/kjg.2010.56.5.285 [ Links ]
36. Moawad FJ, Schoepfer AM, Safroneeva E, Ally MR, Chen YJ, Maydonovitch CL, et al. Eosinophilic oesophagitis and proton pump inhibitor-responsive oesophageal eosinophilia have similar clinical, endoscopic and histological findings. Aliment Pharmacol Ther. 2014;39(6):603-8. https://doi.org/10.1111/apt.12636 [ Links ]
37. Uppal V, Kreiger P, Kutsch E. Eosinophilic Gastroenteritis and Colitis: a Comprehensive Review. Clin Rev Allergy Immunol. 2016;50(2):175-88. https://doi.org/10.1007/s12016-015-8489-4 [ Links ]
38. Fleischer DM, Spergel JM, Assa’ad AH, Pongracic JA. Primary prevention of allergic disease through nutritional interventions. J Allergy Clin Immunol Pract. 2013;1(1):29-36. https://doi.org/10.1016/j.jaip.2012.09.003 [ Links ]
39. Giovannini M, D’Auria E, Caffarelli C, Verduci E, Barberi S, Indinnimeo L, et al. Nutritional management and follow up of infants and children with food allergy: Italian Society of Pediatric Nutrition/Italian Society of Pediatric Allergy and Immunology Task Force Position Statement. Ital J Pediatr. 2014;40:1. https://doi.org/10.1186/1824-7288-40-1 [ Links ]
40. Fiocchi A, Dahda L, Dupont C, Campoy C, Fierro V, Nieto A. Cow’s milk allergy: towards an update of DRACMA guidelines. World Allergy Organ J. 2016;9(1):35. https://doi.org/10.1186/s40413-016-0125-0 [ Links ]
41. Fewtrell M, Bronsky J, Campoy C, Domellöf M, Embleton N, Fidler Mis N, et al. Complementary Feeding: A Position Paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2017;64(1):119-132. https://doi.org/10.1097/MPG.0000000000001454 [ Links ]
42. Molnár K, Pintér P, Győrffy H, Cseh A, Müller KE, Arató A, et al. Characteristics of allergic colitis in breast-fed infants in the absence of cow’s milk allergy. World J Gastroenterol. 2013;19(24):3824-30. https://doi.org/10.3748/wjg.v19.i24.3824 [ Links ]
43. Yu MC, Tsai CL, Yang YJ, Yang SS, Wang LH, Lee CT, et al. Allergic colitis in infants related to cow’s milk: clinical characteristics, pathologic changes, and immunologic findings. Pediatr Neonatol. 2013;54(1):49-55. https://doi.org/10.1016/j.pedneo.2012.11.006 [ Links ]
44. Kokkonen J, Karttunen TJ. Lymphonodular hyperplasia on the mucosa of the lower gastrointestinal tract in children: an indication of enhanced immune response? J Pediatr Gastroenterol Nutr. 2002;34(1):42-6. https://doi.org/10.1097/00005176-200201000-00010 [ Links ]
45. Hwang JB, Park MH, Kang YN, Kim SP, Suh SI, Kam S. Advanced criteria for clinicopathological diagnosis of food protein-induced proctocolitis. J Korean Med Sci. 2007;22(2):213-217. http://dx.doi.org/10.3346/jkms.2007.22.2.213 [ Links ]
46. Mansueto P, Iacono G, Seidita A, D’Alcamo A, Sprini D, Carroccio A. Review article: intestinal lymphoid nodular hyperplasia in children-the relationship to food hypersensitivity. Aliment Pharmacol Ther. 2012;35(9):1000-9. https://doi.org/10.1111/j.1365-2036.2012.05062.x [ Links ]
47. European Academy of Allergy and Clinical Immunology (EAACI). Food Allergy and Anaphylaxis Guidelines. Zúrich: EAACI; 2014. [ Links ]
48. Jones SM, Burks AW. Food Allergy. N Engl J Med. 2017;377(12):1168-1176. https://doi.org/10.1056/NEJMcp1611971 [ Links ]
49. Ebisawa M, Ito K, Fujisawa T; Committee for Japanese Pediatric Guideline for FoodAllergy , The Japanese Society of Pediatric Allergy and Clinical Immunology, The Japanese Society of Allergology. Japanese guidelines for food allergy 2017. Allergol Int. 2017;66(2):248-264. https://doi.org/10.1016/j.alit.2017.02.001 [ Links ]
50. Martorell A, Alonso E, Echeverría L, Escudero C, García-Rodríguez R, Blasco C, et al. Oral Immunotherapy for Food llergy : A Spanish Guideline. Immunotherapy Egg and Milk Spanish Guide (ITEMS Guide). Part I: Cow Milk and Egg Oral Immunotherapy: Introduction, Methodology, Rationale, Current State, Indications, Contraindications, and Oral Immunotherapy Build-up Phase. J Investig Allergol Clin Immunol. 2017;27(4):225-237. https://doi.org/10.18176/jiaci.0177 [ Links ]
51. Kobernick AK, Burks AW. Active treatment for food allergy. Allergol Int. 2016;65(4):388-395. https://doi.org/10.1016/j.alit.2016.08.002 [ Links ]
52. MacGinnite A. Update on Potential Therapies for IgE-Mediated FoodAllergy. Curr Allergy Asthma Rep. 2017;17(1):4. https://doi.org/10.1007/s11882-017-0671-8 [ Links ]
53. Hamad A, Burks WA. Emerging Approaches to Food Desensitization in Children. Curr Allergy Asthma Rep. 2017;17(5):32. https://doi.org/10.1007/s11882-017-0700-7 [ Links ]
54. Vandenplas Y. Prevention and Management of Cow’s Milk Allergy in Non-Exclusively Breastfed Infants. Nutrients. 2017 Jul 10;9(7). pii: E731. https://doi.org/10.3390/nu9070731 [ Links ]
Received: May 13, 2019; Accepted: September 24, 2019











 texto en
texto en