Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.35 no.1 Bogotá Jan./Mar. 2020
https://doi.org/10.22516/25007440.526
Review articles
Basic considerations regarding endoscopic procedures during the COVID-19 pandemic
1Gastroenterología, Universidad Nacional de Colombia, Hospital Universitario Nacional de Colombia y Centro de Gastroenterología y Endoscopia Digestiva. Bogotá, Colombia
2Gastroenterología, Universidad Nacional de Colombia y Hospital Universitario Nacional de Colombia, UGEC. Bogotá, Colombia
3Universidad Nacional de Colombia y Hospital Universitario Nacional de Colombia. Bogotá, Colombia
4Gastroenterología, Universidad Nacional de Colombia y Gut Médica. Bogotá, Colombia
5Gastroenterología, Universidad Nacional de Colombia y Centro de Gastroenterología y Endoscopia Digestiva. Bogotá, Colombia
6Centro de Gastroenterología y Endoscopia Digestiva. Bogotá, Colombia
SARS-CoV-2 is the coronavirus which produces the dreaded COVID-19. Starting in Wuhan, the capital of China’s Hubei province, it has spread it spread throughout the world in less than four months and has caused thousands of deaths. The WHO has declared it to be a pandemic. Humanity is shocked, and many governments have imposed total isolation. It has had varying success due to community negligence. In many cities, institutions and health personnel have not successfully managed this catastrophe. Isolation is the only effective strategy to stop the logarithmic growth of COVID 19. The scientific reason for isolation is that more than 60 % of infections arise from asymptomatic people. SARS-CoV-2 not only produces respiratory symptoms but can also cause nausea, abdominal pain, vomiting, diarrhea, anosmia and ageusia. Fifty percent of those infected may have digestive symptoms which may even precede respiratory symptoms. The fecal-oral route can transmit the virus even when there is no diarrhea. All forms of contagion are found in endoscopy units: aerosols from vomiting, retching, belching, and flatus; fecal matter, close contact, and contamination of the environment. All diagnostic endoscopies should be discontinued. Only urgent and therapeutic endoscopy should be performed. All endoscopy personnel must have strict protection measures. Each patient should be informed, and sign an informed consent form, that the virus can be spread within the endoscopy room. After performance of endoscopy, the patient should be contacted by phone on days 7 and 14 to inquire about all symptoms mentioned.
Keywords: Endoscopy; COVID19; contagion; aerosols; fecal matter; protection
El SARS-Cov-2 es un coronavirus productor de la enfermedad COVID-19. Esta inició en Wuhan, capital de la provincia Hubei, China. En menos de cuatro meses la enfermedad se dispersó por el mundo, lo que dio origen a miles de muertes. La Organización Mundial de la Salud (OMS) la ha declarado pandemia. La humanidad está consternada, múltiples gobiernos han obligado al aislamiento total, con éxito variable debido a la negligencia de parte de la comunidad. En muchas ciudades las instituciones y el personal sanitario no son suficientes para atender la catástrofe. El aislamiento es la única estrategia eficaz para detener el crecimiento logarítmico de COVID-19. El motivo científico del aislamiento es que más del 60 % de los contagios surgen de personas asintomáticas. La enfermedad no solo produce síntomas respiratorios. El SARS-Cov-2, además, puede producir náuseas, dolor abdominal, vómito, diarrea, anosmia y ageusia. El 50% de los infectados pueden tener síntomas digestivos, que incluso preceden a los respiratorios. La ruta fecal-oral trasmite el virus, aún sin diarrea. En las unidades de endoscopia están todas las formas de contagio: aerosoles (vómitos, arcadas, eructos, flatos), materia fecal, contacto estrecho, contaminación del ambiente. Se deben suspender todas las endoscopias programadas para diagnóstico. Solo deben realizarse las urgentes y terapéuticas. Todo el personal de endoscopia debe tener medidas de protección estrictas. El paciente debe saber que en la sala de endoscopia puede contagiarse, con constancia en el consentimiento informado. Debe contactarse al paciente posendoscopia vía telefónica a los días 7 y 14 para indagar sobre todos los síntomas mencionados.
Palabras clave: Endoscopia; COVID-19; contagio; aerosoles; materia fecal; protección
Introduction
COVID-19 is a life-threatening infection that originated in Wuhan, the capital of Hubei province in China. 1 Due to its rapid global spread, the World Health Organization (WHO) has declared it a pandemic. 2 As of March 25, 180 countries had been infected. 2 Since then, humanity has progressively begun to strictly adhere to recommendations issued by world experts whose guidelines have remained unobjectionable as the pandemic has evolved. Endoscopic procedures are of unusual importance since all of this potentially lethal disease’s transmission mechanisms are simultaneously present in endoscopy rooms. Therefore, our group considers this narrative review based on updated information on the most important aspects of SARS-Cov-2 infection and endoscopic procedures to be particularly appropriate.
Methodology
We searched Pubmed using the following terms: (“COVID-19” OR “coronavirus” OR “SARS-Cov-2”) AND (“gastrointestinal” OR “transmission” OR “intestinal” OR “digestive” OR “endoscopy” OR “esophagogastroduodenoscopy” OR “colonoscopy”). There were no restrictions on date, language, or other factors. We found 2,340 references from which we chose the most relevant for a review of this topic. References in primary articles considered important for this study were also consulted manually.
Until recently, the start of the SARS-Cov-2 epidemic in China was believed to have been on December 31, 2019 when patient zero (0) was identified. At that time, an epidemiological alert was issued in that country because of suspected cases of pneumonia with unknown causes. 3 However, recent publications in the Chinese media have reported that the first confirmed case of the new virus was a 55-year-old man on who was diagnosed on November 17, 2019. 4 Whether this patient will be designated “patient zero” is now under study. As of December 31, 2019, there were about 300 COVID-19 patients in Wuhan: soon after there were thousands of fatalities. 2 This pandemic has spread very rapidly. In Italy, after the first case was diagnosed on February 18, 2020, the number of infected has grown exponentially, and there are currently hundreds of deaths every day. 2
SARS-Cov-2 is a single-stranded RNA virus whose diameter is between 60 and140 nm. 1 It belongs to the coronaviruses, whose name derives from the morphology of its envelope which has the form of a “crown” made up of 14 amino acid residues that interact with receptor 2 of the angiotensin-converting enzyme (ACE), which seems to be its receiver. 5-7 There are six species in the coronavirus family that cause mild respiratory diseases in humans, 8,9 but in the last two decades two coronavirus species have caused catastrophic diseases with high mortality rates: SARS-Cov in 2002 and MERS-Cov in 2012. 9 The names of these viruses derives from the fundamental pathologies they produce followed by Cov (coronavirus). SARS-Cov stands for severe acute respiratory syndrome coronavirus and MERS-Cov stands for Middle East respiratory syndrome coronavirus, a name which also identifies the geographical location of its epidemiology. 10 SARS-Cov-2 is the abbreviated name of the disease that produces the “SARS-CoV-2 related disease” that originated in 2019. 10
SARS-Cov-2 was initially called HCoV19 and originally infects bats, civets, badgers, bamboo rats, and wild camels. 1,11 The jump to humans appears to have originated from the consumption of fresh and live animals at the Wuhan seafood market. 11 Genomic and phylogenetic analysis of broncho-alveolar lavage samples in nine hospitalized patients found that SARS-Cov-2 homology of 88% with the genetic sequence of two bat beta-coronaviruses (bat-SL-CoVZC45 and bat-SL- CoVZXC21). 11 SARS-Cov-2-19 is presumed to have infected the human population from a bat reservoir, although it is unknown which animal may have been an intermediate host between bats and humans. It has recently been postulated that the intermediary could be a pangolin, 12 a placental mammal physically similar to an armadillo but genetically different and a member of another species. 13
Clinical manifestations of COVID-19 range from mild asymptomatic disease to severe disease with respiratory failure, multiple organ dysfunction, septic shock, and death. 1,2,14 Fever, dry coughing, and fatigue are common symptoms. Diarrhea and other digestive symptoms such as discomfort and dyspepsia have been reported in less than 5% of patients. 14 However, some authors have found that nausea, abdominal pain, and diarrhea can occur in 50% of patients and may even precede respiratory symptoms. 15-18 The first case identified in the United States had gastrointestinal symptoms including nausea, vomiting, and diarrhea, and viral RNA was documented in that patient’s stool. 19 Nausea and vomiting appeared on the fourth day of illness and diarrhea on the sixth day. 19 A Chinese study of 73 patients hospitalized for COVID-19 found viral RNA in the feces of 53% of the patients. Only 65% had diarrhea, suggesting that fecal infection may occur even if there is no diarrhea. 18 The stools of twenty percent of COVID-19 patients test positive for viral RNA even after respiratory tract tests are negative. 14
Anosmia, dysgeusia or ageusia are recently identified manifestations which may be related by tropism of SARS-Cov-2 with brain tissue. This would make it a neuroinvasive virus. 20 Central nervous system compromise may also play a role in severe respiratory failure through compromising the cardiorespiratory center of the brain stem and neurologically reaching mechanoreceptors and chemoreceptors in the lungs and lower respiratory tract. 20
A breakdown of clinical characteristics of more than 44,000 confirmed cases in China found that 81% had mild cases without pneumonia or mild pneumonia; 14% had severe pneumonia with dyspnea, respiratory rates ≥30, O2 saturation ≤ 93%, fractions of inspired oxygen (FiO2) <300, and/or pulmonary infiltrates > 50%) within 24 to 48 hours; and 5% were critical and suffered respiratory failure, septic shock, and/or multiple organ dysfunction. 21 Clinical suspicion is essential because of the rapid evolution of complicated cases and the fact that 60% or more cases are transmitted by asymptomatic people. 22 SARS-Cov-2 is transmitted from person to person by respiratory secretions, feces (oral-fecal), 14,23,24 and contaminated surfaces. 1 In addition, a 2005 study conducted in China during the SARS epidemic found viral SARS-CoV RNA in wastewater from hospitals that were treating patients with SARS. Even though it was not viable virus, it was found that it could remain for up to 14 days at 4° C and for 2 days at 20° C. 25
The most important transmission modes, and the modes that have been studied most, are droplets of saliva and aerosols. 26 A drop has a diameter greater than 5 µm and contains water. 22 Drops can come into contact with surfaces at a distance of one meter. 26 A sneeze releases about 40,000 microdroplets at a speed of 100 m/s while a cough releases 3,000 drops at a speed of 200 m/s. They remain viable as an aerosol for up to 3 hours. 26,27 Saliva’s viral load is similar to that of fecal matter, but the virus can persist for up to 48 hours longer in feces than in respiratory secretions. 18,24,27 It remains viable in aerosols for 3 hours. 27 The number of copies of the virus in aerosols is not clear, nor is the infective dose. In the case of the influenza virus, concentrations between 48 to 300 copies of the viral RNA have been documented per positive sample filter. This corresponds to a production rate on exhalation of 3.2 to 20 copies of viral RNA per minute. In each cough droplet, there are about 16 copies of the virus. 28,29
SARS-Cov-2 has an affinity for angiotensin-converting enzyme 2 (ACE2) receptors to which its protein S binds. 5 Angiotensin-converting enzyme 2 is not only expressed in alveolar type II cells (AT2) but is also expressed in cells of the oral mucosa, esophagus, ileum, colon, and bile duct. 5-7,30,31,32 Affinity for these digestive system cells may explain transmission through the feces. 17,18 After entering the cytoplasm of digestive system cellss, viral proteins and viral RNA are synthesized and new viruses are assembled. These are released into the intestinal lumen and later reach the feces. 18 The affinity of SARS-Cov-2 with ACE2 receptors has led to a hypothesis now circulating that people taking ACE2 inhibitors may face high risks from COVID-19. 33 Nevertheless, this relationship has not been demonstrated, and the American and European Cardiology Associations recommend continuing ACE inhibitors in patients with COVID-19. 34,35
As mentioned, the epidemiological impact of transmission occurs through aerosols, close contact, inanimate surfaces, and fecal matter. All upper digestive endoscopies and colonoscopies generate aerosols 36,37. Upper digestive endoscopies produce aerosols from coughing and retching from nausea, and colonoscopies produce aerosols from flatus. All of these can contaminate nearby surroundings. 37 The importance of aerosols in the field of medical procedures has been emphasized in an extensive WHO guide on rational use of personal protective equipment for SARS-Cov-2. It contains very strict and precise instructions for health care professionals who perform procedures that generate aerosols. 38 In addition to generating aerosols, other potential sources of contact exist in endoscopy rooms. They include as close person-to-person contact; contact with environments contaminated by splashes of gastrointestinal fluids such as stretchers, pillows, sheets, blankets, floors and walls; and contact with accessories that are removed or inserted through the working channel of endoscopes. Contact with fluids that flow out of a patient that can even contaminate doctors’ shoes, as demonstrated in China. 39 These observations require us to consider endoscopy rooms to be contaminated environments. 40
Endoscopic procedure guidelines are absolutely necessary and must be widely disseminated. Because of the special characteristics of this infection in the gastrointestinal tract, and because endoscopy rooms bring together the most important SARS-Cov-2 transmission mechanisms (aerosols, fecal matter, contaminated surfaces and close contact) in one place, these procedures have high risks for medical and nursing personnel involved in them. Moreover, they could easily become a focus of dissemination of this infection within hospital institutions and their communities.
To prevent transmission of SARS-Cov-2 within endoscopy units, it is equally important that all those involved in endoscopic procedures strictly adhere to protection protocols as all will inevitably be exposed to secretions and patient fluids and that they follow with interest and responsibility recommendations published by international experts in gastroenterology and endoscopy as they are published and updated. 41
Experts from China, Italy and Spain, who have accumulated extensive and respectable experience in the present pandemic, suggest that the following strategy be chosen at this time to avoid transmission of the disease from asymptomatic patients to hospital staff or to healthy patients who undergo endoscopic procedures. Very importantly, this will also reduce health care expenses to free up hospital resources necessary to combat the pandemic. 42
Suspend all scheduled endoscopic procedures for diagnostic purposes. Also, face-to-face outpatient consultation should be done by internet or video call since patients’ visits the hospital can spread the infection to the patient or from the patient to infect others, diminishing the effectiveness of social isolation.
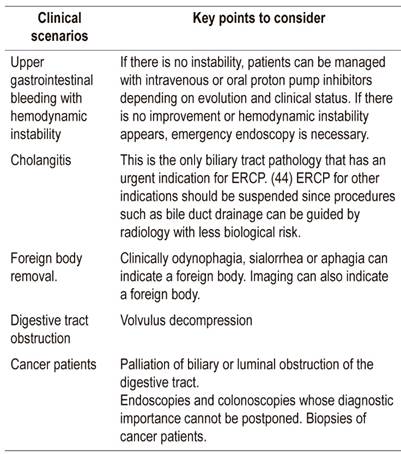
Perform only the therapeutic procedures listed in Table 1 for all patients whether or not they have COVID-19. Biliopancreatic echoendoscopy has no emergency indications. If choledocholithiasis is suspected with low or medium probability, Magnetic resonance cholangiopancreatography can be performed instead since it involves less biological risk. If there is “high probability”, ERCP should be performed.
During a pandemic, all people should be considered to be infected and contagious. Even those who are asymptomatic. 43 Patient risk should be stratified (see below risk stratification) based on the algorithm in Figure 1 in order to correctly use biosecurity measures according to risk and to limit exposure to other people in the waiting or recovery room.
Level 2 protection for medical and healthcare personnel is indicated if a patient is not infected. Level 3 is indicated if a patient is infected (Table 2).
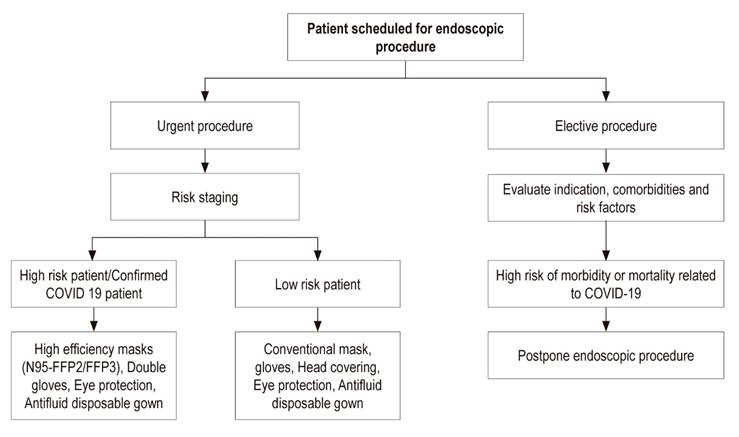
Figure 1 Flow chart of decision-making for endoscopic procedures in the COVID-19 pandemic. Adapted from 49
Table 2 COVID-19 Protection in endoscopy units
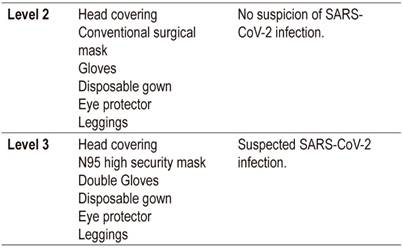
Taken from the recommendations of the Sociedad Española de Endoscopia Digestiva (SEED) and modified
At this moment of pandemic patients already scheduled for appointments should be contacted in order to explain the risks of an endoscopy room. Some may also have pathologies that warrant special treatment that can be identified in the interview. In Colombia, isolation has been initiated in a timely manner in order to avoid even minimal contact between and among people. The rationale for isolation is that most SARS-Cov-2 infections are transferred by asymptomatic individuals. Another consideration is that the face-to-face consultation should be suspended as long as social isolation lasts. These consultations should continue to be done virtually by internet or telephone and the physician should update the patient’s medical history in the usual way.
Risk stratification during scheduling of appointments41,45-48
Endoscopy patients should be stratified for COVID-19 risk, preferably by phone, one day before any procedure.
Patients should clearly be asked about recent history of fever, coughing, dyspnea, runny nose, diarrhea, abdominal pain, nausea, anosmia, and ageusia rather than just respiratory symptoms. Isolating only respiratory symptoms is ineffective for stopping the progression of the pandemic.
Patients should clearly be asked about any relatives with these symptoms.
Patients should clearly be asked about contact with people suspected of COVID-19 or with foreigners from any country.
Patients should clearly be asked about recent trips to high-risk areas (Europe, Latin America, and places in Colombia where there are patients such as hospitals).
During the interview, on the day of the endoscopy, a distance of at least one meter should be maintained. If possible, physical barriers such as glass should be used.
Family members and managers of the company responsible for the patients cannot have access to the endoscopy room. Before anyone is allowed to enter, she or he must undergo the same risk stratification protocol as patients.
Patients who are considered at risk for COVID-19 should be isolated in separate areas before and after any procedure.
All patients entering endoscopy rooms must have protective equipment (face masks and gloves).
Informed consent should include the risk of acquiring the infection in the endoscopy room.
Preventive measures during procedures50,51
Guarantee availability of all the required biosafety items according to the level of risk (see Tables 2 and 3) before entering the procedure room.
Everyone must wash hands strictly according to protocol.
Limit exposure to fomites as much as possible by removing all personal items (watches, rings, cell phones, ID cards, badges).
If orotracheal intubation is required for the procedure, only the anesthesiologist and assistant remain in the room during the intubation sequence. The rest of the team should remain outside.
After completing the procedure, the CDC’s recommendations for removing the biosecurity material should be followed (Table 3).
Only absolutely necessary personnel should be in the endoscopy room.
Table 3 Biosecurity items recommended for health care personnel 63
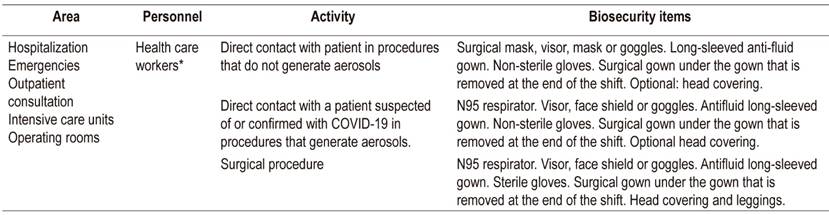
* Health care worker: person whose work requires contact with patients.
Post-procedure recommendations
Contaminated waste and endoscopic accessories from patients with high risk or confirmed COVID-19 should be treated according to recommendations for disposal of high risk material.
Patients should be contacted by phone on days 7 and 14 to inquire about respiratory symptoms of COVID-19 as well as gastrointestinal symptoms such as nausea, vomiting, abdominal pain, and diarrhea.
Reprocessing of post-endoscopy equipment
Commonly described infections associated with endoscopy include bacteria, fungi, parasites, and viruses, but adherence to guidelines of international endoscopy organizations has minimized or eliminated these risks for practically all patients and health care personnel who are present during procedures in endoscopy rooms. 52 Duodenoscopes that have a “nail” or elevator in their distal end are an exception. Because disinfection is more laborious and even impossible in some cases, 53 a disposable distal end has been designed for these endoscopes. 53
The safety of endoscopy procedures is guaranteed if established high-level disinfection guidelines for endoscopy equipment and accessories such as tweezers and papillotomes are complied with, 54 and if cleaning and disinfection procedures for endoscopy units are followed during and at the end of the session. This will directly protect patients and guarantee the safety of personnel who perform procedures as well as their assistants. 55-57
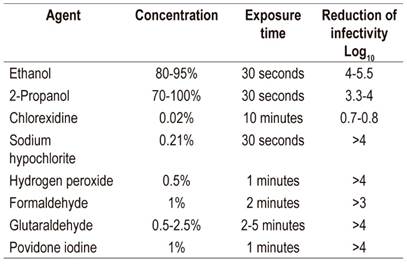
Instructions for washing and disinfecting equipment have not been modified because of the COVID-19 pandemic. Existing instructions are sufficient for the elimination of the virus. 58,59 On the other hand, there are new recommendations to avoid the spread of contagion to patients and health care personnel involved in the procedure. As mentioned, the main recommendations include elimination of diagnostic procedures and limiting performance solely to urgent therapeutic procedures using the measures for protection discussed in this article. Characteristics of antiseptics and susceptibility of the virus must be considered for cleaning work areas, surfaces, elements and hands. In the first instance, removal of organic material must be guaranteed by using soaps and detergents and by rubbing with the cleaning material. Table 4 lists antiseptics and their respective concentrations that guarantee reduction of the virus by at least 3 to 4 logarithms (Table 1). 58
Table 4 Antiseptic concentrations that reduce the concentration of coronavirus by three or more logarithms*
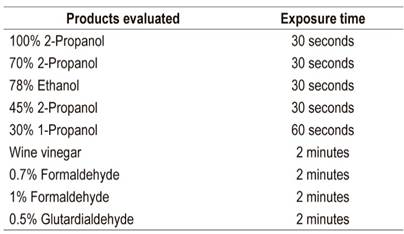
* Virucidal activity for SARS-CoV 58
Gastroenterology residents
Students are integral to most endoscopy units in university hospitals. During the COVID-19 pandemic, their roles in procedures need to be reevaluated. By increasing the duration of procedures, their participation increases risks of exposure. 60 Therefore, they should not perform endoscopic procedures or be in endoscopy rooms.
Their activity should be carried out in clean areas and should be limited to less risky activities such as filling out consent forms and reports. Their academic activities should continue through videoconferences and simulations (when availalbet) in clean rooms. This will allow them to watch and learn the execution and theory of (therapeutic) procedures.
Because gastroenterology residents generally already have one specialty, they can provide support by joining the COVID-19 management workforce. 61 If they do not have a first specialty, they can support the care of patients with COVID-19.
Residents in hospital centers must be reassigned within that institution since displacement carries the risk of accidental spread of the virus between hospitals. 62
Additional general recommendations63
These recommendations and the allocations they require should be the responsibility of epidemiological surveillance departments and the infectious disease departments.
Patients with confirmed diagnoses of COVID-19 and those suspected of having COVID-19 should wear face masks and isolate or separate from other patients at a distance of at least one meter. Ideally, there should be an exclusive recovery area for these patients.
Procedures should be performed in an airborne infection isolation room that meets level 3 biosecurity requirements.
Guarantee biosecurity items for all professionals within the endoscopy unit.
Train all personnel in the correct method of hand hygiene and insist on its use. Handwashing has a protocol which must be compulsory knowledge and must be complied with.
Removal and disposal of biosafety items must be carried out in a clean anteroom outside the procedure room which is separate from the rest of the endoscopy unit facilities.
Additional precautions must be taken to avoid contamination of items and equipment in work stations (computers, desks, pens).
Bathrooms are potential transmission sites. They must be completely separate from the sites used by patients and health care personnel.
Increase the frequency of washing and disinfection
Restrict the number of people in the procedure room to a maximum of 5 to reduce the risk of transmission and reduce use of critical biosafety material.
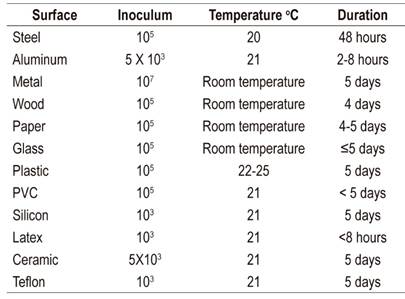
SARS-Cov-2 is very stable on surfaces. It has been found to remain viable in aerosols for 3 hours and on plastic and stainless steel for up to 72 hours although no viable virus is found on copper after 4 hours. No viable virus was found on cardboard after 24 hours. SARS-Cov-2’s stability is similar to that of SARS-CoV-1 for most substances. 27
The persistence of SARS-Cov-2 is shown in Table 5 while chemical agents that neutralize it are shown in Table 6. 62
Endoscopy services must maintain their independence from potential pressure from hierarchically superior administrative authorities. These recommendations are based on the most recently published scientific literature about clinical characteristics and modes of transmission as well as recently published universal guidelines on management of the COVID-19 pandemic by the WHO and by experts from the American Association of Gastrointestinal Endoscopy (ASGE), the Chinese Association of Gastroenterology, the Spanish Association of Digestive Diseases plus the experience of gastroenterologists and endoscopists.
Acknowledgments
We would like to thank Professors Diego Aponte and Fernando García del Risco for reviewing the manuscript and making recommendations
REFERENCES
1. Zhou W. The coronavirus prevention handbook: 101 science-based tips that could save your life [Internet]. 2020 [acceso 2020 Mar 26]. Disponible en: Disponible en: https://www.overdrive.com/search?q=76E6C249-9E76-47F7-B887-592DCDB1B16E [ Links ]
2. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020 [Internet]. [acceso 2020 Mar 25]. Disponible en: Disponible en: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 [ Links ]
3. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497-506. https://doi.org/10.1016/S0140-6736(20)30183-5 [ Links ]
4. China’s first confirmed Covid-19 case traced back to November 17 [Internet]. South China Morning Post. 2020 [acceso 2020 Mar 24]. Disponible en: Disponible en: https://www.scmp.com/news/china/society/article/3074991/coronavirus-chinas-first-confirmed-covid-19-case-traced-back [ Links ]
5. Hoffmann M, Kleine-Weber H, Krüger N, Müller M, Drosten C, Pöhlmann S. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells [Internet]. Molecular Biology; 2020 Jan [acceso 2020 Mar 23]. Disponible en: Disponible en: http://biorxiv.org/lookup/doi/10.1101/2020.01.31.929042 Disponible en: https://doi.org/10.1101/2020.01.31.929042 . [Epub ahead of print]. [ Links ]
6. Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med [Internet]. 2020 Mar 12 [acceso 2020 Mar 23]; Disponible en: Disponible en: http://link.springer.com/10.1007/s11684-020-0754-0 Disponible en: https://doi.org/10.1007/s11684-020-0754-0 [ Links ]
7. Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov [Internet]. Bioinformatics; 2020 Jan [acceso 2020 Mar 23]. Disponible en: Disponible en: http://biorxiv.org/lookup/doi/10.1101/2020.01.26.919985 Disponible en: https://doi.org/10.1101/2020.01.26.919985 [ Links ]
8. Smith JA, Judd J. COVID-19: Vulnerability and the power of privilege in a pandemic. Health Promot J Austr [Internet]. 2020 Mar 20 [acceso 2020 Mar 20]; Disponible en: Disponible en: http://doi.wiley.com/10.1002/hpja.333 Disponible en: https://doi.org/10.1002/hpja.333 . [Epub ahead of print]. [ Links ]
9. WHO. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. [Internet]. Dec 31, 2003 [acceso 2020 Mar 19]. https://www.who.int/csr/sars/country/table2004_04_21/en/ [ Links ]
10. Li J-Y, You Z, Wang Q, Zhou Z-J, Qiu Y, Luo R, et al. The epidemic of 2019-novel-coronavirus (2019-nCoV) pneumonia and insights for emerging infectious diseases in the future. Microbes Infect. 2020;22(2):80-5. https://doi.org/10.1016/j.micinf.2020.02.002 [ Links ]
11. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. 2020;395(10224):565-74. https://doi.org/10.1016/S0140-6736(20)30251-8 [ Links ]
12. Zhang T, Wu Q, Zhang Z. Probable Pangolin Origin of SARS-CoV-2 Associated with the COVID-19 Outbreak. Curr Biol. 2020 Mar;S0960982220303602. https://doi.org/10.1016/j.cub.2020.03.022. [Epub ahead of print]. [ Links ]
13. Pangolin. In: Wikipedia [Internet]. 2020 [acceso 2020 Mar 23]. Disponible en: Disponible en: https://en.wikipedia.org/w/index.php?title=Pangolin&oldid=947412030 [ Links ]
14. Gu J, Han B, Wang J. COVID-19: Gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020;S001650852030281X. https://doi.org/10.1053/j.gastro.2020.02.054 [ Links ]
15. WEO ALERT: Wuhan proposal for Safety in Digestive Endoscopy [Internet]. [acceso 2020 Mar 23]. Disponible en: Disponible en: http://www.worldendo.org/2020/02/05/weo-alert-wuhan-proposal-for-safety-in-digestive-endoscopy/ [ Links ]
16. Pan L, Mu M, Yang P, Sun Y, Yan J, Li P, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Soy J Gastroenterol. 2020 14 de abril. Doi: 10.14309/ajg.0000000000000620 Article in press [ Links ]
17. Luo S, Zhang X, Xu H. Don’t overlook digestive symptoms in patients with 2019 novel coronavirus disease (COVID-19).Clin Gastroenterol Hepatol. 2020 Mar 20. pii: S1542-3565(20)30401-8. doi: 10.1016/j.cgh.2020.03.043. [Epub ahead of print] [ Links ]
18. Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;S0016508520302821. doi: 10.1053/j.gastro.2020.02.055 [Epub ahead of print] [ Links ]
19. Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382(10):929-36. https://doi.org/10.1056/NEJMoa2001191 [ Links ]
20. Li Y, Bai W, Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may be at least partially responsible for the respiratory failure of COVID‐19 patients. J Med Virol. 2020;jmv.25728. https://doi.org/10.1002/jmv.25728 [ Links ]
21. Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA [Internet]. 2020 Feb 24 [acceso 2020 Mar 26]; Disponible en: Disponible en: https://jamanetwork.com/journals/jama/fullarticle/2762130 Disponible en: https://doi.org/10.1001/jama.2020.2648 [ Links ]
22. Center for Disease Control and Prevention. Filtering out Confusion: Frequently Asked Questions about Respiratory Problems [Internet]. [cited 2020 Mar 23]. Available from: Available from: https://www.cdc.gov/niosh/docs/2018-128/pdfs/2018- 128.pdf?id=10.26616/NIOSHPUB2018128 [ Links ]
23. Yeo C, Kaushal S, Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet. 2020;5(4):335-337. https://doi.org/10.1016/S2468-1253(20)30048-0 [ Links ]
24. CUHK Finds that the Coronavirus Can Persist in Stool after Its Clearance in Respiratory Tract Will Conduct Stool Test for People in Quarantine Camps for Early Identification [Internet]. [acceso 2020 Mar 21]. Disponible en: Disponible en: https://www.med.cuhk.edu.hk/press-releases/cuhk-finds-that-the-coronavirus-can-persist-in-stool-after-its-clearance-in-respiratory-tract-will-conduct-stool-test-for-people-in-quarantine-camps-for-early-identification [ Links ]
25. Wang XW, Li J, Guo T, Zhen B, Kong Q, Yi B, et al. Concentration and detection of SARS coronavirus in sewage from Xiao Tang Shan hospital and the 309th Hospital of the Chinese People’s Liberation Army. Water Sci Technol. 2005;52(8):213-21. https://doi.org/10.2166/wst.2005.0266 [ Links ]
26. Wei J, Li Y. Airborne spread of infectious agents in the indoor environment. Am J Infect Control. 2016;44(9):S102-8. https://doi.org/10.1016/j.ajic.2016.06.003 [ Links ]
27. Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1 [published online ahead of print, 2020 Mar 17]. N Engl J Med . 2020; DOI:10.1056/NEJMc2004973. [ Links ]
28. Fabian P, McDevitt JJ, DeHaan WH, Fung ROP, Cowling BJ, Chan KH, et al. Influenza Virus in Human Exhaled Breath: An Observational Study. Fouchier RAM, editor. PLoS ONE. 2008;3(7):e2691. https://doi.org/10.1371/journal.pone.0002691 [ Links ]
29. Lindsley WG, Blachere FM, Thewlis RE, Vishnu A, Davis KA, Cao G, et al. Measurements of Airborne Influenza Virus in Aerosol Particles from Human Coughs. Pekosz A, editor. PLoS ONE. 2010;5(11):e15100. https://doi.org/10.1371/journal.pone.0015100 [ Links ]
30. Zhang H, Kang Z, Gong H, Xu D, Wang J, Li Z, et al. The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes [Internet]. Microbiology; 2020 Jan [acceso 2020 Mar 23]. Disponible en: Disponible en: http://biorxiv.org/lookup/doi/10.1101/2020.01.30.927806 Disponible en: https://doi.org/10.1101/2020.01.30.927806 [ Links ]
31. Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):1-5. https://doi.org/10.1038/s41368-020-0074-x [ Links ]
32. Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, et al. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection [Internet]. Genomics; 2020 Feb [acceso 2020 Mar 22]. Disponible en: Disponible en: http://biorxiv.org/lookup/doi/10.1101/2020.02.03.931766 Disponible en: https://doi.org/10.1101/2020.02.03.931766 [ Links ]
33. Diaz JH. Hypothesis: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19. J Travel Med. 2020;taaa041. https://doi.org/10.1093/jtm/taaa041 [ Links ]
34. HFSA/ACC/AHA Statement Addresses Concerns Re: Using RAAS Antagonists in COVID-19 [Internet]. American College of Cardiology. [acceso 2020 Mar 22]. Disponible en: https://www.acc.org/latest-in-cardiology/articles/2020/03/17/08/59/hfsa-acc-aha-statement-addresses-concerns-re-using-raas-antagonists-in-covid-19 [ Links ]
35. Simone C. Position Statement of the ESC Council on Hypertension on ACE-Inhibitors and Angi [Internet]. [acceso 2020 Mar 26]. Disponible en: Disponible en: https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang [ Links ]
36. Muscarella LF. Recommendations for the prevention of transmission of SARS during GI endoscopy. Gastrointest Endosc. 2004;60(5):792-5. https://doi.org/10.1016/S0016-5107(04)01858-9 [ Links ]
37. Chapman S. Hot air? BMJ. 2001;323(7327):1449-1449. https://doi.org/10.1136/bmj.323.7327.1449 [ Links ]
38. World Health Organization. Rational use of personal protective equipment for coronavirus disease (COVID-19): interim guidance, 27 February 2020. 2020 [acceso 2020 Mar 22]; Disponible en: Disponible en: https://extranet.who.int/iris/restricted/handle/10665/331215 [ Links ]
39. Johnston ER, Habib-Bein N, Dueker JM, Quiroz B, Corsaro E, Ambrogio M, et al. Risk of bacterial exposure to the endoscopist’s face during endoscopy. Gastrointest Endosc. 2019;89(4):818-24. https://doi.org/10.1016/j.gie.2018.10.034 [ Links ]
40. Repici A, Maselli R, Colombo M, Gabbiadini R, Spadaccini M, Anderloni A, et al. Coronavirus (COVID-19) outbreak: what the department of endoscopy should know. Gastrointest Endosc. 2020;S0016510720302455. https://doi.org/10.1016/j.gie.2020.03.019 [ Links ]
41. JOINT GI SOCIETY MESSAGE: COVID-19 Clinical Insights for Our Community of Gastroenterologists and Gastroenterology Care Providers [Internet]. Default. [acceso 2020 Mar 20]. Disponible en: Disponible en: https://www.asge.org/home/joint-gi-society-message-covid-19 [ Links ]
42. Ayittey FK, Ayittey MK, Chiwero NB, Kamasah JS, Dzuvor C. Economic impacts of Wuhan 2019-nCoV on China and the world.J Med Virol. 2020;92(5):473-475. https://doi.org/10.1002/jmv.25706 [ Links ]
43. World Health Organization. Disease commodity package - Novel Coronavirus (nCoV). Operational Support & Logistics Disease Commodity Packages [Internet]. [acceso 2020 Mar 20]. Disponible en: Disponible en: https://www.who.int/emergencies/what-we-do/prevention-readiness/disease-commodity-packages/dcp-ncov.pdf [ Links ]
44. Shah SL, Carr-Locke D. ERCP for acute cholangitis: timing is everything. Gastrointestinal Endoscopy. 2020;91(4):761-762. https://doi.org/10.1016/j.gie.2019.12.010 [ Links ]
45. ESGE and ESGENA Position Statement on gastrointestinal endoscopy and the COVID-19 pandemic - European Society of Gastrointestinal Endoscopy (ESGE) [Internet]. [acceso 2020 Mar 22]. Disponible en: Disponible en: https://www.esge.com/esge-and-esgena-position-statement-on-gastrointestinal-endoscopy-and-the-covid-19-pandemic/ [ Links ]
46. Sociedad Española de Endoscopia Digestiva (SEED). Recomendaciones de la SEED: Protección en Unidades de Endoscopia frente al COVID-19 [Internet]. [acceso 2020 Mar 23]. Disponible en: Disponible en: https://wseed.es/images/site/guia_clinica/2020/RecomendacionesSEED_ProteccionUnidadesEndoscopia_Coronavirus.pdf [ Links ]
47. Johnston ER, Habib-Bein N, Dueker JM, et al. Risk of bacterial exposure to the endoscopists face during endoscopy. Gastrointest Endosc. 2019; 89: 818-824. https://doi.org/10.1016/j.gie.2018.10.034 [ Links ]
48. Cao Z, Zhang Q, Lu X, Pfeiffer D, Jia Z, Song H, Zeng DD. Estimating the effective reproduction number of the 2019-nCoV in China. [Internet]. [acceso 2020 Mar 23]. Disponible en: medRxiv 2020.01.27.20018952; doi: Disponible en: medRxiv 2020.01.27.20018952; doi: https://doi.org/10.1101/2020.01.27.20018952 [ Links ]
49. Zhang Y, Zhang X, Liu L, Wang H, Zhao Q. Suggestions of Infection Prevention and Control in Digestive Endoscopy During Current 2019-nCoV Pneumonia Outbreak in Wuhan, Hubei Province, China. [acceso 2020 Mar 23]; Disponible en: Disponible en: http://www.worldendo.org/wp-content/uploads/2020/02/Suggestions-of-Infection-Prevention-and-Control-in-Digestive-Endoscopy-During-Current-2019-nCoV-Pneumonia-Outbreak-in-Wuhan-Hubei-Province-China.pdf [ Links ]
50. Wang X, Pan Z, Cheng Z. Association between 2019-nCoV transmission and N95 respirator use. J Hosp Infect [Internet]. 2020 Mar 3. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3214288 [ Links ]
51. Beilenhoff U, Biering H, Blum R, et al. Reprocessing of flexible endoscopes and endoscopic accessories used in gastrointestinal endoscopy: Position Statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA) - Update 2018. Endoscopy. 2018;50:1205-1234. [ Links ]
52. Petersen BT, Cohen J, Hambrick RD, Buttar N, Greenwald DA, Buscaglia JM, et al. Multisociety guideline on reprocessing flexible GI endoscopes: 2016 update. Gastrointest Endosc. 2017;85(2):282-294.e1. https://doi.org/10.1016/j.gie.2016.10.002 [ Links ]
53. Forbes N, Elmunzer BJ, Allain T, Chau M, Koury HF, Bass S, et al. Infection control in ERCP using a duodenoscope with a disposable cap (ICECAP): rationale for and design of a randomized controlled trial. BMC Gastroenterol. 2020;20(1):64. https://doi.org/10.1186/s12876-020-01200-7 [ Links ]
54. Yang R, Ng S, Nichol M, Laine L. A cost and performance evaluation of disposable and reusable biopsy forceps in GI endoscopy. Gastrointest Endosc. 2000;51(3):266-70. https://doi.org/10.1016/S0016-5107(00)70353-1 [ Links ]
55. Rutala WA, Weber D. Center for Disease Control and Prevention (CDC). Disinfection of Healthcare Equipment. Guideline for Disinfection and Sterilization in Healthcare Facilities (2008). Disponible en: https://www.cdc.gov/infectioncontrol/pdf/guidelines/disinfection-guidelines-H.pdf [ Links ]
56. Calderwood AH, Chapman FJ, Cohen J, Cohen LB, Collins J, Day LW, et al. Guidelines for safety in the gastrointestinal endoscopy unit. Gastrointest Endosc. 2014;79(3):363-72. https://doi.org/10.1016/j.gie.2013.12.015 [ Links ]
57. Calderwood AH, Day LW, Muthusamy VR, Collins J, Hambrick RD, Brock AS, et al. ASGE guideline for infection control during GI endoscopy. Gastrointest Endosc. 2018;87(5):1167-79.https://doi.org/10.1016/j.gie.2017.12.009 [ Links ]
58. Geller C, Varbanov M, Duval R. Human Coronaviruses: Insights into Environmental Resistance and Its Influence on the Development of New Antiseptic Strategies. Viruses. 2012; 4(11): 3044-68. https://doi.org/10.3390/v4113044 [ Links ]
59. Rio C del, Malani PN. COVID-19-New Insights on a Rapidly Changing Epidemic. JAMA [Internet]. 2020 Feb 28 [acceso 2020 Mar 22]; Disponible en: Disponible en: https://jamanetwork.com/journals/jama/fullarticle/2762510 Disponible en: https://doi.org/10.1001/jama.2020.3072 [ Links ]
60. Soetikno R, Teoh A, Kaltenbach T, Lau J, Asokkumar R, Cabral-Prodigalidad P. Considerations in performing endoscopy during the COVID-19 pandemic; Joint GI Society Message: Gastroenterology COVID-19 Guide: Telehealth March 20, 2020. [ Links ]
61. Bai Y, Yao L, Wei T, Tian F, Jin D-Y, Chen L, et al. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA [Internet]. 2020 Feb 21 [acceso 2020 Mar 22]; Disponible en: Disponible en: https://jamanetwork.com/journals/jama/fullarticle/2762028 Disponible en: https://doi.org/10.1001/jama.2020.2565 [ Links ]
62. Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104(3):246-51. https://doi.org/10.1016/j.jhin.2020.01.022 [ Links ]
63. World Health Organization. Rational use of personal protective equipment for coronavirus disease 2019 (COVID-19). Interim guidance 27 February 2020. [ Links ]
Received: March 24, 2020; Accepted: March 26, 2020











 text in
text in