Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista colombiana de Gastroenterología
versão impressa ISSN 0120-9957
Rev Col Gastroenterol vol.35 no.1 Bogotá jan./mar. 2020
https://doi.org/10.22516/25007440.325
Case report
Case report of glucosamine-chondroitin induced hepatotoxicity in a public hospital in Lima, Peru
1 Médico cirujano, Gastroenterólogo, Servicio de Gastroenterología, Clínica San Gabriel, Lima, Perú
2 Médico cirujano, Gastroenterólogo. Hospital Nacional Arzobispo Loayza. Lima, Perú
The human body naturally produces glucosamine and chondroitin which are important components of the cartilaginous system. There are multiple clinical indications for them as medicines, but they are primarily used for osteoarthritis. Hepatotoxicity induced by these biomolecules is uncommon, and the only reports in the world literature are isolated individual cases. This article presents the case of a patient with glucosamine-chondroitin-induced hepatocellular damage who was admitted to the hospital with respiratory symptoms and malaise. Marked hypertransaminemia was found in laboratory tests. Etiologies such as alcohol, viral hepatitis and autoimmune liver diseases were ruled out, and abdominal ultrasound found no evidence of chronic liver disease. Discontinuance of glucosamine and chondroitin led to a considerable decrease in hypertransaminemia after one week with total improvement two months of hospital discharge. This case adds to the small number reported worldwide and is relevant for future systematic studies to clarify the outlook for this disease.
Keywords: Glucosamine; chondroitin; toxicity; transaminases; osteoarthritis
En el cuerpo humano tenemos glucosamina y condroitina de forma natural. Estas sustancias constituyen un componente importante del sistema cartilaginoso. Como medicamentos, tienen múltiples indicaciones clínicas, principalmente la osteoartritis. La hepatotoxicidad inducida por estas biomoléculas es infrecuente, pues cuentan solo con reportes de casos aislados en la literatura mundial. En este trabajo, presentamos el caso de una paciente con una lesión hepática inducida por glucosamina-condroitina del tipo hepatocelular, que fue admitida en el hospital por causa de una sintomatología respiratoria y malestar general. En ella, se destacó una marcada hipertransaminasemia durante los exámenes de laboratorio. Asimismo, se descartaron etiologías como el alcohol, hepatitis virales y hepatopatías autoinmunes, principalmente. De igual forma, no se llegó a evidenciar una enfermedad hepática crónica mediante la ecografía abdominal. Al suspenderse el medicamento, se observó una disminución considerable de la hipertransaminasemia luego de 1 semana, y una mejoría total de esta a los 2 meses del alta hospitalaria. Este caso se añade a los pocos reportados a nivel mundial y cobra una importancia relevante para la publicación de posteriores estudios sistemáticos que aclaren el panorama de esta enfermedad.
Palabras clave: Glucosamina; condroitina; toxicidad; transaminasas; osteoartritis
Introduction
Glucosamine and chondroitin biomolecules, natural components of the human body, are vital to the structure of the cartilage system, but they can have both favorable and harmful effects when applied exogenously.
Although the Peruvian Dirección General de Medicamentos, Insumos y Drogas (DIGEMID - General Directorate of Medicines, Supplies and Drugs) has not approved glucosamine and chondroitin for treatment of osteoarthritis due to the high cost, 1 prescription has been approved in the United States and the United Kingdom because of the treatment’s beneficial effects. 2
Nevertheless, despite apparent harmlessness, this treatment can have adverse effects including gastrointestinal complaints, drowsiness, dermatological disorders, and headaches, and it may even lead to cardiovascular events. 3
Biochemical alterations such as hepatocellular, cholestatic and mixed liver damage can also occur together with their clinical and biochemical repercussions. Use can in fact lead to extreme situations such as sudden liver failure and death. These outcomes have been reported in several cases some of which have been demonstrated by liver biopsies. 4-10
We present the case of a patient who had hepatocellular damage induced by glucosamine-chondroitin in a public hospital in Lima, Peru.
Case presentation
The patient was a 65-year-old woman from Abancay who had a history of osteoarthritis in her right knee. She had been taking naproxen irregularly for one year together with sporadic treatment for chronic asthma with salbutamol. She had begun using an over-the-counter drug based on glucosamine-chondroitin ten days prior to coming to the emergency room. She reported no use of alcohol or herbal products and no significant family history.
After suffering from chest pain associated with general malaise and three episodes of hemoptysis (total volume of approximately 300 cm3) for one day, she came to the emergency department. During physical examination, she appeared to be slightly pale and in apparently poor general condition but was hemodynamically stable.
There were no stigmas of chronic liver disease or signs of visceromegaly or lymphadenopathy. Multislice spiral chest tomography was then performed, and bronchiectasis and diffuse interstitial lung disease were diagnosed.
Emergency laboratory test results
Hemoglobin (Hb): 11.3
Leukocyte count: 3,940
Platelet count: 191,000
Aspartate transaminase (AST): 672
Alanine aminotransferase (ALT): 689
Total bilirubin (TB): 0.71
Direct bilirubin (DB): 0.38
Total proteins (TP): 8.21
Albumin (Alb): 4.2
Alkaline phosphatase (AP): 160
Gamma-glutamyl transpeptidase (GGT): 244
Prothrombin time (PT): 14.2
International Normalized Ratio (INR): 1.2
Creatine-phosphokinase (CPK) total: 136
CPK-MB: 24
Lactate dehydrogenase (LDH): 764
Human immunodeficiency virus (HIV): nonreactive
Ferritin: 124.5
Free T4: 1.51
Thyroid-Stimulating Hormone (TSH): 3.36
Tests for viral markers for hepatitis A, B, C, D, and E were negative while tests for antinuclear antibody (ANA) markers, anti-smooth muscle antibodies (ASMA), antimitochondrial serotype M2 (AMA M2), Liver Kidney Microsome Antibodies (LKM-1) and Antineutrophil Cytoplasmic Antibody (ANCA) were negative. Immunoglobulin G was 1,842 and Immunoglobulin M was 31.7 (both within normal ranges).
Abdominal ultrasound during hospitalization showed biliary mud, increased flatulence, a normal sized liver, uniform edges and homogeneous parenchyma. No focal or diffuse lesions were evident. Intrahepatic bile ducts did not appear to be dilated. The common bile duct and portal vein were of normal caliber.
Two weeks later, after the patient’s respiratory process had improved, she was discharged. Medication to treat her underlying disease were prescribed and consumption of the substances containing glucosamine-chondroitin that she had stopped consuming a few days before admission to the hospital was prohibited.
Two months after discharge, a follow-up examination at the hospital showed clinical improvement.
Follow-up laboratory results
Hb: 12.1
Hematocrit (Hcto): 36.2%
Leukocyte count: 4,260
Ab: 0%
Platelet count: 208,000
PT: 14.7
INR: 1.31
AST: 37
ALT: 25
TB: 0.57
DB: 0.24
AP: 223
GGT: 57
PT: 7.29
Alb: 4.06
Table 1 describes the patient’s liver biochemistry data.
Table 1 Results of the patient’s liver biometry exams for glucosamine-chondroitin hepatotoxicity.
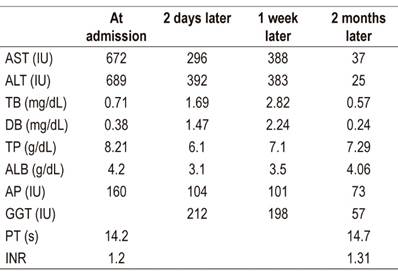
AST: Aspartate transaminase (negative value [NV]: 0-35 IU); ALT: Alanine aminotransferase (NV: 0-35 IU); TB: total bilirubin (NV: 0.3-1.2 mg/dL); DB: direct bilirubin (NV: 0-0.3 mg/dL); PT: total proteins (NV: 6-8 g/dL); ALB: albumin (NV: 3.5-5.5 g/dL); AP: alkaline phosphatase (36-129 IU); GGT: gamma-glutamyl transpeptidase (NV: 8-78 IU); PT: prothrombin time (NV: 10-14 s); INR: International Normalized Ratio (NV: <1.4)
Discussion
Glucosamine and chondroitin are biomolecules that are found naturally within the human body. They maintain the integrity of the cartilage system through multiple processes. 11
Glucosamine, part of the glycosaminoglycan family, is found in the cartilage matrix and synovial fluid. It can have medicinal effects when administered externally, 12 especially in bone and joint diseases such as osteoarthritis for which its effectiveness at mitigating symptoms and its safety have been proven. 13 Nevertheless, prophylactic use to prevent appearance of osteoarthritis has not been demonstrated. 14
The patient described here had hepatocellular liver damage that was directly related to an over-the-counter drug based on glucosamine-chondroitin. According to a recent update on glucosamine, onset of liver disorders occurs from one to four weeks after intake of the drug begins, and its injury pattern is typically hepatocellular, or - as in our case- mixed. The mechanism by which hepatotoxicity occurs is still unknown. 15
It is important to emphasize that our patient never had clinical indications of liver disease such as asthenia, pruritus, jaundice, and nausea. In contrast, of the three cases of liver disease in patients who consumed glucosamine-containing herbal preparations reported by Smith and Dillon, two had hepatocellular damage and one progressed to fulminant liver failure and death. 4
Two of those three patients had jaundice and pruritus which are clinical indications associated with liver disease. These symptoms and their liver biochemistry improved after they discontinued taking the herbal supplements. Similarly, the patient who had no symptoms experienced biochemical remission when supplements were discontinued.
Ebrahim et al. have reported a patient who had general malaise and jaundice whose laboratory tests revealed a mixed pattern of hepatocellular and cholestatic liver injury. 5 These changes were associated with ingestion of glucosamine supplements two weeks prior to admission. Clinical and biochemical improvement were seen one week after suspension of the drug and became completely normal four weeks after suspension.
The details of these cases are similar to those of our patient who stopped taking the medication a few days before entering the hospital after which transaminase levels decreased to 50%. After eight weeks they returned to normal.
Yang et al. reported another case of a patient who had mixed liver damage after consuming an over-the-counter product that contained glucosamine-chondroitin and herbal products. 6 This exposure to glucosamine-chondroitin is similar to the case of our patient as well. An important point in that case was demonstration of liver damage by biopsy. In our case, a biopsy could not be performed, since the patient was asymptomatic. Similarly, Linnebur et al. have demonstrated an association between this OTC product and liver damage in a report of two cases of glucosamine-chondroitin-related hepatotoxicity. 7
Ossendza et al. described a case with elevated transaminases and bilirubin indicating hepatocellular damage. Clinical indications were pruritus and jaundice which were associated with therapeutic doses of glucosamine. All indications disappeared after suspension of the drug. 8 It is important to note that our patient’s bilirubin levels began to increase at a certain point in her evolution.
A unique case has been reported by Stephen et al. 9 The patient presented with jaundice and cholestasis but the etiology was uncertain during the three-month study. Subsequently, a direct association of these symptoms with ingestion of glucosamine-chondroitin was determined and a cholestatic lesion was demonstrated by means of a liver biopsy.
It is important to mention the work carried out by Cerda et al. to determine association of glucosamine-chondroitin ingestion and alterations in liver tests. 10 Their study was based on completion of a questionnaire by 151 patients with underlying chronic liver disease. The authors were able to establish that two patients, one of whom had clinical indications of liver alterations, had altered transaminase levels and levels of cholestasis enzymes associated with the drug.
In summary, our patient joins the statistics of the few cases registered so far of glucosamine-chondroitin-induced hepatotoxicity. Indeed, this is the first case reported in Peru.
Therefore, we recommend continuing investigation of etiologies of all abnormal liver tests in patients who are admitted to emergency rooms, outpatient clinics and to inpatient care. Only in this way can we increase the casuistry of drug-induced liver injury and have a better overview of this increasingly frequent pathology.
Referencias
1. Ministerio de Salud. Informe técnico N° 22-2007: glucosamina sulfato 1,5 g/condroitina 1,2 g (polvo). Lima: Dirección General de Medicamentos, Insumos y Drogas; 2007. [ Links ]
2. Hochberg MC, Martel-Pelletier J, Monfort J, Möller I, Castillo JR, Arden N, et al. Combined chondroitin sulfate and glucosamine for painful knee osteoarthritis: a multicentre, randomised, double-blind, non-inferiority trial versus celecoxib. Ann Rheum Dis. 2016;75(1):37-44. http://dx.doi.org/10.1136/annrheumdis-2014-206792 [ Links ]
3. Sawitzke AD, Shi H, Finco MF, Dunlop DD, Harris CL, Singer NG, et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis. 2010;69(8):1459-64. http://dx.doi.org/10.1136/ard.2009.120469 [ Links ]
4. Smith A, Dillon J. Acute liver injury associated with the use of herbal preparations containing glucosamine: three case studies. BMJ Case Rep. 2009;2009. pii: bcr02.2009.1603. https://doi.org/10.1136/bcr.02.2009.1603 [ Links ]
5. Ebrahim V, Albeldawi M, Chiang DJ. Acute liver injury associated with glucosamine dietary supplement. BMJ Case Rep. 2012;2012. pii: bcr2012007665. https://doi.org/10.1136/bcr-2012-007665 [ Links ]
6. Yang L, Aronsohn A, Hart J, Jensen D. Herbal hepatoxicity from Chinese skullcap: A case report. World J Hepatol. 2012;4(7):231-233. https://doi.org/10.4254/wjh.v4.i7.231 [ Links ]
7. Linnebur SA, Rapacchietta OC, Vejar M. Hepatotoxicity associated with chinese skullcap contained in Move Free Advanced dietary supplement: two case reports and review of the literature. Pharmacotherapy. 2010;30(7):750, 258e-262e. https://doi.org/10.1592/phco.30.7.750 [ Links ]
8. Ossendza RA, Grandval P, Chinoune F, Rocher F, Chapel F, Bernardini D. Acute cholestatic hepatitis due to glucosamine forte. Gastroenterol Clin Biol. 2007;31(4):449-50. https://doi.org/10.1016/S0399-8320(07)89410-3 [ Links ]
9. Ip S, Jeong R, Schaeffer DF, Yoshida EM. Unusual case of drug-induced cholestasis due to glucosamine and chondroitin sulfate. World J Hepatol. 2015;7(24):2559-2562. https://doi.org/10.4254/wjh.v7.i24.2559 [ Links ]
10. Cerda C, Bruguera M, Parés A. Hepatotoxicity associated with glucosamine and chondroitin sulfate in patients with chronic liver disease. World J Gastroenterol. 2013;19(32):5381-4. https://doi.org/10.3748/wjg.v19.i32.5381 [ Links ]
11. Fransen M, Agaliotis M, Nairn L, Votrubec M, Bridgett L, Su S, et al. Glucosamine and chondroitin for knee osteoarthritis: a double-blind randomized placebo-controlled clinical trial evaluating single and combination regimens. Ann Rheum Dis. 2015;74(5):851-8. https://doi.org/10.1136/annrheumdis-2013-203954 [ Links ]
12. Bruyère O, Altman RD, Reginster JY. Efficacy and safety of glucosamine sulfate in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin Arthritis Rheum. 2016;45(4 Suppl):S12-7. https://doi.org/10.1016/j.semarthrit.2015.11.011 [ Links ]
13. Clegg DO, Reda DJ, Harris CL, Klein MA, O’Dell JR, Hooper MM, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354(8):795-808. https://doi.org/10.1056/NEJMoa052771 [ Links ]
14. de Vos BC, Landsmeer MLA, van Middelkoop M, Oei EHG, Krul M, Bierma-Zeinstra SMA, et al. Long-term effects of a lifestyle intervention and oral glucosamine sulphate in primary care on incident knee OA in overweight women. Rheumatology (Oxford). 2017;56(8):1326-1334. https://doi.org/10.1093/rheumatology/kex145 [ Links ]
15. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Glucosamine. [actualizada el 12 de marzo de 2020]. Disponible en: https://www.ncbi.nlm.nih.gov/books/NBK547949/ [ Links ]
Received: December 06, 2018; Accepted: March 02, 2019











 texto em
texto em 


