Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.35 no.1 Bogotá Jan./Mar. 2020
https://doi.org/10.22516/25007440.306
Case report
Primary gastric sarcoma: Case report and literature review
1Especialista en Cirugía Gastrointestinal y Endoscopia Digestiva. Instituto Nacional de Cancerología. Bogotá, Colombia
2Especialista en Cirugía Gastrointestinal y Endoscopia Digestiva. Instituto Nacional de Cancerología. Bogotá, Colombia
3Especialista en Patología Oncológica. Instituto Nacional de Cancerología. Bogotá, Colombia
Gastric cancer, a neoplastic pathology of undeniable importance, accounts for 90% of cases to adenocarcinoma. GIST lymphomas and gastrointestinal stromal tumors are the majority of the other 10%. However, non-GIST sarcomas remain a possible differential diagnosis to keep in mind and constitute a neoplastic pathology whose treatment is fundamentally surgical. Leiomyosarcoma represents less than 1% of malignant stomach tumors, and the available literature consists of case reports or case series. Because of its rarity, we present this clinical case and review the literature.
Keywords: Leiomyosarcoma; gastric neoplasms; mesenchymal tumors
El cáncer gástrico, patología neoplásica de innegable importancia, corresponde en el 90 % de los casos a un adenocarcinoma. Dentro del 10 % restante, los linfomas y los tumores estromales gastrointestinales (Gastrointestinal Stromal Tumor, GIST) constituyen la mayoría. Sin embargo, los sarcomas no GIST siguen siendo un diagnóstico diferencial posible para tener en cuenta y configuran una patología neoplásica de tratamiento fundamentalmente quirúrgico.
En particular, el leiomiosarcoma representa menos del 1 % de los tumores malignos del estómago y la literatura disponible al respecto consiste en reportes de caso o serie de casos. Por su rareza, presentamos este caso clínico y revisamos la literatura relacionada.
Palabras clave: Leiomiosarcoma; neoplasias gástricas; tumores mesenquimales
Introduction
Primary gastric leiomyosarcoma is a malignant non-epithelial tumor which originates in cells of the muscular stroma and which frequently metastasizes to the liver and lungs. It is the most common of sarcomas of the stomach which account for less than 2% of gastric cancers around the world. 1 Its clinical presentation is subtly different from that of adenocarcinoma, and preoperative diagnosis can be difficult.
A classic 1948 JAMA article by Doctors Bassler and Peters discussed the clinical differences between gastric sarcomas and carcinomas as well as the possibility of diagnosing sarcoma in the clinical setting of gastric malignancy. 2 Among the relevant characteristics of sarcomas are patient’s age at onset (on average 38 years), higher frequency among men than women, long-term clinical symptoms with less cachexia than in adenocarcinoma, severe pain as a cardinal symptom and accompanied by massive bleeding, especially in advanced stages.
Since that publication, our knowledge of sarcomas has expanded and its classification has been revised on several occasions. However, these observations are still clinically valid and useful.
We present the case of a young man with a family history of gastric adenocarcinoma who developed pain and digestive bleeding for whom we confirmed a diagnosis of primary gastric leiomyosarcoma. We also review the literature.
Case presentation
This patient was a 42-year-old man who had experienced abdominal pain and melena for three months prir to diagnosis. His grandfather and father had had gastric adenocarcinoma at ages 70 and 53, respectively. Upper endoscopy found a 5 cm in diameter vegetative and ulcerated subcardial mass in the greater curvature. A biopsy found a non-malignant hyperplastic polyp.
Upon arrival at the National Cancer Institute, a new endoscopy and biopsy found a 6 x 8 cm lobed, firm and ulcerated subcardial polypoid lesion with a 3 cm implanted base. Its pathology corresponded to a spindle cell mesenchymal lesion that immunohistochemical study classified as a leiomyoma. Meanwhile, the patient presented two new episodes of digestive bleeding that required transfusion of blood products. Difficult to manage abdominal pain continued.
A contrast CT scan of the chest and abdomen showed a gastric mass. Metastatic lesions were ruled out (Figure 1). The mass was then resected surgically using total gastrectomy with D1 nodal dissection and Roux-en-Y reconstruction. The patient’s postoperative evolution was favorable, and he was discharged seven days later.
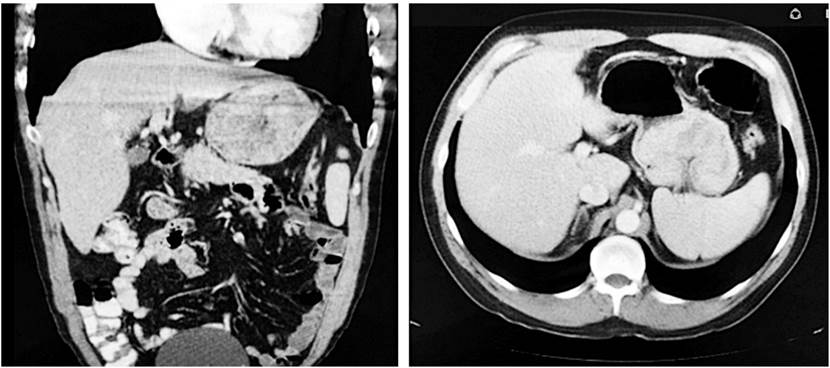
Figure 1 Abdominal computerized axial tomography (CAT) scan showing a gastric mass. Coronal and axial cuts.
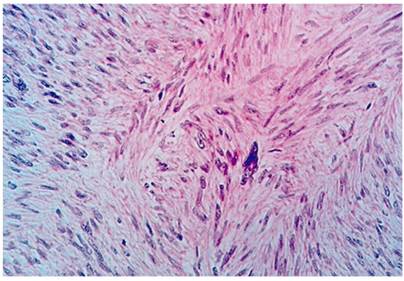
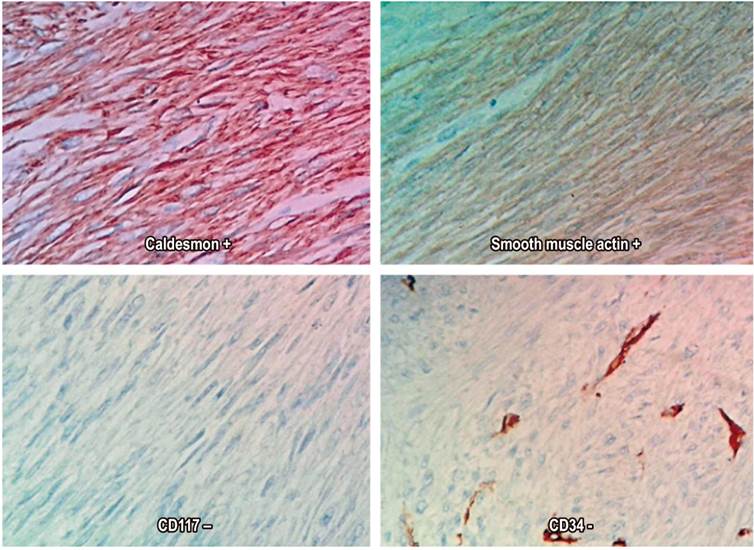
The definitive pathological diagnosis was a high-grade leiomyosarcoma that measured 14 x 12 cm. There were 50 mitoses in 50 high-power fields, Ki67 was 40%, the resected specimen was free of tumor at the margins, and there were 15 tumor-free nodes (Figures 2 and 3). The patient was evaluated by the clinical oncology service which did not prescribe adjuvant therapy. Follow-up at 28 months found no recurrence and the general condition of the individual was good. Digestive pain and bleeding disappeared completely after surgery.
Discussion
Sarcomas account for 1% to 2% of gastrointestinal malignancies. Most are leiomyosarcomas, and the most frequent location is the stomach. 1,3 Other types of sarcomas such as synovial, granulocytic, and Ewing sarcoma have been reported in isolation in the gastrointestinal tract but are true rarities. 4-9
Mitotic activity has been shown to be the most important isolated indicator for prognosis of this disease, and surgery continues to be the mainstay of treatment. 8-12 High grade tumors, metastasis at the time of diagnosis, tumor size and incomplete surgical resection are independent factors that have poor prognoses. 13,14
The nature of these malignancies and the peculiarities of their biological behavior make surgical resection en bloc, even with neighboring organs if they are involved, of paramount importance for reducing the possibility of recurrence. Unlike adenocarcinomas, sarcomas grow locally and invade contiguously. Metastases to lymph nodes are unusual, 15 but the extent of lymph node dissection needed is a matter of discussion. There have been reports of lymph node involvement in up to 15% of cases, according to a series by R. Álvarez et al. 16 Adjuvant therapies, such as chemotherapy and radiation therapy, have a marginal role and are mainly used for palliation of unresectable or recurrent primary lesions. 17
The place of leiomyosarcomas in the classification of sarcomas has been relatively stable despite revisions of the classification system. Leiomyosarcomas are fundamentally different from GIST as shown by immunohistochemical techniques. 18 Diagnosis by endoscopy and biopsy is not always easy because they are intramural and extramucosal lesions. Clinical suspicion, in-depth biopsies, and imaging techniques are key for establishing a pre-surgical diagnosis. 19 Differentiation between leiomyoma and leiomyosarcoma is also difficult to determine in biopsies, and the surgical piece is often required to establish a correct diagnosis since cellular atypia and mitosis are keys to this differential diagnosis. 20
The literature contains numerous classifications for sarcomas in general and specifically for soft tissue sarcomas. The National Federation of French Cancer Centers (Fédération nationale des centres de lutte contre le cancer - FNCLCC) haves proposed a classification system for visceral and soft tissue sarcomas based on the degrees of differentiation and ulceration plus the mitotic rate. This system has been validated and is widely used. 21,22
Tumor size using 5 cm as a cut-off point is important for patient prognosis, as is the absence or presence of metastases at diagnosis. They are included in the TNM staging system of the American Joint Committee on Cancer (AJCC). The overall 5-year survival for patients with gastric sarcomas varies and has been reported to be between 16% and 56%. It depends on tumor grade and success of complete surgical resection. Recurrences usually occur around two years after resection and occur in 36% to 60% of cases. 17
The patient presented here had high-grade leiomyosarcoma according to the FNCLCC classification His total score was seven points: two for differentiation, three for mitosis, and two for necrosis (Table 1).
Table 1 FNCLCC sarcoma classification parameters.
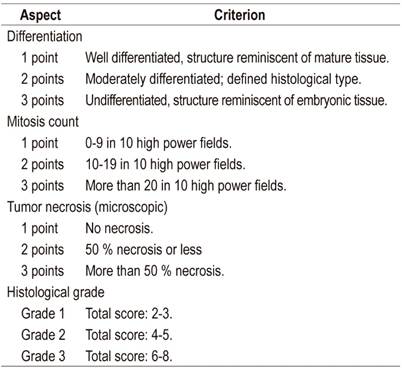
Modified from Evaluation of performance of various histological grading systems of soft tissue sarcomas and the prognosis (metastatic risk and survival rate)21
There is no unanimity of opinion about the need for a formal lymph node dissection. In general, metastases from this neoplasm are known to be uncommon, but its preference for the hematogenous route is recognized. The surgeon’s decision prevails, based on clinical and surgical findings. In this case, there was no macroscopic evidence of lymphadenopathy. A D1 gastrectomy was performed. The fifteen lymph nodes obtained were all negative for tumor.
A particular characteristic of this case was the history of gastric adenocarcinoma in two of the patient’s first degree relatives. The literature contains reports of coexistence between the histology of adenocarcinoma and GIST in one patient and of coexistence of adenocarcinoma and gastric lymphomas in one patient. 23,24 Nevertheless, a family association between gastric sarcoma and adenocarcinoma had not been previously reported among the known syndromes of hereditary-family gastric cancer as far as the authors have been able to discern. This should be considered for clinical observation and future studies.
Conclusions
Primary gastric leiomyosarcoma is a rare but clearly established entity whose treatment is based on radical surgical resection. Its prognosis is superior to that of gastric adenocarcinoma. Its clinical characteristics make suspicion possible, but diagnosis with certainty is difficult in the pre-surgical phase. It is based on histopathology findings with special emphasis on immunohistochemistry.
Referencias
1. McGrath PC, Neifeld JP, Lawrence W, Kay S, Horsley JS, Parker GA. Gastrointestinal sarcomas. Analysis of prognostic factors. Ann Surg. 1987;206(6):706-10. https://doi.org/10.1097/00000658-198712000-00004 [ Links ]
2. Bassler A, Peters AG. Distinctions between gastric sarcoma and carcinoma with special reference to the infiltrating types of sarcoma. J Am Med Assoc. 1948;138(7):489-94. https://doi.org/10.1001/jama.1948.02900070021005 [ Links ]
3. Peñaherrera V, Abarca J, Carrillo L. Leiomiosarcoma: reporte de un caso clínico. Rev Med. 2003;9(4):327-330. [ Links ]
4. Billings SD, Meisner LF, Cummings OW, Tejada E. Synovial sarcoma of the upper digestive tract: a report of two cases with demonstration of the X;18 translocation by fluorescence in situ hybridization. Mod Pathol. 2000;13(1):68-76. https://doi.org/10.1038/modpathol.3880011 [ Links ]
5. Zhou W, Vasquez JC, O’Donnell MR, Paz BI. Clinical manifestations of gastrointestinal granulocytic sarcoma requiring surgical treatment. Am Surg. 2001;67(8):764-6. [ Links ]
6. Agaimy A, Gaumann A, Schroeder J, Dietmaier W, Hartmann A, Hofstaedter F, et al. Primary and metastatic high-grade pleomorphic sarcoma/malignant fibrous histiocytoma of the gastrointestinal tract: an approach to the differential diagnosis in a series of five cases with emphasis on myofibroblastic differentiation. Virchows Arch. 2007;451(5):949-57. https://doi.org/10.1007/s00428-007-0495-3 [ Links ]
7. Kim HS, Kim S, Min YD, Kee KH, Hong R. Ewing’s Sarcoma of the Stomach; Rare Case of Ewing’s Sarcoma and Suggestion of New Treatment Strategy. J Gastric Cancer. 2012;12(4):258-261. http://dx.doi.org/10.5230/jgc.2012.12.4.258 [ Links ]
8. Dougherty MJ, Compton C, Talbert M, Wood WC. Sarcomas of the gastrointestinal tract. Separation into favorable and unfavorable prognostic groups by mitotic count. Ann Surg. 1991;214(5):569-574. https://doi.org/10.1097/00000658-199111000-00006 [ Links ]
9. Weledji EP, Enoworock G, Ngowe MN. Gastric leiomyosarcoma as a rare cause of gastric outlet obstruction and perforation: a case report. BMC Res Notes. 2014;7:479. https://doi.org/10.1186/1756-0500-7-479 [ Links ]
10. Carson W, Karakousis C, Douglass H, Rao U, Palmer ML. Results of aggressive treatment of gastric sarcoma. Ann Surg Oncol. 1994;1(3):244-51. https://doi.org/10.1007/BF02303530 [ Links ]
11. Lehnert T, Sinn HP, Wolf O. Diagnosis and surgical treatment of gastric sarcoma. Onkologie 1994;17:391-396. https://doi.org/10.1159/000218444 [ Links ]
12. Koga H, Ochiai A, Nakanishi Y, Sasako M, Mizuno S, Kinoshita T, et al. Reevaluation of prognostic factors in gastric leiomyosarcoma. Am J Gastroenterol. 1995;90(8):1307-12. [ Links ]
13. Medina-Franco H, Eltoum IE, Urist MM, Heslin MJ. Primary gastrointestinal sarcomas. Am Surg. 2000;66(12):1171-5. [ Links ]
14. Farrugia G, Kim CH, Grant CS, Zinsmeister AR. Leiomyosarcoma of the stomach: determinants of long-term survival. Mayo Clin Proc. 1992;67(6):533-6. https://doi.org/10.1016/S0025-6196(12)60459-5 [ Links ]
15. Yao KA, Talamonti MS, Langella RL, Schindler NM, Rao S, Small W, et al. Primary gastrointestinal sarcomas: analysis of prognostic factors and results of surgical management. Surgery. 2000;128(4):604-12. https://doi.org/10.1067/msy.2000.108056 [ Links ]
16. Álvarez R, Arancibia A, Klaassen R, Gutiérrez G, González R, Molina H, et al. Tumores gástricos de estirpe muscular. Rev Chil Cir. 2003;55(5):470-475. [ Links ]
17. Beyrouti MI, Beyrouti R, Amar M, Frikha F, Abid M, Affes N, et al. Sarcomes gastriques. La Presse Médicale. 2008;37(3):e60-e66. https://doi.org/10.1016/j.lpm.2007.05.004 [ Links ]
18. Fletcher CD. The evolving classification of soft tissue tumours - an update based on the new 2013 WHO classification. Histopathology. 2014;64(1):2-11. https://doi.org/10.1111/his.12267 [ Links ]
19. ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 Suppl 3:iii102-12. https://doi.org/10.1093/annonc/mdu254 [ Links ]
20. Miettinen M. Smooth muscle tumors of soft tissue and non-uterine viscera: biology and prognosis. Mod Pathol. 2014;27 Suppl 1:S17-29. https://doi.org/10.1038/modpathol.2013.178 [ Links ]
21. Baig MA. Evaluation of performance of various histological grading systems of soft tissue sarcomas and the prognosis (metastatic risk and survival rate). Int J Res Med Sci. 2015;3(9):2394-2401. https://doi.org/10.18203/2320-6012.ijrms20150637 [ Links ]
22. Guillou L, Coindre JM, Bonichon F, Nguyen BB, Terrier P, Collin F, et al. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol. 1997;15(1):350-62. https://doi.org/10.1200/JCO.1997.15.1.350 [ Links ]
23. Khoshnevis J, Rakhshan A, Sobhiyeh MR, Gholizadeh B, Rahbari A, Adhami F, et al. Simultaneous gastric adenocarcinoma and gastrointestinal stromal tumor of the stomach: a case report. Iran J Cancer Prev. 2013;6(1):55-58. [ Links ]
24. Weng CH, Wang RC, Wu CC, Lin TH. Treatment of synchronous adenocarcinoma and lymphoma of the stomach: A case report. Mol Clin Oncol. 2016;5(6):783-785. https://doi.org/10.3892/mco.2016.1044 [ Links ]
Received: October 22, 2018; Accepted: December 01, 2018











 text in
text in