Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.35 no.3 Bogotá July/Sept. 2020 Epub Mar 01, 2021
https://doi.org/10.22516/25007440.488
Original article
Demographic characterization of the population with Barrett’s esophagus in two medical centers of Bogotá, Colombia
1Médica egresada de la Universidad de los Andes. Residente de Medicina Interna, Universidad del Rosario. Durante la ejecución del estudio: interna, Universidad de los Andes. Bogotá, Colombia
2Médico egresado de la Universidad de los Andes. Durante ejecución del estudio: interno, Universidad de los Andes. Bogotá, Colombia
3Medicina Interna, Gastroenterología y endoscopia digestiva, Fisiología digestiva. Profesora de Gastroenterología, Universidad de los Andes. Miembro institucional, sección de Gastroenterología, Endoscopia, Hepatología y Fisiología, Hospital Universitario Fundación Santa Fe de Bogotá. Bogotá, Colombia
Introduction:
Barrett’s esophagus occurs when the stratified squamous epithelium of the esophagus changes to a specialized columnar epithelium as a result of chronic gastroesophageal reflux. Its current prevalence in Colombia is unknown and the population suffering from it has not been characterized. The present study aims to determine the main demographic characteristics of the population diagnosed with Barrett’s esophagus treated at two medical centers in Bogotá, Colombia.
Materials and methods:
A multicenter cross-sectional study was conducted to assess the endoscopy and histopathology reports of 3,000 patients who underwent this procedure for any reason. A descriptive statistical analysis of the data was performed.
Results:
The prevalence of Barrett’s esophagus in the sample was 0.73%. The endoscopic-histology correlation was low (28.5%). Of the diagnosed cases, the most frequent age range was 60-80 years, with an average age of 65.5 years. This condition is predominant in the female sex (63.6%), in people with a BMI over 25 kg/m², with a history of smoking, and no history of alcohol consumption. Most patients underwent endoscopy for symptoms associated with gastroesophageal reflux (50%). The length of the observed segment was not reported in most endoscopies.
Conclusions:
In the medical centers included in this study, Barrett’s esophagus is a rare pathology, found predominantly in elderly women with symptoms of gastroesophageal reflux, overweight, and with a history of smoking.
Keywords: Barrett’s esophagus: Sex; Age distribution; Signs and symptoms; Prevalence
Introducción:
el esófago de Barrett es un trastorno en el que ocurre un cambio del epitelio escamoso estratificado del esófago por uno columnar especializado, lo cual se da como consecuencia del reflujo gastroesofágico crónico. En Colombia no se conoce la prevalencia actual de esta patología, ni se ha caracterizado a la población que la padece. El presente estudio tiene como objetivo conocer cuáles son las características demográficas principales de la población diagnosticada con esófago de Barrett en dos instituciones médicas de Bogotá.
Material y métodos:
se realizó un estudio de corte transversal multicéntrico, en el cual se evaluaron los reportes de endoscopias y de histopatología de 3000 pacientes que asistieron a estas instituciones por cualquier indicación. A partir de estos reportes se tomaron los datos requeridos. Asimismo, se realizó un análisis estadístico descriptivo de dichos datos.
Resultados:
la prevalencia del esófago de Barrett en la muestra es del 0,73 %. Se observó, además, que la correlación endoscópico-patológica es baja (28,5 %). De los casos diagnosticados, el rango de edad más frecuente se ubica entre los 60 y 80 años, con una edad promedio de 65,5 años. Asimismo, existe una predominancia de esta patología en el sexo femenino (63,6 %), en personas con un índice de masa corporal (IMC) >25 kg/m² y en aquellas con antecedentes de tabaquismo, sin historial de consumo de alcohol. En la mayoría de pacientes, se realizó la endoscopia por síntomas de reflujo gastroesofágico (50 %). La longitud del segmento observado no fue reportada en una gran cantidad de endoscopias.
Conclusiones:
en las instituciones analizadas, el esófago de Barrett es una patología de muy baja prevalencia y predominante en mujeres de edad avanzada con síntomas de reflujo gastroesofágico, sobrepeso y antecedente de tabaquismo.
Palabras clave: Esófago de Barrett; sexo; distribución por edad; signos y síntomas; prevalencia
Introduction
Barrett’s esophagus (BE) is the most relevant etiological factor for the development of esophageal adenocarcinoma 1. Diagnosing BE is important since the risk of esophageal adenocarcinoma in these patients increases 10 to 30 times compared to the general population 2-4. In this context, the possibility of the formation of an adenocarcinoma in patients with BE is greater sexing males and, in the elderly, as well as in patients with >10 years BE, and those with esophagitis and long-segment BE 5-7.
In a study conducted at the Fundación Santa Fe de Bogotá, the incidence of esophageal cancer after 11 years of follow-up of patients with BE was 4% 8. Recently, the annual incidence of esophageal adenocarcinoma in patients with BE has been estimated to be between 0.2 and 0.3% 9,10.
In this context, BE is not a rare pathology and, in fact, it occurs in 2% of the general population in other countries 11, as well as in 10-15% of patients with gastroesophageal reflux 1,12. Even in asymptomatic individuals over the age of 50, its prevalence can be up to 25% 13. Given these alarming figures, it is important to know the prevalence of this disease in Colombia.
According to the literature that was reviewed, the current prevalence of BE in the country is unknown, and the population that suffers from this condition has not been characterized either, making clear the relevance of conducting research on this matter. Considering that epidemiology data in Colombia are limited, and that BE is a precursor of esophageal adenocarcinoma —which has a survival rate of 17% at 5 years 14—, it is fundamental to know the characteristics of patients with this disease and to be certain of its prevalence in the country.
Consequently, expanding epidemiological knowledge of this pathology may help optimize the diagnosis process by characterizing the population and based on its prevalence, determine the importance of BE in Colombia.
Therefore, this study aims to know the main demographic characteristics of people diagnosed with BE in two medical centers located in Bogotá, Colombia. The secondary objectives include establishing the prevalence of BE in the target population, determining the proportion between short- and long-segment BE in confirmed cases, and analyzing the endoscopic-histological correlation.
Materials and methods
Target population
Patients who attended both medical centers for undergoing upper endoscopy between March and May 2018. These two centers were chosen so that the target population was enriched by including a wider range of health care service providers and socio-economic conditions.
Likewise, patients who underwent an upper endoscopy were included and those with a diagnosis of esophageal adenocarcinoma or a history of treatment for BE with dysplasia were excluded. A total of 3 000 patients met these criteria. The sample size was calculated using a 95% confidence interval (CI), an estimated prevalence of 2%, and a precision level of 0.5%. In fact, the estimated prevalence was taken from a study conducted in Mexico 11, in which the prevalence of BE in the general population undergoing endoscopy was 1.8%.
Source of information
Endoscopy and histopathology reports, as well as the medical records of the selected patients, were reviewed to collect the data required for conducting the study. Specifically, the percentage of patients with endoscopic findings in which intestinal metaplasia was confirmed through biopsy was obtained from pathology reports. The number of patients with a final diagnosis of BE was established in order to calculate the prevalence of BE in the study population.
Information analysis
Microsoft Excel, version 2017, was used for data processing. A descriptive analysis of information was performed using mean, median, mode and standard deviations. It was possible to observe the distribution of the outcome according to the selected characteristics.
In addition, the endoscopic prevalence of BE was determined by taking the 3 000 patients selected as the total population and placing the number of people diagnosed with BE in the numerator.
Number of patients diagnosed with BE (endoscopy + biopsy)
3 000 patients who underwent upper gastrointestinal endoscopy (UGIE)
Results
During the study period, that is between March and May 2018, 3 000 patients underwent upper endoscopy: 1 500 were referred to one center and the remaining 1 500 to the other center. In all cases, endoscopies were performed using the Olympus EVIS Exera III-CLV 190 video system, which provides high-definition images.
Out of the 3000 patients, 1 633 (54.4%) were women and 1 367 (45.6%), men; their average age was 51 years (minimum 15 and maximum 93 years). The most frequent indications for endoscopic examination were symptoms of gastroesophageal reflux in 16.9% of the participants (n=508), followed by epigastric pain in 10.8% (n=324), and history of gastritis confirmed by endoscopy in 6.7% (n=201). The reason for endoscopy was not mentioned in 13.5% (n=405) of the reports.
Based on the endoscopic findings, BE was suspected in 77 patients (2.5% of the total sample). Of these, intestinal metaplasia was confirmed in 22 after the histopathology report was obtained. This means that the endoscopy-histopathology correlation was 28.57%, and BE was ruled out in 52 patients (67.53%). Likewise, 2 (2.5%) pathology reports were not found, and in 1 patient (1.2%) the biopsy could not be taken due to active digestive bleeding (Figure 1).
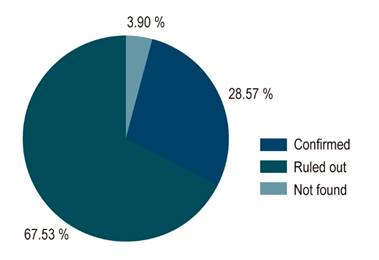
Figure 1 Correlation between the findings of the digestive tract endoscopy and the outcome of the pathology report. 100 % represent all patients in whom BE was suspected after the endoscopy was performed. Thus, Confirmed means that BE was confirmed by the pathology service; Ruled out means that the diagnosis of BE was ruled out based on the pathology report; and Not found means that the result of the biopsy was not known or was the biopsy was not performed for some reason.
In 70% of the patients with suspected BE, 1 or 2 biopsies were performed, while in 30%, 3 to 6 biopsies were performed. In 84.7%, the biopsy site was the Z line, and in 10.86%, the precise location of the biopsy was not described in the report. 93% of the histopathology reports were reviewed by a pathologist (Table 1).
Table 1 Descriptive statistics of digestive tract endoscopy and histopathology reports of patients with suspected or final diagnosis of BE
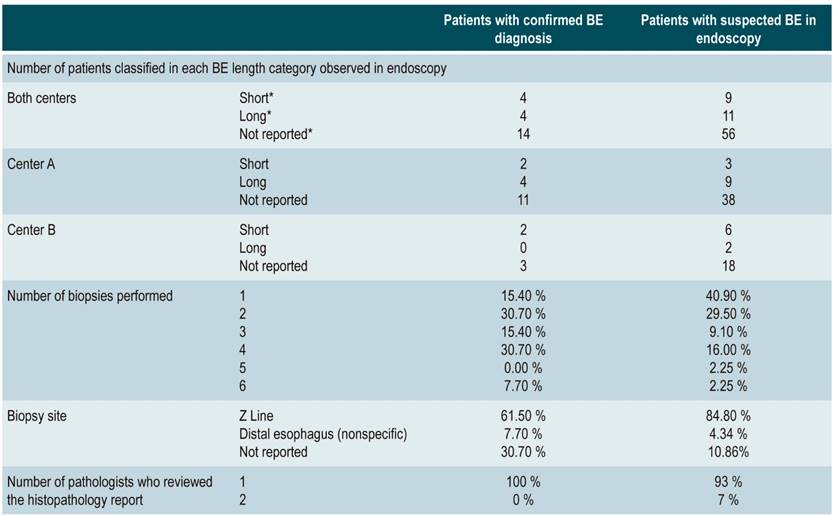
BE: Barrett’s esophagus. *Short; <3 cm; Long: >4 cm; not reported: the length of the observed segment, suggestive of BE, was not mentioned in the endoscopy report.
In terms of demographic characterization, out of the 22 cases with confirmed diagnosis, 14 (63.6%) were women and 8 (36.3%) were men (Figure 2). The age of patients with BE ranged from 40 to 79 years, with an average age of 65.5 years. 77.2% (n=17) were within the age range of 60-79 years, 13.6% (3) were between 50-59 years and 9% (2) between 40-49 years (Figure 3). In addition, 67% of these 22 individuals were obese or overweight according to their BMI, while 33% had a normal weight (Table 2).
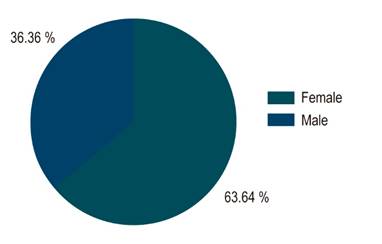
Figure 2 Sex distribution of BE patients. Of the overall sample, 36.3% were men and 63.6% were women.
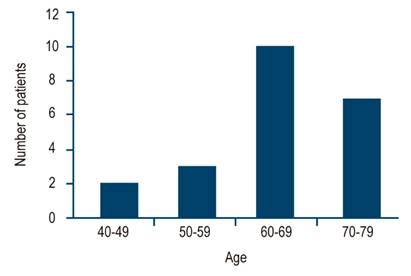
Figure 3 Age of BE patients. Age ranges are on the X-axis: 40 to 49, 50 to 59, 60 to 69 and 70 to 79 years
Regarding the history of smoking or alcohol consumption, 50% of the patients were smokers (current or suspended), while 67% had no history of alcohol consumption. On socio-demographic conditions, 58% were from middle-income households (strata 3 and 4, according to the Colombian system) (Table 2).
Table 2 Descriptive statistics for different personal variables of patients with BE
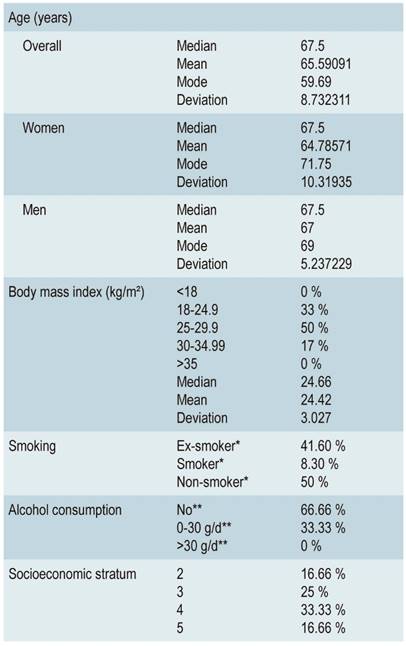
*Ex-smoker: patient with a history of smoking, but not a current smoker; Current smoker: smoker at the time of assessment; Non-smoker: no history of smoking, either in the past or in the present.
**No: no consumption; 0-30 g/d is modified as 14 g/d in women; >30 g/d is modified as >14 g/d in women (values taken from the World Health Organization and the National Institute on Alcohol Abuse and Alcoholism)
On the other hand, the main reason for endoscopy in patients with BE was gastroesophageal reflux in 11 (50%) cases, dyspepsia in 3 (13.6%), endoscopic history of gastritis in 3 (13.6%), peptic acid disease in 2 (9%), anemia in 1 (4.5%), family history of gastric cancer in 1 (4.5%); in 1 (4.5%) there was no indication (Figure 4).
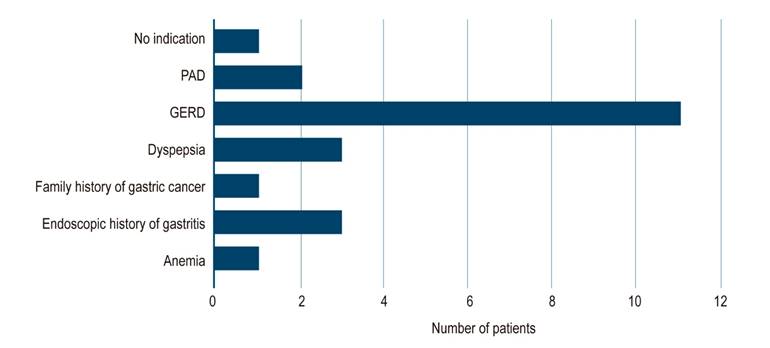
Figure 4 Reasons for digestive tract endoscopy in patients with BE. GERD: chronic gastroesophageal reflux disease; PAD: peptic acid disease; Not reported: the reason for requesting the study was not mentioned in the endoscopy report.
Also, 8.1% of patients had short-segment BE, and 18.1% had long-segment BE. Furthermore, 63.6% of the patients were not categorized at all. None of the endoscopy reports reviewed described the use of any other rating scale. Similarly, no biopsy revealed dysplasia or malignancy in these patients. Reports did not mention whether electronic chromoendoscopy was used at the time of suspected BE, nor there are any descriptions of the mucosal pattern. The endoscopic prevalence obtained for BE was 0.73% (22 cases out of 3 000).
Discussion
With respect to countries with similar characteristics than Colombia (Latin American countries), a small BE diagnosis endoscopy-histopathology correlation is evident: in the present study, it was of 28.5%, while in others, a prevalence of up to 40% has been reported 15. In the study carried out in Mexico, this correlation was 60% 11, while in a research conducted in Peru, a correlation of 25% was found (11 of 44 patients), which is similar to that found in the present study 16.
All this could suggest that the endoscopic characteristics of BE are not predictive at all for its diagnosis, that they are variable (since they are in the short-segment BE or even focal, which makes it difficult to perform an accurate biopsy) or that an appropriate number of biopsies have not been performed in relation to the size of the possible BE observed (data that cannot be known with certainty by us, since the length of the observed segment was not mentioned in 63% of the cases).
Although most patients diagnosed with BE are over 60 years old, the age range in which they are is wide (from 40 to 79 years old) and the standard deviation is 8 years. Taking this into account, it can be deduced that this pathology should not be ruled out only in patients over 60 years of age, but that it is necessary to carefully visualize the esophagus in patients of any age. Moreover, if necessary, endoscopy follow-ups could be implemented in patients with gastroesophageal reflux at younger ages. The mean age obtained is consistent with what has been described in the literature 17.
Interestingly, the relationship between men and women was reversed. Contrary to what the literature suggests 17, the prevalence of BE was higher in females. In fact, for every man diagnosed with BE, the disease was confirmed in 2 women. This is an unexpected result, which should be considered more carefully when making the general evaluation of patients since it could be explained by the fact that most of individuals making up the target population were women (54%).
However, this explanation loses validity when the population of each center is studied specifically. In the first center, most patients diagnosed were women, although most of the general target population consisted of men, and vice versa in the second center. The question remains as to why the relationship was reversed.
As is well known, BE is largely associated with chronic gastroesophageal reflux 1,18. Results obtained here confirm this fact since this digestive disease was the main indication for performing an endoscopy in patients who were eventually diagnosed with BE. However, we cannot ignore that in the remaining 50% BE cases, an endoscopy was performed for a reason unrelated to chronic gastroesophageal reflux. This could suggest that in many cases individuals with BE do not experience BE specific symptoms and that, regardless of the reason for performing the endoscopy, a detailed visualization of the esophagus should be carried out in all cases.
Also, data obtained here revealed that most patients with BE had a BMI >25 kg/m², which is consistent with available evidence, which states that there is a 2 to 3 times greater risk of developing this condition if the patient is overweight or obese to some degree 19,20.
Furthermore, 50% of BE patients had a history of smoking (either as a current smoker or an ex-smoker). This finding agrees with what has been described in the literature, in which smoking (being or having been a smoker) is a risk factor for BE 21,22. In contrast, our study does not suggest that alcohol consumption is a risk factor for the development of this condition, since most of the patients diagnosed with it did not consume it, which is consistent with the evidence available un the relevant literature.
It is also important to point out the lack of homogeneity in the descriptions of possible BE in the endoscopy reports. Worldwide, tools that help characterize the lesion in an objective manner have been described, including the Prague classification for long-segment BE. They have implications for the prognosis and risk of cancer development or for the length classification of the observed segment 23, thus descriptions should be detailed and objective. Some of them even only mentioned the presence of BE without any other detailed description.
Conversely, in studies conducted in other countries, all confirmed BE cases were classified as long-and short-segment 11,16,24. Thus, when considering the only 8 patients in our study in which length was reported, the amount of short- vs. long-segment BE was the same, while in studies carried out in other countries, short-segment BE was 3 times more frequent than the long-segment type 16,24.
Consequently, it is of great importance to emphasize that these tools should be used as a standard to describe these lesions in endoscopic reports, since the use of the Prague criteria or the length of the lesions allows having a broader idea to assess the risk of adenocarcinoma and to support more solidly a possible treatment 23.
Thus, in comparison with countries with similar characteristics (Latin American countries), the prevalence of BE in Colombia is very low, even lower than expected: 0.73 %. In this sense, the prevalence in similar studies conducted in Mexico and Chile was higher than the prevalence estimated in the present study: 1.8% 11 and 1.6 % 24, respectively.
However, in a study carried out in Peru with a similar sample population, a prevalence of 0.48% 16 was found, which is similar to that of the present study. In that sense, the low prevalence described here can be explained by the following possible reasons. Firstly, it is likely that the prevalence of BE in Colombian population is actually low, similar to the case of Japan, where it ranges between 0.9-1.2% 25. In fact, in this Asian country, some studies have proposed a high prevalence of Helicobacter pylori infection as a possible explanation for these values 25, which could also be applicable to Colombia. In addition, as mentioned above, the prevalence of our study is similar to that reported in Peruvian population.
Secondly, it is possible that the time frame of data collection was very short; actually, the data from the 1500 patients treated at one of the medical centers were collected in only 20 days. Since BE is a condition with such a low prevalence, it is possible that, in that short period, very few patients with the disease were randomly assessed.
Thirdly, although in the present study a high-definition endoscope was used and the endoscopists who performed the procedure have extensive experience in this field, low endoscopic diagnosis may be a consequence of not giving enough importance to the visualization of the esophagus in patients who underwent this procedure for reasons that were not primarily focused on the search of BE. This would go hand in hand with the lack of use of appropriate tools for the description of endoscopic findings suggestive of BE.
Finally, based on the scarce information provided in most endoscopy reports, it was not possible to determine if the number of biopsies performed was adequate in relation to the size of the possible BE observed. This could also contribute to yielding false negatives in biopsies and finding a lower prevalence than the actual number of cases.
Some of the limitations of the study are that, although a careful and extensive review was done, the population was not completely representative, and that the choice of the evaluated sites was based on the researchers’ individual criteria (possible selection bias). Likewise, there could be a risk of memory bias when inquiring about the exact magnitude of exposure to risk factors in previous stages of life (in this case smoking and alcohol consumption), and a bias of confusion with variables not contemplated that explain the true reason for certain associations (for example, concluding that BE is not more prevalent in women when considering certain variables).
Implications in the area of research are broad. This study seeks to take a first step in the recognition of this condition in the Colombian population, as well as to know the possible shortcomings related to the diagnosis of the disease. Based on these results, a second study is planned in which diagnostic tools such as segment length and the Prague classification will be implemented, as well as a more detailed visualization of the esophagus. All this with the purpose of finding out if a greater prevalence and endoscopy-histopathology correlation are obtained from the implementation of these tools, or if the results obtained in this study show the actual prevalence.
Conclusions
In the centers selected for conducting the study, BE has a very low prevalence. It is found predominantly in elderly women, with symptoms of gastroesophageal reflux, who are overweight or obese and have a history of smoking. In general, after comparing data obtained from other studies, it could be concluded that, even though the prevalence of BE in countries such as Chile and Mexico is higher than in Colombia, the demographic characteristics are similar, with the exception of sex 11,16,24.
It should be noted that in the studies mentioned above the population sample is much less significant compared to our sample. In addition, the results described for Peruvian population are similar to our results in terms of prevalence, demographic characteristics and the endoscopy-histopathology correlation 16.
Acknowledgments
The authors would like to thank both medical centers in which the study was conducted, especially their gastroenterology services.
REFERENCES
1. Spechler SJ, Souza RF. Barrett’s esophagus. N Engl J Med. 2014;371(9):836-845. http://doi.org/10.1056/NEJMra1314704 [ Links ]
2. Hani A. Esófago de Barrett: Una problemática para analizar, editorial. Rev Col Gastroenterol. 2008;23(1):1-2. [ Links ]
3. Van der Veen AH, Dees J, Blankensteijn JD, Van Blankenstein M. Adenocarcinoma in Barrett’s oesophagus: an overrated risk. Gut. 1989;30(1):14-18. http://doi.org/10.1136/gut.30.1.14 [ Links ]
4. Hvid-Jensen F, Pedersen L, Drewes AM, Sørensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med. 2011;365(15):1375-1383. http://doi.org/10.1056/NEJMoa1103042 [ Links ]
5. Pohl H, Pech O, Arash H, Stolte M, Manner H, May A, Kraywinkel K, Sonnenberg A, Ell C. Length of Barrett’s oesophagus and cancer risk: implications from a large sample of patients with early oesophageal adenocarcinoma. Gut. 2016;65(2):196-201. http://doi.org/10.1136/gutjnl-2015-309220 [ Links ]
6. Sikkema M, Looman CW, Steyerberg EW, Kerkhof M, Kastelein F, van Dekken H, van Vuuren AJ, Bode WA, van der Valk H, Ouwendijk RJ, Giard R, Lesterhuis W, Heinhuis R, Klinkenberg EC, Meijer GA, ter Borg F, Arends JW, Kolkman JJ, van Baarlen J, de Vries RA, Mulder AH, van Tilburg AJ, Offerhaus GJ, ten Kate FJ, Kusters JG, Kuipers EJ, Siersema PD. Predictors for neoplastic progression in patients with Barrett’s Esophagus: a prospective cohort study. Am J Gastroenterol. 2011;106(7):1231-8. http://doi.org/10.1038/ajg.2011.153 [ Links ]
7. Yousef F, Cardwell C, Cantwell MM, Galway K, Johnston BT, Murray L. The incidence of esophageal cancer and high-grade dysplasia in Barrett’s esophagus: a systematic review and meta-analysis. Am J Epidemiol. 2008;168(3):237-249. http://doi.org/10.1093/aje/kwn121 [ Links ]
8. Sierra F. Incidencia de adenocarcinoma en esófago de Barrett, Fundación Santa Fe de Bogotá, 11 años de seguimiento. Rev Col Gastroenterol. 2008;23(1):13-25. [ Links ]
9. Desai TK, Krishnan K, Samala N, Singh J, Cluley J, Perla S, Howden CW. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett’s oesophagus: a meta-analysis. Gut. 2012;61(7):970-6. http://doi.org/10.1136/gutjnl-2011-300730 [ Links ]
10. Bhat S, Coleman HG, Yousef F, Johnston BT, McManus DT, Gavin AT, Murray LJ. Risk of malignant progression in Barrett’s esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2011;103(13):1049-57. http://doi.org/10.1093/jnci/djr203 [ Links ]
11. Herrera JL, Monreal R, García D, González EI, Borjas OD, Maldonado HJ, González JA. Prevalencia de esófago de Barrett: estudio observacional en una clínica de gastroenterología. Revista de Gastroenterología de México. 2017;82(4):296-300. https://doi.org/10.1016/j.rgmx.2017.01.006 [ Links ]
12. Olmos JA, Piskorz MM, Vela MF. Revisión sobre enfermedad por reflujo gastroesofágico (ERGE). Acta Gastroenterol Latinoam. 2016;46(2):160-172. [ Links ]
13. Gerson LB, Shetler K, Triadafilopoulos G. Prevalence of Barrett’s esophagus in asymptomatic individuals. Gastroenterology. 2002;123(2):461-467. http://doi.org/10.1053/gast.2002.34748 [ Links ]
14. Brunicardi FC, Andersen DK, Billiar TR, Dunn DL, Hunter JG, Matthews JB, Pollock RE. Schwartz’s Principles of Surgery. Nueva York: McGraw-Hill, 9a edición; 2010. [ Links ]
15. Angarita OR, Granados CE, Ricaurte O. Frecuencia de esófago de Barrett en una serie de biopsias endoscópicas de esófago (2002-2007). Rev Col Gastroenterol. 2008;23(1):4-12. [ Links ]
16. Chacaltana A, Urday C, Ramon W, Rodríguez C, Espinoza J, Velarde H, Rodríguez I, Lucho E, Rauch E. Prevalencia, características clínico-endoscópicas y factores predictivos de Esófago de Barrett. Rev Gastroenterol Perú. 2009;29(1):24-32. [ Links ]
17. Odze RD. Gastrointestinal Pathology, An Issue of Gastroenterology Clinics (The Clinics: Internal Medicine). Filadelfia: Saunders; 2007. [ Links ]
18. Spechler SJ. Barrett esophagus and risk of esophageal cancer: a clinical review. JAMA. 2013;310(6):627-636. http://doi.org/10.1001/jama.2013.226450 [ Links ]
19. Shinkai H, Iijima K, Koike T, Abe Y, Dairaku N, Inomata Y, Kayaba S, Ishiyama F, Oikawa T, Ohyauchi M, Ito H, Asonuma S, Hoshi T, Kato K, Ohara S, Shimosegawa T. Association between the body mass index and the risk of Barrett’s esophagus in Japan. Digestion. 2014;90(1):1-9. http://doi.org/10.1159/000357776 [ Links ]
20. El-Serag HB, Kvapil P, Hacken-Bitar J, Kramer JR. Abdominal obesity and the risk of Barrett’s esophagus. Am J Gastroenterol. 2005;100(10):2151-2156. http://doi.org/10.1111/j.1572-0241.2005.00251.x [ Links ]
21. Iyer PG, Kaul V. Barrett Esophagus. Mayo Clin Proc. 2019;94(9):1888-1901. http://doi.org/10.1016/j.mayocp.2019.01.032 [ Links ]
22. Vargas G. Prevalencia y factores de riesgo en el Hospital Nacional Arzobispo Loayza, Lima-Perú. Rev Gastroenterol. 2010;30(4):284-304. [ Links ]
23. Sharma P, Dent J, Armstrong D, Bergman JJ, Gossner L, Hoshihara Y, Jankowski JA, Junghard O, Lundell L, Tytgat GN, Vieth M. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C & M criteria. Gastroenterology. 2006;131(5):1392-9. http://doi.org/10.1053/j.gastro.2006.08.032 [ Links ]
24. Csendes A, Smok G, Burdiles P, Quesada F, Huertas C, Rojas J, Korn O. Prevalence of Barrett’s esophagus by endoscopy and histologic studies: a prospective evaluation of 306 control subjects and 376 patients with symptoms of gastroesophageal reflux. Dis Esophagus. 2000;13(1):5-11. http://doi.org/10.1046/j.1442-2050.2000.00065.x [ Links ]
25. Hongo M. Review article: Barrett’s oesophagus and carcinoma in Japan. Aliment Pharmacol Ther. 2004;20 Suppl 8:50-54. http://doi.org/10.1111/j.1365-2036.2004.02230.x [ Links ]
Citation: Bernal Vaca ML, García HF, Mendoza de Molano B. Demographic characterization of the population with Barrett’s esophagus in two medical centers of Bogotá, Colombia. Rev Colomb Gastroenterol. 2020;35(3):311-318. https://doi.org/10.22516/25007440.488
Received: December 06, 2019; Accepted: June 16, 2020











 text in
text in 



