Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.35 no.1 Bogotá Jan./Mar. 2020
https://doi.org/10.22516/25007440.379
Review articles
Diagnostic and therapeutic approach to cow’s milk protein allergy
1Médico; magíster en Epidemiología; docente en la Fundación Universitaria San Martín y en la Universidad del Valle. Cali, Colombia
2Pediatra; docente en la Fundación Universitaria San Martín, en la Universidad Santiago de Cali y en la Universidad Icesi. Cali, Colombia
3Médico; residente de especialidad en Medicina Familiar, Universidad del Valle. Cali, Colombia
4Médico cirujano, residente de especialidad en Medicina Familiar, Universidad del Valle. Cali, Colombia
5Pediatra, Hospital Mario Correa Rengifo; docente en la Universidad del Valle, la Universidad Santiago de Cali, la Fundación Universitaria San Martín, la Universidad Icesi. Investigador Junior, Colciencias. Cali, Colombia
The worldwide prevalence of cow’s milk protein allergy (CMPA) is approximately 1.9% to 4.9%. Its prevalence in Colombia is unknown. A high percentage of cases are unsuspected by medical personnel resulting in delayed diagnosis and treatment which increase the time and resources used to establish the etiology of this condition in children. The clinical history is fundamental for diagnosis of CMPA, especially the background evaluation. Of special importance are early exposure to the protein and atopy in first degree relatives. CMPA’s initial presentation may be digestive, cutaneous or respiratory. Digestive symptoms can include vomiting and acute diarrhea, and cutaneous symptoms include hives, dermatitis and angioedema. Respiratory and systemic manifestations occur less frequently. The wide variety of clinical manifestations and signs can challenge health care professionals who are not alert to this pathology to the point that the diagnosis is not even considered event though delaying the suspension of cow’s milk protein from the diet delays access to an effective treatment. The well-recognized ideal treatment is an exclusion diet which requires strict compliance. For children who are exclusively breastfed, the mother’s diet must restrict milk and its derivatives. Children who are not breastfed, should be fed formulas of extensively hydrolyzed milk proteins based on amino acids. The prognosis is favorable, and most children will tolerate cow’s milk proteins at two years. The process may take more years for polysensitive patients. Oral immunotherapy is an option that is available for patients who do not achieve toleration.
Keywords: Cow’s milk protein allergy; clinical practice; guidelines; diagnosis; treatment; prevention
La prevalencia de la alergia a las proteínas de la leche de vaca (APLV) en el ámbito mundial es, aproximadamente, de 1,9 a 4,9 %. En Colombia, esta cifra se desconoce. En un alto porcentaje de los casos, no existe la sospecha por parte del personal de salud, y, por lo tanto, el diagnóstico y el tratamiento se retrasan. Esto acarrea un aumento en el tiempo y en los recursos que emplean los profesionales de la salud y los padres en procura de establecer la etiología del padecimiento de los niños que presentan esta enfermedad. Dentro de este contexto, la historia clínica es fundamental en la sospecha de la APLV, y es especialmente relevante la evaluación de antecedentes, en los cuales se destacan la presencia de la exposición temprana a la proteína, así como la atopia en familiares en primer grado de consanguinidad. La presentación de la APLV puede manifestarse con reacciones inmediatas a nivel digestivo (vómitos, diarrea aguda), cutáneo (urticaria, dermatitis, angioedema) y, con menos frecuencia, a partir de signos respiratorias y sistémicos. Sin embargo, la amplia variedad de manifestaciones clínicas y signos puede ser un reto para el profesional que no se encuentre sensibilizado con la patología, e incluso soslayar este diagnóstico retrasa la suspensión de la proteína de la leche de vaca de la dieta y demora el acceso a un tratamiento eficaz. El tratamiento ideal reconocido es la dieta de exclusión, la cual requiere un estricto cumplimiento. En los niños alimentados con lactancia materna exclusiva, será necesaria la dieta restrictiva de leche y sus derivados en la madre. En quienes no reciben lactancia, se deberá tratar mediante fórmulas de proteínas lácteas extensamente hidrolizadas (FEH) o a base de aminoácidos (FAA). Así bien, el pronóstico es favorable y la mayoría de niños tolerarán las proteínas de la leche de vaca a los 2 años, mientras que en pacientes con polisensibilización el proceso puede prolongarse. En el caso de no alcanzar la tolerancia, la inmunoterapia oral es una opción disponible.
Palabras clave: Alergia a la proteína de la leche de vaca; práctica clínica; guías; diagnóstico; tratamiento; prevención
Introduction
The antigens that most frequently produce hypersensitivity reactions in infants are cow’s milk proteins ingested directly in formula or through breast milk. Cow’s milk protein allergy (CMPA) leads to a wide variety of clinical manifestations among which digestive manifestations predominates. Digestive tract segments or the entire length can be affected. They are frequently followed by skin and respiratory compromises. 1
Food allergies can be mediated by immunoglobulin E (IgE) type antibodies, not mediated by IgE, or mediated by mixed mechanisms. During the first year of life, CMPA is the most frequent presentation of food allergy. Its symptoms are nonspecific and frequently include pathological gastroesophageal reflux (GER), blood in stools, lack of appetite, colic, diarrhea, and constipation. Cases of enteropathy with poor weight gain occur less frequently. 1,2. Lower calcium and lipid intake as well as lower serum retinol, beta-carotene, lycopene, and 25-hydroxyvitamin D levels have also been observed in these pediatric patients. 3
This study is based on a review of the literature using the keywords “cow’s milk protein allergy”, “clinical practice”, “guidelines”, “diagnosis”, “treatment” and “prevention”. A search for relevant articles published in English and Spanish was carried out in Pubmed from March 2017 to June 2019.
Randomized trials, literature reviews, and case studies were included. The terms were developed with help from an epidemiologist and a pediatric specialist.
All titles and abstracts were retrieved from the original publication for selection. Articles were subsequently analyzed independently by two literature reviewers. A total of 95 articles were reviewed.
Definition
CMPA is defined as a reproducible adverse reaction to one or more milk proteins, usually caseins, α-lactalbumin, and/or β-lactoglobulin. They are mediated by one or more immune mechanisms. 4,5. The immunological mechanism distinguishes CMPA from other adverse reactions to cow’s milk such as lactose intolerance.
Natural history
Generally, symptoms develop one week after the introduction of cow’s milk although they can appear after 24 and 36 weeks later. 6,7 The mean age of onset is similar to 2.8 +/- 1.8 months and 3.5 +/- 2.8 months. 5,8
In most children, CMPA symptoms appear before 6 months of age, as described in multiple literature reviews and in an Argentine study by Mehaudy et al. 5-9 The trigger is cow’s milk and formulas or food based on it. This may occur because it is the first dietary protein to which children are exposed. A smaller proportion of infants develop a reaction to breastfeeding. 10
Studies prior to 2005 showed that CMPA had a good prognosis, since between 80% and 90% of children developed tolerance during school age. 10-12
Epidemiology
Although there are no comparable international epidemiological data on the prevalence of CMPA, given that there are different methods of clinical evaluation, the results of cohort studies and metaanalyses show that of CMPA’s overall prevalence is between 1.9% and 4.9% with a peak prevalence (2% to 3%) in the first year of life. A study by Eggesbo et al. has described a prevalence of less than 1% in children older than six years of age in Norway. 12
Parents recognize CMPA in their children much more frequently than can be confirmed by diagnostic studies, and symptoms suggesting adverse reactions to cow’s milk protein occur in 5% to 15% of children, exceeding true approximations of the prevalence of the CMPA. 13
Accurate diagnosis is important for preventing infants from being subjected to inappropriate exclusion diets which can have a long-term effect on growth and development. The prognosis of CMPA in childhood is good, and the remission rate is up to 90% at 3 years with better prognoses in cases of gastrointestinal symptoms. 14
Most children have two or more symptoms while CMPA is clinically active. However, presence of only one symptom does not rule out the possibility of allergy. It is important to mention that available data comes centers that specialize in allergies and gastrointestinal disorders where incidences as high as 14% at first-time consultations, of which 71% are CMPA, have been reported. 2
Pathophysiology
Cow’s milk whey and casein contains about 20 potentially sensitizing proteins. They include α-lactalbumin, β-lactoglobulin, bovine immunoglobulins, and casein allergens. 15 The effect of the industrial process on the antigenic/allergenic properties of cow’s milk proteins is minimal. 16
As with other food allergies, factors that promote oral tolerance or sensitization to cow’s milk include genetic predisposition, infections and alterations of the intestinal microflora, first exposure, maternal diet, transmission of the antigen through breast milk, and the amount and frequency of antigen load. 17
The organs primarily affected by allergies are the gastrointestinal tract, the skin, and the respiratory tract. In some settings systemic compromise can occur. The antigen, in this case the proteins from cow’s milk, passes through the intestinal lumen and is recognized by the M cells of the intestinal mucosa which carry the information to antigen presenting cells (usually dendritic cells of the submucosa).
Thus, presenting cells show the antigen to helper T lymphocytes (T helper 0 or Th 0) which causes an overexpression of the response of the helper T lymphocytes type 2 (Th2) through cytokines such as interleukins (IL) 4 and 13 which it secretes. 18 Th2 stimulates B lymphocytes which are prepared to synthesize specific IgE against that antigen.
In clinical expressions not mediated by IgE, IL-5 and tumor necrosis factor alpha (TNFα), cytokines secreted by the Th0 cell, promote recruitment of neutrophils and eosinophil activation and can determine the appearance of edema, pain and abnormal functioning of organs. Thus, when a child is exposed to the antigen again, an antigen-antibody reaction occurs that triggers the response of previously prepared B lymphocytes, or the degranulation of mast cells/eosinophils, which generates manifestations in different organs. 19
Clinical manifestations
Onset of symptoms occurs when exposure to cow’s milk protein occurs due to consumption of dairy products by the mother who is breastfeeding her baby, due to feeding an infant formulas, or from consumption of cow’s milk. Depending on the severity and time of the reaction, clinical manifestations can present in three ways:
Immediate reactions occur within 30 min and are characterized by local skin reactions such as urticaria, rashes, oral allergic syndrome, facial angioedema, anaphylaxis and IgE elevation.
Medium term reactions occur after a few hours and are not mediated by IgE. Generally they appear as gastrointestinal symptoms.
Delayed reactions appear between days 1 and 5. Participation of the IgE-mediated response is uncertain. They are characterized by gastrointestinal, respiratory and/or cutaneous symptoms such as rhinitis, eczema, urticaria, angioedema and anaphylaxis that are all associated with IgE, but they may also appear as pulmonary hemosiderosis, villous atrophy malabsorption, eosinophilic proctocolitis, enterocolitis, and esophagitis which are not associated with IgE. 20
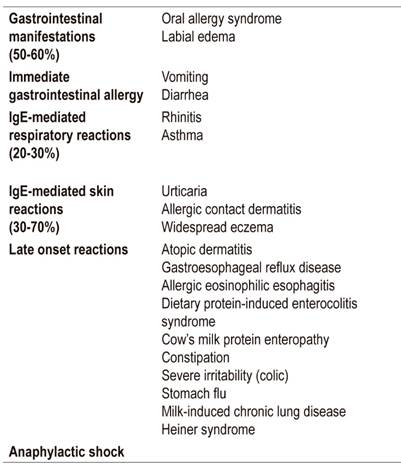
It is estimated that between 50% and 60% of clinical manifestations are digestive, 21,22 and that the most frequent digestive manifestations are bloody stools. 5 Respiratory manifestations occur in 20% to 30%, while 30% to70% have cutaneous manifestations, 23,24 the most common of which are rashes. 25
In addition, neurological and systemic manifestations such as insomnia, edema and other conditions can occur. 26 Of the gastrointestinal symptoms, only a small proportion are IgE-mediated. A relationship between gastroesphogeal reflux and CMPA has been described in more than 50% of cases, evidence of the two entities coexisting.
Vandenplas et al. reported gastroesphogeal reflux disease (GERD), diagnosed with pH testing, in 50% of children with CMPA. Only 10% of healthy children have GERD (Table 1). 26,27
Diagnosis
The non-specificity of CMPA’s clinical manifestations is the reason the disease is not recognized early. One form or another of Cow’s milk is ingested daily by infants in greater amounts than any other individual food. It is important to describe the age of onset, type and frequency of symptoms, time between ingestion and onset of symptoms, dietary details, and any personal and/or family history of atopy. 26
It is necessary to define whether a mother who is breast feeding a baby is herself consuming dairy products and their derivatives because cow’s milk proteins may appear in breast milk and expose the baby to development of CMPA.
Similarly, it must be established whether a child who is exclusively breastfed was exposed to breast milk substitute formulas during the 24 hours after birth because of institutional care protocols for newborns or in cases of hypoglycemic prevention. It has been observed that children subjected to this practice have a risk of developing CMPA that is seven times greater than controls who were exclusively breast fed during the first 24 hours. 27
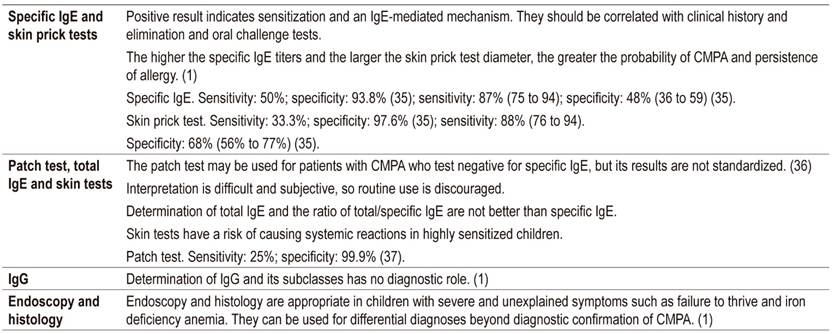
None of the diagnostic tests available in routine clinical situations fully demonstrate or exclude CMPA. (26) Doctors can perform a skin prick test, specific IgE determination, and/or patch tests. However, these only indicate sensitization to the substrate which does not necessarily constitute confirmation of an allergic reaction.
Some studies have shown that the sensitivity of skin prick tests is 31.8, their specificity is 90.3%, their negative predictive value (NPV) is greater than 95%, and their positive predictive value is less than 50 %. They serve to rule out specific antibody reactions and to make etiological diagnoses of asthma, rhinitis, and food allergies. 28 In cases where a dietary challenge cannot be performed, both the skin test and IgE can be used. 29,30
Diagnostic detection procedures
The double-blind, placebo-controlled food challenge (DBPCFC) is considered the gold standard for diagnosing CMPA, but in practice only one open challenge is performed. 31 The patient suspected of having CMPA must follow an exclusion diet for two to four weeks. Formula-fed infants begin extensively hydrolyzed formula (EHF), and mothers who exclusively breast feed start a diet free of cow’s milk protein. If CMPA is present, clinical manifestations will disappear.
Cow’s milk protein is gradually reintroduced, and clinical symptoms are monitored. The risk of an open challenge that the diagnosis will be overestimated. 32 A double-blind, placebo-controlled challenge will blind the parent and physician to the introduction of cow’s milk protein and is the only objective way to make the diagnosis.
Unfortunately, this process is expensive, requires extensive preparation, is time consuming, and is difficult to perform. Medical supervision during a challenge is necessary because the severity of symptoms cannot be predicted. When additional serum specific IgE, and skin tests are negative, life-threatening manifestations are extremely rare, but hospital care with an established protocol is indicated for patients with a history of severe reactions or high levels of IgE.
When CMPA is confirmed, the elimination diet should be continued until the patient is between 9 and 12 months of age, or at least 6 months. After that, a new challenge can be performed. Children who do not develop allergy-related manifestations within a week can resume their normal diets.
Skin tests and measurement of specific IgE make it possible to establish a child’s sensitization to cow’s milk protein and to predict possible new reactions. The probability of a positive result in the controlled oral challenge test is over 95% when the specific IgE concentration is greater than one IU/mL in children under two, and when it is greater than 15 IU/mL in children older than two years. 33 The PPV of a skin test reaction to milk consumption is over 95% when a 6 mm wheal is formed in children younger than 2 years and when a 8 mm wheal is formed in older children (Table 2). 34
Non-IgE mediated tests such as the atopic patch test can be non-invasive and allow evaluation of cellular response but are not standardized. There are also other tests such as cellular function, precipitins, intestinal permeability, eosinophils and TNFα. Invasive gastrointestinal endoscopy for biopsies requires histological study and is not usually recommended.
Histological diagnosis of samples from the small intestine requires a pathologist’s report of more than 60 eosinophils in six high-power fields, or more than 15 to 20 eosinophils per field with more than 25% inflammatory infiltrate, and the presence of intraepithelial eosinophils plus eosinophilic abscesses in the crypts.
Table 2 Diagnostic detection procedures
IgE: immunoglobulin E; IgG: immunoglobulin G; CMPA: cow’s milk protein allergy
In the colon, focal erythema, friable mucosa, lymphoid follicular hyperplasia (present in 75% of cases) and allergic vasculitis must be found macroscopically. Microscopically, it is necessary to find local eosinophil infiltrate in all compartments. 33
Diagnostic tools
Correct diagnosis of CMPA can be delayed because its symptoms and signs are broad and nonspecific. Diagnosis involves a two to four week elimination diet followed by a cow’s milk protein challenge.
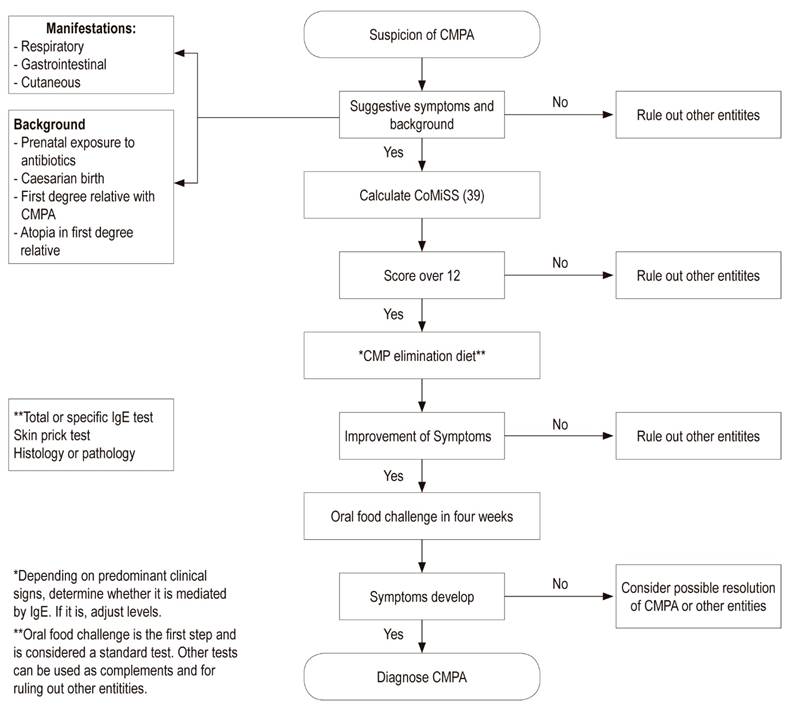
Consequently, the Cow’s Milk-related Symptom Score (CoMiSS) has been developed to facilitate the diagnostic process. 38 It includes gastrointestinal manifestations of regurgitation and impaired bowel movements, skin signs of eczema and urticaria), respiratory tract symptoms, and general symptoms such as crying time.
The CoMiSS varies from 0 to 33 points, from the absence of symptoms and signs to multiple manifestations and degrees of severity. A cut-off point of 12 was proposed by the expert panel. Existing data shows that the predictive value of the tool for identifying babies at risk of CMPA may be 80%.
CoMiSS was validated in a study that found its sensitivity to be 87.5% and its specificity to be 78.6%. 39 This tool is a step towards reducing delays and difficulties in diagnosis of CMPA (Figure 1). 38
Differential diagnosis
The list of possible differential diagnoses for CMPA includes recurrent viral infections and transient lactose intolerance. In addition, GERD has been mentioned as a possible manifestation of CMPA, 40,41 and CMPA has also been associated with about 10% of infant colic cases. 42,43
Although a correlation between atopic dermatitis and CMPA is suggested in some infants, many cases of this type of dermatitis are not associated with an allergy. The younger the infant, or the more severe the atopic dermatitis, the stronger the association seems to be. 44
Reactions to other foods (especially egg and soy, wheat, and fish) frequently occur in combination with CMPA. 45 Therefore, these foods should be avoided during diagnostic exclusion testing.
Treatment of CMPA
The safest strategy for treating CMPA is strict avoidance of cow’s milk protein. The decision to use a substitute formula and the option chosen to meet the nutritional needs of children with CMPA should be made primarily on the basis of the patient’s age and any history of other food allergies. For infants who are exclusively breastfed, elimination implies exclusion of milk derivatives from the maternal diet. Mothers need to receive calcium supplements and dietary advice to avoid nutritional deficiencies. 1
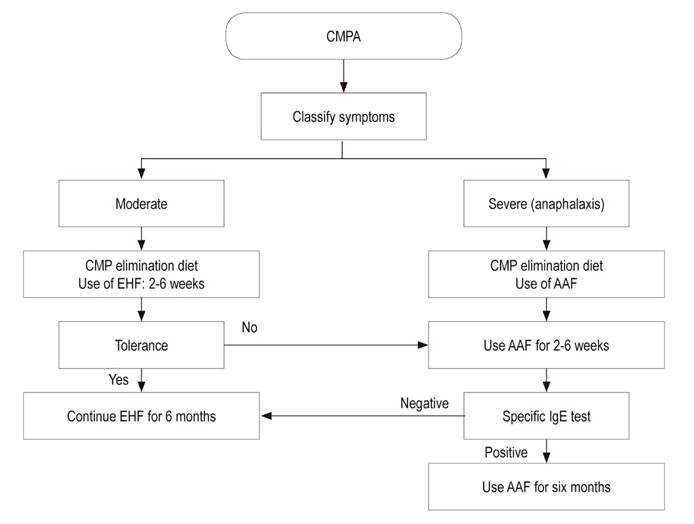
All formulas based on cow’s milk and all complementary food containing cow’s milk derivatives should be avoided in exclusively breastfed infants. When allergy symptoms occur in infants who are fed formula, either exclusively or as a supplement to breastfeeding, they should be given a therapeutic formula that has been clinically proven to reduce allergenicity and has high tolerability. 46,47 EHFs and amino acid-based formulas (AAFs) are the two alternatives recommended for formula fed infants with CMPA. 25,46,48,49
EHFs are indicated for treatment and prevention of CMPA and are tolerated by most infants and children with this condition. 1,48 AAFs were developed to overcome hypersensitivity to residual proteins in EHF, particularly in patients with severe enteropathy or with multiple food allergies. 48 For this reason, AAFs can be considered only for infants with severe reactions such as anaphylaxis, enteropathy, eosinophilic esophagitis, protein-induced enterocolitis and for patients who have compromises of multiple systems, several food allergies, and intolerance to EHF. 14,48,50
While soy formulas are associated with lower allergenicity than those based on cow’s milk 51,52, concerns have been raised about their high isoflavone (phytoestrogen) content. 53
The European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the American Academy of Pediatrics (AAP) consider the use of EHF and AAF to be the first treatment option for infants with CMPA. 52-54 Similarly, they do not recommend the use of partially hydrolyzed formulas (PHF) based on cow’s milk or other mammals’ milk. 55-57 Despite this, the Middle Eastern consensus has included the use of PHF as a bridge therapy between EHF and AAF in their management algorithm for selected cases.
For infants with documented CMPA who are fed exclusively with breast milk and formula, transitional foods should be free of cow’s milk protein until the development of tolerance is confirmed by oral exposure tests. Dietary supervision by a health professional who specializes in, or is trained in, pediatric nutrition is recommended for children with CMPA who are over 12 months of age who are on an exclusion diet. This supervision is important for making decisions about sufficient amounts of nutrients, proteins, calcium, vitamin D and vitamin A in the child’s diet. This is also essential for choosing any nutritional formula or supplements needed for achievement of normal growth according to the child’s age. 49,56,57
Similarly, it is essential to determine a child’s tolerance to cow’s milk protein in order to avoid prolonging restrictive diets that affect a child’s growth and development of the child and that may also compromise the nutritional status of a breastfeeding mother. 2,49
Exclusively breastfed children
During exclusive breastfeeding, any food containing milk protein must be removed from the mother’s diet. The physician must tell the woman that foods whose labels indicate that they contain milk, whey, milk solids, casein and caseinate are prohibited.
At the end of six months, complementary feeding should begin but the consumption of food containing cow’s milk protein should be delayed. During the mother’s elimination diet, she should receive nutritional counseling and supplements of 1000 mg of calcium per day and 800 IU of vitamin D per day. 50
Children fed with formula or formula plus breast milk
These patients should receive a diet that excludes dairy products with a therapeutic formula for CMPA.
Formulas Indicated for Patients with CMPA
Formulas indicated for patients with CMPA do not generate reactions in 90% of infants and children with confirmed CMPA.
AAF are synthetic formulas based that have no amino acids. Because they are lactose free, they are the first line option for treatment of CMPA.
EHF are formulas produced by enzymatic hydrolysis, heat treatment and ultrafiltration processes which are adapted for use in infants. These processes break cow’s milk protein into shorter peptide chains.
Amino acid formulas with oligosaccharide supplementation of breast milk are now being developed. They are proposed as supplements for EHF with 2’fucosyl-lactose (2’FL) and lacto-N-neotetraose (LNnT). These two supplements are based on the use of oligosaccharides found in breast milk whose addition they could reduce the risk of enteric infections. Furthermore, they may provide a substrate for colonization of the child’s intestine with beneficial bifidobacteria thereby reducing colonization by pathogens.
These supplements could positively affect intestinal epithelial integrity, apoptosis, and intestinal permeability. In fact, they have reduced allergic symptoms to cow’s milk protein in animal models although this is an area that requires more investigation. 2,58
Inappropriate Formulas for CMPA
PHF are formulas in which peptide epitopes capable of producing allergic reactions are conserved. 49
Milk from other mammals is not nutritionally appropriate for use in infants. 49
Milk made from almonds, hazelnuts, rice, soy, coconut and other vegetables is not nutritionally adequate. In fact, they are juices that are inappropriately called milk since they do not come from the any mammary glands. They provide low caloric intake and low bioavailability. 49
Soy formulas, unlike the so-called milks of other vegetables, are adapted for use by infants. Nevertheless, the availability of minerals such as zinc, iron, magnesium and phosphorus may be low due to their phytate content. In addition, cross-reactions have been reported in 10% to 30% of cases with CMPA. 49
Cross-reactions with soy-based infant nutritional formulas have been found in 17.3% of infants with CMPA, regardless of whether they were positive or negative in tests for cow’s milk protein specific IgE. 52,59
In particular, infants with multiple food allergies and eosinophilic enterocolitis react to soy protein. 60 Not surprisingly, specialist groups have different positions on the use of soy formula for CMPA, but they generally agree that they should not be used before 6 months of age. 46,52,58
CMPA prevention
The risk of developing allergies has a genetic component that can be determined by a family factor. Historical data shows that the incidence of atopic disease is around 15% but that it is higher in children with a family history of atopic disease. If a family member has an allergy, the risk of it also occurring in siblings is 10 times greater than in the general population. 61
Breast milk is universally recognized as the ideal food for infants, and breastfeeding as the ideal way to provide that milk for healthy growth and development of infants. The World Health Organization recommends that infants be exclusively breastfed for the first six months of life to achieve healthy growth and development. After this period, breastfeeding should continue together with nutritionally adequate and safe complementary foods until the child is two years or older. Exclusive breastfeeding has proven to be the best method for preventing allergies.
Children who are exclusively breastfed have been identified as having lower risks of developing CMPA. It has also been observed that if CMPA appears during childhood it is less severe for breastfed children than for those fed with formulas or breast milk and formulas. The reason for this lower risk is that breast milk has 100,000 times less protein than cow’s milk, and breast milk also contains immunomodulators. 50
There is conflicting evidence about whether delaying the introduction of solid foods into an infant’s diet helps prevent the incidence of allergies. Some studies suggest that restriction and delay in the introduction of food can prevent allergies, 62-64 but other authors argue that early introduction has no adverse effects and may even be protective against allergies. 64,65 In addition, restricting solid foods after a child reaches six months of age can lead to inadequate nutrient intake, feeding problems, and growth deficits. 65 In summary, the evidence suggests that there is no benefit in delaying introduction or imposing a specific restriction on potentially allergenic foods beyond four to six months. 2
Meanwhile, prebiotics and probiotics are often marketed with the promise that they may help prevent allergies. 66,67 In fact, some studies suggest that mothers who take probiotic supplements during pregnancy and lactation may help prevent early atopic disease in infants. 68
A systematic review has found that children who had received probiotics acquired greater tolerance of cow’s milk protein at the end of three years than did children who received placebos. However, the level of evidence is low given the quality of the studies included. 69
There is also evidence to suggest that supplementing EHF with prebiotics may decrease the incidence of allergic manifestations such as atopic dermatitis, recurrent wheezing, and allergic urticaria in childhood. 70,71 However, no studies have been published that demonstrate that this also occurs with PHF supplemented with prebiotics.
Nevertheless, these data suggest that prebiotics and probiotics are safe and that some evidence indicates that they can reduce the incidence of allergy even though more testing is needed to make them a routine recommendation.
Another proposal for modulating CMPA is to induce changes in the structure of cow’s milk proteins through thermal treatments of cow’s milk. These studies have been done in vitro, so their results are not yet conclusive. Consequently, it is not advisable to offer dairy products that are boiled, baked or cooked for long periods of time since they do not offer any demonstrated benefit in terms of tolerance to cow’s milk protein (Figure 2). 72,73
Conclusions
CMPA can occur in exclusively breastfed infants and formula-fed infants. Since its manifestations are not pathognomonic, a complete medical history with a thorough examination is the basis of diagnosis. Confirmation using a skin prick test, serum-specific IgE, or atopic patch test lacks specificity, so placebo-controlled double-blind dietary challenges remain the reference treatment. 14
The debate on the management of CMPA will continue according to the predominant clinical manifestations and the context of the patient. Breastfeeding is the best and cheapest option for feeding healthy children and those with CMPA. Meanwhile, EHF based on cow’s milk remains the recommended and preferred therapeutic option while AAF are reserved for the most severe cases.
Referencias
1. Koletzko S, Niggemann B, Arato A, Dias JA, Heuschkel R, Husby S, et al. Diagnostic approach and management of cow’s-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr. 2012;55(2):221-9. https://doi.org/10.1097/MPG.0b013e31825c9482 [ Links ]
2. Vandenplas Y, Abuabat A, Al-Hammadi S, Aly GS, Miqdady MS, Shaaban SY, et al. Middle East Consensus Statement on the Prevention, Diagnosis, and Management of Cow’s Milk Protein Allergy. Pediatr Gastroenterol Hepatol Nutr. 2014;17(2):61-73. http://dx.doi.org/10.5223/pghn.2014.17.2.61 [ Links ]
3. Boaventura RM, Mendonça RB, Fonseca FA, Mallozi M, Souza FS, Sarni ROS. Nutritional status and food intake of children with cow’s milk allergy. Allergol Immunopathol (Madr). 2019;47(6):544-550. http://dx.doi.org/10.1016/j.aller.2019.03.003 [ Links ]
4. Hill DJ, Firer MA, Shelton MJ, Hosking CS. Manifestations of milk allergy in infancy: clinical and immunologic findings. J Pediatr. 1986;109(2):270-6. https://doi.org/10.1016/S0022-3476(86)80384-5 [ Links ]
5. Mehaudy R, Parisi C, Petriz N, Eymann A, Jauregui MB, Orsi M. Prevalence of cow’s milk protein allergy among children in a university community hospital. Arch Argent Pediatr. 2018;116(3):219-223. https://doi.org/10.5546/aap.2018.219 [ Links ]
6. Høst A, Halken S. A prospective study of cow milk allergy in Danish infants during the first 3 years of life. Clinical course in relation to clinical and immunological type of hypersensitivity reaction. Allergy. 1990;45(8):587-96. https://doi.org/10.1111/j.1398-9995.1990.tb00944.x [ Links ]
7. Jakobsson I, Lindberg T. A prospective study of cow’s milk protein intolerance in Swedish infants. Acta Paediatr Scand. 1979;68(6):853-9. https://doi.org/10.1111/j.1651-2227.1979.tb08223.x [ Links ]
8. Santos A, Dias A, Pinheiro JA. Predictive factors for the persistence of cow’s milk allergy. Pediatr Allergy Immunol. 2010;21(8):1127-34. https://doi.org/10.1111/j.1399-3038.2010.01040.x [ Links ]
9. Kvenshagen B, Halvorsen R, Jacobsen M. Adverse reactions to milk in infants. Acta Paediatr. 2008;97(2):196-200. https://doi.org/10.1111/j.1651-2227.2007.00599.x [ Links ]
10. Bishop JM, Hill DJ, Hosking CS. Natural history of cow milk allergy: clinical outcome. J Pediatr. 1990;116(6):862-7. https://doi.org/10.1016/S0022-3476(05)80641-9 [ Links ]
11. Høst A, Halken S, Jacobsen HP, Christensen AE, Herskind AM, Plesner K. Clinical course of cow’s milk protein allergy/intolerance and atopic diseases in childhood. Pediatr Allergy Immunol. 2002;13(s15):23-8. https://doi.org/10.1034/j.1399-3038.13.s.15.7.x [ Links ]
12. Vanto T, Helppilä S, Juntunen-Backman K, Kalimo K, Klemola T, Korpela R, Koskinen P. Prediction of the development of tolerance to milk in children withcow’s milk hypersensitivity. J Pediatr. 2004;144(2):218-22. https://doi.org/10.1016/j.jpeds.2003.10.063 [ Links ]
13. Høst A. Frequency of cow’s milk allergy in childhood. Ann Allergy Asthma Immunol. 2002;89(6 Suppl 1):33-7. https://doi.org/10.1016/S1081-1206(10)62120-5 [ Links ]
14. De Greef E, Hauser B, Devreker T, Veereman-Wauters G, Vandenplas Y. Diagnosis and management of cow’s milk protein allergy in infants. World J Pediatr. 2012;8(1):19-24. https://doi.org/10.1007/s12519-012-0332-x [ Links ]
15. Wal JM. Cow’s milk proteins/allergens. Ann Allergy Asthma Immunol. 2002;89(6 Suppl 1):3-10. https://doi.org/10.1016/S1081-1206(10)62115-1 [ Links ]
16. Nowak-Wegrzyn A, Fiocchi A. Rare, medium, or well done? The effect of heating and food matrix on food protein allergenicity. Curr Opin Allergy Clin Immunol. 2009;9(3):234-7. https://doi.org/10.1097/ACI.0b013e32832b88e7 [ Links ]
17. Karlsson MR, Rugtveil J, Brandtzaeg P. Allergen-responsive CD41CD251 regulatory cells in children who have outgrown cow’s milk allergy. J. Exp. Med. 2004; 199: 1679-1688. https://doi.org/10.1084/jem.20032121 [ Links ]
18. Salvatore S, Vandenplas Y. Gastroesophageal reflux and cow milk allergy: is there a link? Pediatrics. 2002;110(5):972-84. https://doi.org/10.1542/peds.110.5.972 [ Links ]
19. Daza W, Dadán S, Rojas AM. Alergia alimentaria en la infancia. Programa de Educación Continuada (Precop), Soc Col de Pediat. 2014;13(3):49-58. [ Links ]
20. Magazzù G, Scoglio R. Gastrointestinal manifestations of cow’s milk allergy.Ann Allergy Asthma Immunol. 2002;89(6 Suppl 1):65-8. https://doi.org/10.1016/S1081-1206(10)62126-6 [ Links ]
21. Sicherer SH. Determinants of systemic manifestations of food allergy. J Allergy Clin Immunol. 2000;106(5 Suppl):S251-7. https://doi.org/10.1067/mai.2000.110158 [ Links ]
22. Walker-Smith J. Cow’s milk allergy: a new understanding from immunology. Ann Allergy Asthma Immunol. 2003;90(6 Suppl 3):81-3. https://doi.org/10.1016/S1081-1206(10)61666-3 [ Links ]
23. Huang J, Walker WA. Food allergic enteropathy. En: Huang J, Walker WA. Review of pediatric gastroenterology disease and nutrition. Hamilton: BC Decker Inc; 2005. p. 145-146. [ Links ]
24. Nosan G, Jakic M, Jager M, Paro-Panjan D. Prognostic accuracy of clinical signs and diagnostic tests in cow’s milk allergy in newborns. Pediatr Neonatol. 2017;58(5):449-454. https://doi.org/10.1016/j.pedneo.2016.09.009 [ Links ]
25. Bahna SL. Clinical expressions of food allergy. Ann Allergy Asthma Immunol. 2003;90(6 Suppl 3):41-4. https://doi.org/10.1016/S1081-1206(10)61659-6 [ Links ]
26. Fiocchi A, Schünemann HJ, Brozek J, Restani P, Beyer K, Troncone R, et al. Diagnosis and Rationale for Action Against Cow’s Milk Allergy (DRACMA): a summary report. J Allergy Clin Immunol. 2010;126(6):1119-28.e12. https://doi.org/10.1016/j.jaci.2010.10.011 [ Links ]
27. Kelly E, DunnGalvin G, Murphy BP, O’B Hourihane J. Formula supplementation remains a risk for cow’s milk allergy in breast-fed infants. Pediatr Allergy Immunol. 2019;30(8):810-816. https://doi.org/10.1111/pai.13108 [ Links ]
28. Vanto T, Juntunen-Backman K, Kalimo K, Klemola T, Koivikko A, Koskinen P, et al. The patch test, skin prick test, and serum milk-specific IgE as diagnostic tools in cow’s milk allergy in infants. Allergy. 1999;54(8):837-42. https://doi.org/10.1034/j.1398-9995.1999.00134.x [ Links ]
29. Costa AJ, Sarinho ES, Motta ME, Gomes PN, de Oliveira de Melo SM, da Silva GA. Allergy to cow’s milk proteins: what contribution does hypersensitivity in skin tests have to this diagnosis? Pediatr Allergy Immunol. 2011;22(1 Pt 2):e133-8. https://doi.org/10.1111/j.1399-3038.2010.00988.x [ Links ]
30. García-Ara MC, Boyano-Martínez MT, Díaz-Pena JM, Martín-Muñoz MF, Martín-Esteban M. Cow’s milk-specific immunoglobulin E levels as predictors of clinical reactivity in the follow-up of the cow’s milk allergy infants. Clin Exp Allergy. 2004;34(6):866-70. https://doi.org/10.1111/j.1365-2222.2004.01976.x [ Links ]
31. Vandenplas Y, Koletzko S, Isolauri E, Hill D, Oranje AP, Brueton M, et al. Guidelines for the diagnosis and management of cow’s milk protein allergy in infants. Arch Dis Child. 2007;92(10):902-8. https://doi.org/10.1136/adc.2006.110999 [ Links ]
32. Venter C, Pereira B, Voigt K, Grundy J, Clayton CB, Gant C, et al. Comparison of open and double-blind placebo-controlled food challenges in diagnosis of food hypersensitivity amongst children. J Hum Nutr Diet. 2007;20(6):565-79. https://doi.org/10.1111/j.1365-277X.2007.00828.x [ Links ]
33. Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001;107(5):891-6. https://doi.org/10.1067/mai.2001.114708 [ Links ]
34. Nowak-Wegrzyn A, Bloom KA, Sicherer SH, Shreffler WG, Noone S, Wanich N, et al. Tolerance to extensively heated milk in children with cow’s milk allergy. J Allergy Clin Immunol. 2008;122(2):342-7, 347.e1-2. https://doi.org/10.1016/j.jaci.2008.05.043 [ Links ]
35. Lifschitz C, Szajewska H. Cow’s milk allergy: evidence-based diagnosis and management for the practitioner. Eur J Pediatr. 2015;174(2):141-150. https://doi.org/10.1007/s00431-014-2422-3 [ Links ]
36. Cuomo B, Indirli GC, Bianchi A, Arasi S, Caimmi D, Dondi A, et al. Specific IgE and skin prick tests to diagnose allergy to fresh and baked cow’s milk according to age: a systematic review. Ital J Pediatr. 2017;43(1):93. https://doi.org/10.1186/s13052-017-0410-8 [ Links ]
37. Celakovska J, Krcmova I, Bukac J, Vaneckova J. Sensitivity and specificity of specific IgE, skin prick test and atopy patch test in examination of food allergy.Food and agricultural immunology. 2016;28(2):238-247. http://dx.doi.org/10.1080/09540105.2016.1258548 [ Links ]
38. Vandenplas Y, Dupont C, Eigenmann P, Host A, Kuitunen M, Ribes-Koninckx C, et al. A workshop report on the development of the Cow›s Milk-related Symptom Score awareness tool for young children. Acta Paediatr. 2015;104(4):334-9. https://doi.org/10.1111/apa.12902 [ Links ]
39. Zeng Y, Zhang J, Dong G, Liu P, Xiao F, Li W, et al. Assessment of Cow›s milk-related symptom scores in early identification of cow›s milk protein allergy in Chinese infants. BMC Pediatr. 2019;19(1):191. https://doi.org/10.1186/s12887-019-1563-y [ Links ]
40. Vandenplas Y, Mukherjee R, Dupont C, Eigenmann P, Høst A, Kuitunen M, et al. Protocol for the validation of sensitivity and specificity of the Cow›s Milk-related Symptom Score (CoMiSS) against open food challenge in a single-blinded, prospective, multicentre trial in infants. BMJ Open. 2018;8(5):e019968. https://doi.org/10.1136/bmjopen-2017-019968 [ Links ]
41. Xanthakos SA, Schwimmer JB, Melin-Aldana H, Rothenberg ME, Witte DP, Cohen MB. Prevalence and outcome of allergic colitis in healthy infants with rectal bleeding: a prospective cohort study. J Pediatr Gastroenterol Nutr. 2005;41(1):16-22. https://doi.org/10.1097/01.MPG.0000161039.96200.F1 [ Links ]
42. Hill DJ, Heine RG, Cameron DJ, Catto-Smith AG, Chow CW, Francis DE, et al. Role of food protein intolerance in infants with persistent distress attributed to reflux esophagitis. J Pediatr. 2000;136(5):641-7. https://doi.org/10.1067/mpd.2000.104774 [ Links ]
43. Jakobsson I, Lindberg T. Cow›s milk proteins cause infantile colic in breast-fed infants: a double-blind crossover study. Pediatrics. 1983;71(2):268-71. [ Links ]
44. Isolauri E, Tahvanainen A, Peltola T, Arvola T. Breast-feeding of allergic infants. J Pediatr. 1999;134(1):27-32. https://doi.org/10.1016/S0022-3476(99)70368-9 [ Links ]
45. Saarinen UM, Kajosaari M. Breastfeeding as prophylaxis against atopic disease: prospective follow-up study until 17 years old. Lancet. 1995;346(8982):1065-9. https://doi.org/10.1016/S0140-6736(95)91742-X [ Links ]
46. American Academy of Pediatrics. Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics. 2000;106(2 Pt 1):346-9. https://doi.org/10.1542/peds.106.2.346 [ Links ]
47. Giampietro PG, Kjellman NI, Oldaeus G, Wouters-Wesseling W, Businco L. Hypoallergenicity of an extensively hydrolyzed whey formula. Pediatr Allergy Immunol. 2001;12(2):83-6. https://doi.org/10.1034/j.1399-3038.2001.012002083.x [ Links ]
48. Vandenplas Y, De Greef E, Devreker T. Treatment of Cow›s Milk Protein Allergy. Pediatr Gastroenterol Hepatol Nutr. 2014;17(1):1-5. http://dx.doi.org/10.5223/pghn.2014.17.1.1 [ Links ]
49. Isolauri E, Sütas Y, Mäkinen-Kiljunen S, Oja SS, Isosomppi R, Turjanmaa K. Efficacy and safety of hydrolyzed cow milk and amino acid-derived formulas in infants with cow milk allergy. J Pediatr. 1995;127(4):550-7. https://doi.org/10.1016/S0022-3476(95)70111-7 [ Links ]
50. Cordero C, Prado F, Bravo P. Actualización en manejo de alergia a la proteína de leche de vaca: fórmulas lácteas disponibles y otros brebajes. Rev. chil. Pediatra. 2018;89(3):310-317. https://doi.org/10.4067/S0370-41062018005000503 [ Links ]
51. Halpern SR, Sellars WA, Johnson RB, Anderson DW, Saperstein S, Reisch JS. Development of childhood allergy in infants fed breast, soy, or cow milk. J Allergy Clin Immunol. 1973;51(3):139-51. https://doi.org/10.1016/0091-6749(73)90019-5 [ Links ]
52. Bhatia J, Greer F; American Academy of Pediatrics Committee on Nutrition. Use of soy protein-based formulas in infant feeding. Pediatrics. 2008;121(5):1062-8. https://doi.org/10.1542/peds.2008-0564 [ Links ]
53. Vandenplas Y, Castrellon PG, Rivas R, Gutiérrez CJ, Garcia LD, Jimenez JE, et al. Safety of soya-based infant formulas in children. Br J Nutr. 2014;111(8):1340-60. https://doi.org/10.1017/S0007114513003942 [ Links ]
54. ESPGHAN Committee on Nutrition, Agostoni C, Axelsson I, Goulet O, Koletzko B, Michaelsen KF, et al. Soy protein infant formulae and follow-on formulae: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2006;42(4):352-61. https://doi.org/10.1097/01.mpg.0000189358.38427.cd [ Links ]
55. Høst A, Koletzko B, Dreborg S, Muraro A, Wahn U, Aggett P, et al. Dietary products used in infants for treatment and prevention of food allergy. Joint Statement of the European Society for Paediatric Allergology and Clinical Immunology (ESPACI) Committee on Hypoallergenic Formulas and the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Committee on Nutrition. Arch Dis Child. 1999;81(1):80-4. https://doi.org/10.1136/adc.81.1.80 [ Links ]
56. Järvinen KM, Chatchatee P. Mammalian milk allergy: clinical suspicion, cross-reactivities and diagnosis. Curr Opin Allergy Clin Immunol. 2009;9(3):251-8. https://doi.org/10.1097/ACI.0b013e32832b3f33 [ Links ]
57. Laitinen K, Kalliomäki M, Poussa T, Lagström H, Isolauri E. Evaluation of diet and growth in children with and without atopic eczema: follow-up study from birth to 4 years. Br J Nutr. 2005;94(4):565-74. https://doi.org/10.1079/BJN20051503 [ Links ]
58. Nowak-Wegrzyn A, Czerkies L, Reyes K, Collins B, Heine RG. Confirmed Hypoallergenicity of a Novel Whey-Based Extensively Hydrolyzed Infant Formula Containing Two Human Milk Oligosaccharides. Nutrients. 2019;11(7). pii: E1447. https://doi.org/10.3390/nu11071447 [ Links ]
59. Dupont C, Chouraqui JP, Linglart A, Bocquet A, Darmaun D, Feillet F, et al. Nutritional management of cow’s milk allergy in children: An update. Arch Pediatr. 2018;25(3):236-243. https://doi.org/10.1016/j.arcped.2018.01.007 [ Links ]
60. Klemola T, Vanto T, Juntunen-Backman K, Kalimo K, Korpela R, Varjonen E. Allergy to soy formula and to extensively hydrolyzed whey formula in infants with cow’s milk allergy: a prospective, randomized study with a follow-up to the age of 2 years. J Pediatr. 2002;140(2):219-24. https://doi.org/10.1067/mpd.2002.121935 [ Links ]
61. Nowak-Wegrzyn A, Sampson HA, Wood RA, Sicherer SH. Food protein-induced enterocolitis syndrome caused by solid food proteins. Pediatrics. 2003;111(4 Pt 1):829-35. https://doi.org/10.1542/peds.111.4.829 [ Links ]
62. American College of Allergy, Asthma, & Immunology. Food allergy: a practice parameter. Ann Allergy Asthma Immunol. 2006;96(3 Suppl 2):S1-68. [ Links ]
63. Fergusson DM, Horwood LJ, Shannon FT. Early solid feeding and recurrent childhood eczema: a 10-year longitudinal study. Pediatrics. 1990;86(4):541-6. [ Links ]
64. Morgan J, Williams P, Norris F, Williams CM, Larkin M, Hampton S. Eczema and early solid feeding in preterm infants. Arch Dis Child. 2004;89(4):309-314. https://doi.org/10.1136/adc.2002.020065 [ Links ]
65. Zutavern A, Brockow I, Schaaf B, Bolte G, von Berg A, Diez U, et al. Timing of solid food introduction in relation to atopic dermatitis and atopic sensitization: results from a prospective birth cohort study. Pediatrics. 2006;117(2):401-11. https://doi.org/10.1542/peds.2004-2521 [ Links ]
66. Koplin JJ, Osborne NJ, Wake M, Martin PE, Gurrin LC, Robinson MN, et al. Can early introduction of egg prevent egg allergy in infants? A population-based study. J Allergy Clin Immunol. 2010;126(4):807-13. https://doi.org/10.1016/j.jaci.2010.07.028 [ Links ]
67. Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel report. Nutr Res. 2011;31(1):61-75. https://doi.org/10.1016/j.jaci.2010.10.008 [ Links ]
68. Osborn DA, Sinn JK. Prebiotics in infants for prevention of allergy. Cochrane Database Syst Rev. 2013;(3):CD006474. https://doi.org/10.1002/14651858.CD006474.pub3 [ Links ]
69. Qamer S, Deshmukh M, Patole S. Probiotics for cow’s milk protein allergy: a systematic review of randomized controlled trials. Eur J Pediatr. 2019;178(8):1139-1149. https://doi.org/10.1007/s00431-019-03397-6 [ Links ]
70. Kalliomäki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet. 2003;361(9372):1869-71. https://doi.org/10.1016/S0140-6736(03)13490-3 [ Links ]
71. Arslanoglu S, Moro GE, Schmitt J, Tandoi L, Rizzardi S, Boehm G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J Nutr. 2008;138(6):1091-5. https://doi.org/10.1093/jn/138.6.1091 [ Links ]
72. Bavaro SL, De Angelis E, Barni S, Pilolli R, Mori F, Novembre EM, et al. Modulation of Milk Allergenicity by Baking Milk in Foods: A Proteomic Investigation. Nutrients. 2019;11(7). pii: E1536. https://doi.org/10.3390/nu11071536 [ Links ]
73. Chen M, Sutherland A, Birrueta G, Laubach S, Leonard S, Peters B, et al. Analysis of Allergen-Specific T Cell and IgE Reactivity to Different Preparations of Cow’s Milk-Containing Food Extracts. Cells. 2019;8(7):667. https://doi.org/10.3390/cells8070667 [ Links ]
Received: March 19, 2019; Accepted: August 06, 2019











 text in
text in