Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957On-line version ISSN 2500-7440
Rev. colomb. Gastroenterol. vol.35 no.4 Bogotá Oct./Dec. 2020 Epub July 12, 2021
https://doi.org/10.22516/25007440.478
Original article
Risk factors for gallbladder polyp malignancy in two public hospitals of Peru
1Escuela de medicina humana, Universidad Continental. Huanxayo, Perú
2Facultad de medicina, Universidad San Martín de Porres. Lima, Perú
3Asociación Médica de Investigación y Servicios de Salud. Lima, Perú
4Facultad de medicina humana, Universidad Ricardo Palma. Lima, Perú
5Hospital Nacional Daniel Alcides Carrión. Callao, Perú
Introduction:
In most patients, gallbladder polyps, both benign and malignant, are usually an incidental finding. However, imaging studies cannot accurately establish their degree of malignancy.
Objective:
To determine the risk factors for gallbladder polyp malignancy in two Peruvian public hospitals.
Methodology:
Retrospective cohort study conducted on secondary data from patients who underwent cholecystectomy between 2004 and 2012 in Lima and another in Callao, Peru. The malignancy of the polyp was established according to the histopathological type of adenocarcinoma. Relative risks and their 95% confidence intervals (95%CI) were obtained. Moreover, ROC curves were used to determine sensitivity and specificity according to the size of the polyp.
Results:
Of 368 biopsies, 26 (7%) were adenocarcinomas. The median size of the polyps was 4mm (range: 1-65mm). 176 patients (51%) had multiple polyps, and 85 (23%) had associated gallstones. Multivariate analysis showed that the risk of malignancy increased by 26% (95%CI:14-40%, p-value:<0.001) per millimeter of polyp size and by 182% (95%CI:46-445%, p-value=0.002) based on vesicular wall size, adjusted for patient age, lithiasis and vesicular size. For a size of 6mm, sensitivity was 81%, and specificity was 85%.
Conclusion:
The size of the polyp and the thickness of the vesicular wall are associated with the malignancy of vesicular polyps.
Keywords: Gallbladder neoplasms; polyps; adenocarcinoma; risk factors
Introducción:
los pólipos de vesícula biliar, benignos y malignos, en la mayoría de pacientes tienen un diagnóstico generalmente incidental; a través de estudios de imágenes, que no se pueden distinguir con precisión según su grado de malignidad.
Objetivo:
determinar los factores de riesgo para la malignidad de los pólipos vesiculares en dos hospitales públicos peruanos.
Metodología:
estudio de cohorte retrospectiva, de datos secundarios, en colecistectomizados del 2004 al 2012 en un hospital de Lima y otro de Callao. Se definió como pólipo maligno según el tipo histopatológico de adenocarcinoma. Se obtuvieron los riesgos relativos y sus intervalos de confianza del 95 % (IC 95 %). Además, mediante curvas ROC (característica operativa del receptor), se obtuvieron la sensibilidad y especificidad según el tamaño de pólipo.
Resultados:
de las 368 biopsias, 26 (7 %) fueron adenocarcinomas. La mediana del tamaño de los pólipos fue de 4 mm (rango: 1-65 mm), 176 (51 %) tuvieron múltiples pólipos y 85 (23 %) tuvieron litiasis biliar asociada. En el análisis multivariado, se incrementó el riesgo de malignidad por cada milímetro del tamaño del pólipo en 26 % (IC 95 %:14 %-40 %, valor p < 0,001) y del tamaño de la pared vesicular en 182 % (IC 95 %:46 %-445 %, valor p: 0,002), ajustados por la edad del paciente, la litiasis y el tamaño vesicular. Para un tamaño de 6 mm se tuvo una sensibilidad de 81 % y especificidad del 85 %.
Conclusión:
se concluye que el tamaño del pólipo y el grosor de la pared vesicular estuvieron asociados con la malignidad de pólipos vesiculares.
Palabras clave: Neoplasias de la vesícula biliar; pólipos; adenocarcinoma; factores de riesgo
INTRODUCTION
Gallbladder cancer is the fifth most common gastrointestinal cancer worldwide1. It has a poor prognosis once detected, with an average survival time of 6 months2,3. Its incidence varies, ranging from the lowest rates in developed countries to the highest in some South American ethnic groups4. In Peru, it is one of the most frequent neoplasms in elderly women5.
The presence of gallbladder polyps is one of the main risk factors associated with the development of this type of cancer since they occur in 4% to 7% of healthy individuals6. Of the gallbladder polyps that will be classified as malignant, 85 % are adenocarcinomas7,8 and are usually diagnosed incidentally through abdominal ultrasound9. Other factors associated with gallbladder malignancy include being over 60 years old and polyp size larger than 10 mm10-12, the occurrence of symptoms11,13, vesicular lithiasis10,14, and the number of polyps11,14, among others15.
In Peru, several studies have been conducted to identify the characteristics of this type of cancer, such as the work carried out in a population being treated in a private healthcare center in a suburban area of the country16, yet analyses determining the association between different variables and this disease have not been conducted. Therefore, the aim of this study is to determine the risk factors for gallbladder polyp malignancy in two public hospitals from Peru.
Materials and methods
Study design and population
An observational retrospective cohort study was carried out using secondary data analysis. Databases were obtained from the archives of the Hospital Nacional Arzobispo Loayza (HNAL) and the Hospital Nacional Daniel Alcides Carrión (HNDAC) during the 2004-2012 period. These are public hospitals and most patients come from socioeconomic groups C, D and E.
Reports of gallbladder biopsies performed in patients who underwent cholecystectomy and in which a gallbladder polyps anatomic pathology diagnosis was later confirmed were included. Patients without complete primary data were excluded. Non-probabilistic quota sampling was used.
Procedures and variables
Permission was requested at both hospitals for using the data. After creating a database in Excel for Windows 2013, data were cleared and those that met the selection criteria were chosen. This research was approved by a local committee endorsed by the National Health Institute of Peru.
The dependent variable was the diagnosis of polyp malignancy according to the anatomopathological characteristics of gallbladder polyps. Adenoma, hyperplastic, cholesterol, and inflammatory polyps were considered benign, while adenocarcinoma polyps were classified as malignant.
Independent variables included the patient’s age and sex (for statistical analysis, the female sex was considered the variable of interest); anatomopathological characteristics: polyp size (measured in millimeters; in cases of multiple polyps, only the largest polyp size measurement was considered and number of polyps (multiple polyps were defined as the presence of 2 or more polyps); and gallbladder characteristics: size of the gallbladder diameter (measured in centimeters according to the length of the gallbladder) and thickness of the gallbladder wall (measured in millimeters according to the wall thickness at the polyp site).
Statistical analysis
Stata 11.1 for Windows was used for data analysis. Frequencies and percentages were used to describe categorical variables and means and standard deviations for quantitative variables. The chi-square (χ2) and Student’s t statistical tests were used to calculate p-values for variations in categorical and quantitative variables, respectively. For the bivariate and multivariate analyses, crude (cRR) and adjusted (aRR) relative risks were calculated with their corresponding 95 % confidence intervals (95 % CI). The receiver operating characteristic (ROC) curve was used exploratorily to determine sensitivity and specificity based on the cut-off points of gallbladder polyp size. P-values < 0.05 were considered statistically significant.
Results
Of the 368 biopsies performed on cholecystectomized patients diagnosed with gallbladder polyps, 264 (71.7 %) were treated at HNAL and 104 (28.3 %) at HNDAC; 288 (78.3 %) were female and the mean age was 45.5 years (±15.5 years, range: 12-86 years). The median size of polyps was 4mm (range: 1-65mm). 176 patients (50.7%) had multiple polyps and 85 (23.1%) had associated cholelithiasis. There were 26 (7.1 %) malignant adenocarcinoma polyps, and the remaining polyps were benign: 237 (64.6 %) cholesterol, 53 (14.4 %) hyperplastic, 48 (13.1 %) adenoma, and 3 (0.8 %) inflammatory polyps.
Depending on the malignancy, differences were found regarding patient’s age (p = 0.001), polyp size (p < 0.001), associated cholelithiasis (p < 0.001), size of the gallbladder (p = 0.001), and gallbladder wall thickness (p < 0.001) (Table 1).
Table 1 Variables according to the type of polyp found in patients who underwent cholecystectomy at two hospitals from Peru.
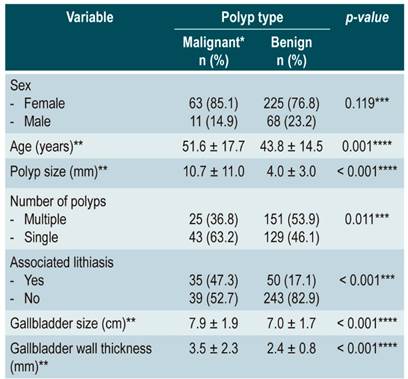
*Malignant polyp: adenocarcinoma + adenoma. **Median and range. ***Obtained using χ2 test. ****Obtained using Student’s t-test.
When the analysis was based on the type of gallbladder polyp, statistically significant differences were observed in the median values of the following variables: age (p = 0.006), polyp size (p < 0.001), gallbladder wall thickness (p < 0.001), and gallbladder size (p < 0.001), (Figure 1).
P-values obtained using the ANOVA statistical test to determine the difference in each variable average value:
1A: Depending on the patient’s age (years of age);
1B: Depending on polyp size (mm);
1C: Depending on gallbladder size (cm);
1D: Depending on gallbladder wall thickness (mm).
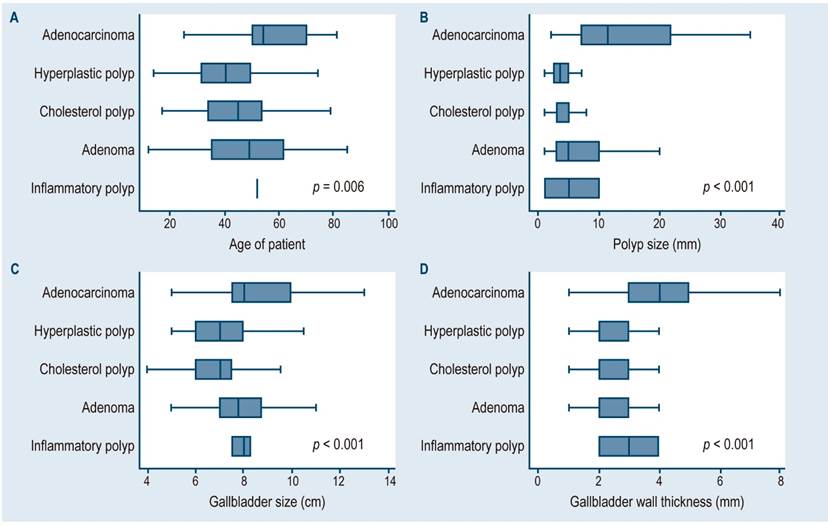
Figure 1 Differences in median values according to the type of gallbladder polyp in cholecystectomized patients treated at two hospitals in Lima and Callao.
In the bivariate analysis, polyp size (p < 0.001), gallbladder wall thickness (p < 0.001), cholelithiasis (p < 0.001), patient age (p = 0.003), and gallbladder size (p = 0.004) were associated with gallbladder polyp malignancy. The multivariate analysis showed that the risk of malignancy increased by 26 % per millimeter of polyp size (95 % CI: 14 %-40 %, p-value < 0.001) and by 182 % per millimeter of gallbladder wall size (95 % CI: 46 %-445 %, p-value = 0.002), adjusted for patient age, lithiasis and gallbladder size (Table 2).
Table 2 Risk factors for malignancy of gallbladder polyps in cholecystectomized patients treated at two hospitals in Lima and Callao.
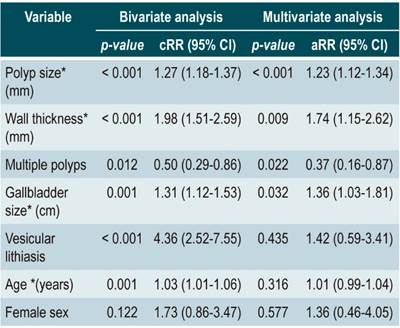
*Mean and standard deviation/p-value. 95 %CI: 95 % confidence interval; aRR: adjusted relative risk; cRR: crude relative risk.
Figure 2 shows the ROC curves for polyp size (A = 0.78), gallbladder wall thickness (B = 0.67), gallbladder size (C = 0.66), and all variables in the multivariate model (D = 0.86).
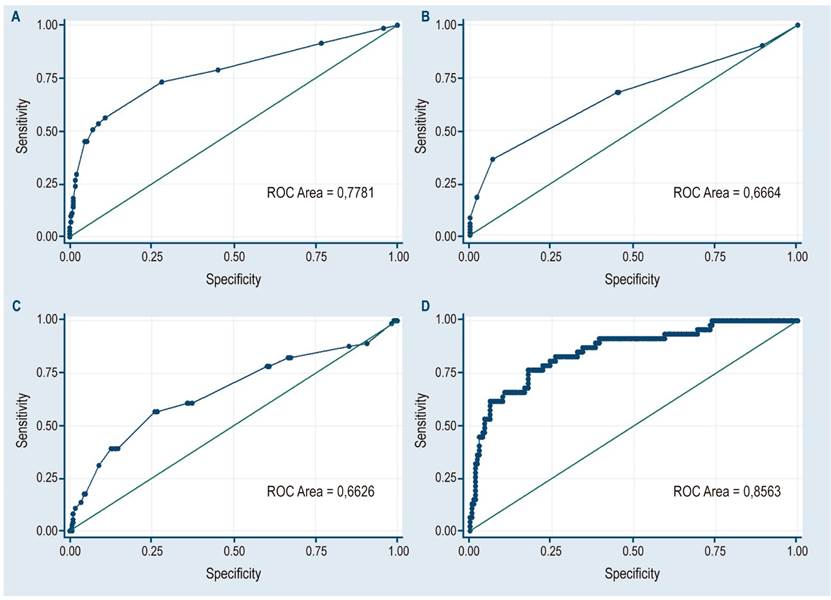
Figura 2 ROC curves for polyp size (A = 0.78), gallbladder wall thickness (B = 0.67), gallbladder size (C = 0.66) and all variables in the multivariate model (D = 0.86).
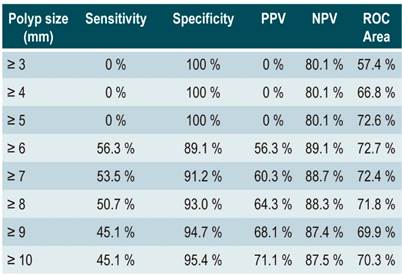
The cut-off points for the continuous variable polyp size were determined using the ROC curve; the best cut-off point would be a size of 6 mm, with a sensitivity of 80.8 % and a specificity of 84.9 % (Table 3).
Discussion
In our study, 7% of gallbladder polyps were malignant. This is important since the diagnosis of gallbladder polyps was incidental in most patients, usually following a routine abdominal ultrasound or after undergoing cholecystectomy for treating biliary lithiasis or biliary colic17. However, due to the development of new diagnostic imaging devices, the detection of gallbladder polyps is becoming more common11. Although most gallbladder polyps are benign, differentiating them from malignant masses is important because gallbladder carcinoma usually has a late onset and a poor prognosis. Consequently, they must be detected early to change their prognosis18.
Polyp size was associated with malignancy, which is consistent with most findings reported in similar studies11,19; also, there was almost a 13 mm difference between size averages depending on the malignancy. Various studies show that a polyp size ≥ 10 mm is a predictor of malignancy20, while others describe that, a polyp size greater than 10-15 mm was observed in 45 % to 67 % of malignant polyp cases the cases10,21-23. In some studies, temporal follow-up has been reported in patients with polyps smaller than 10 mm, concluding that no adenocarcinomas or progression to malignancy were identified during the follow-up period24. However, in our study several reports of malignancy in polyps smaller than 5 mm were found. Therefore, the cut-off points for each measurement were determined using the ROC curve and it was observed that the best measure was found in polyps ≥ 6 mm, with acceptable sensitivity and specificity values. This is consistent with the study of Zielinski et al. con ducted in a US population25, which suggests surgical resection of gallbladder polyps ≥ 6 mm based on preoperative ultrasound testing. Furthermore, evidence of malignancy in polyps > 6 mm is easily detected during biopsies or examinations performed by qualified gastroenterologists, but not so much in imaging studies26.
Gallbladder wall thickness was another factor associated with malignancy. This is consistent with the findings of Kim et al.27, who concluded that wall thickness > 10 mm is a predictive factor for neoplastic thickening of the gallbladder wall. Similarly, Aldouri et al.28 noted that severe thickening of the gallbladder wall is associated with an increased risk of developing gallbladder cancer. In turn, Sun et al.29 included patients with a locally thickened, irregular gallbladder wall in their surgical indications for gallbladder polyps. Considering the above, further research into the thickness of the gallbladder wall is suggested to assess its relevance regarding the presence of malignant polyps.
No significant differences were found between sexes as a risk factor for malignancy in our study; however, female predominance was observed in the group of malignant polyps, which is in consistent with the findings of Manrique et al.30) in their study of gallbladder cancer conducted in a population from Arequipa, Peru. This may be explained by the different reports on this variable since some show male predominance31-34) and others the opposite11,14,35. Although some studies have reported an association between age and gallbladder polyp malignancy10,11,28, in our study age was not associated, nor was the prevalence of vesicular lithiasis, which is not always associated with malignancy11.
One of the limitations of the study was the operator-dependent bias, as samples were analyzed by multiple physicians before being entered into the database. Nonetheless, since pathologists are highly qualified and the pathology report to be evaluated does not raise significant diagnostic doubt, we consider that this situation did not have a major impact on the results obtained.
Based on the data analyzed here, it is concluded that polyp size and gallbladder wall thickness were associated with the malignancy of gallbladder polyps.
Acknowledgments
We thank Emperatriz Centeno for helping us to prepare some sections of the article.
REFERENCES
1. Peña Dávila FE, Sánchez Renteria FA, Fernandez Mogollon J, Rodríguez Rodríguez MR. Frecuencia y perfil clínico de cáncer de vesícula biliar en pacientes colecistectomizados en 3 hospitales referenciales de Chiclayo entre 2011 y 2015. Rev gastroenterol Perú. 2017;37(2):142-5. [ Links ]
2. Navarro Rosenblatt D, Durán Agüero S. Cáncer de vesícula biliar en Chile y factores nutricionales de riesgo. Nutr Hosp. 2016;33(1):105-110. https://doi.org/10.20960/nh.37 [ Links ]
3. Uribe M, Heine TC, Brito MF, Bravo LD. Actualización en cáncer de vesícula biliar. Revista Médica Clínica Las Condes. 2013;24(4):638-643. https://doi.org/10.1016/S0716-8640(13)70202-5 [ Links ]
4. Torres Rodríguez JK. Características ecográficas y anatomopatológicas en pacientes colecistectomizados por pólipo vesicular en el Hospital Nacional Edgardo Rebagliati Martins de enero 2016 a julio del 2018. Lima: Universidad Peruana Unión; 2019. [ Links ]
5. Datos Epidemiológicos Perú cáncer 2000-2009 [Internet]. INEN [acceso el 20 de noviembre de 2016]. Disponible en: Disponible en: http://www.inen.sld.pe/portal/estadisticas/datos-epidemiologicos.html [ Links ]
6. Contreras Castro E, Alfaro Fernández P, Contreras Castro F, Luna Victoria R, Contreras Alomía I. Correlación entre diagnóstico ecográfico e histopatológico de poliposis vesicular en la Clínica Good Hope 2008-2014. Horiz Med. 2016;16(2):27-32. https://doi.org/10.24265/horizmed.2016.v16n2.05 [ Links ]
7. Ramírez AS, Martínez E, Román R, Gamarra E, Villalba W. Cáncer de la vesícula biliar. Experiencia de 10 años del Instituto Nacional del Cáncer. Rev. Cir. Parag. 2016;40(2):8-11. http://dx.doi.org/10.18004/sopaci.noviembre.8-11 [ Links ]
8. Justo I, Marcacuzco A, Nutu OA, Manrique A, Calvo J, Caso Ó, Cambra F, García A, Jiménez C. Análisis retrospectivo en pacientes con cáncer de vesícula biliar: tratamiento quirúrgico y supervivencia en función del estadio tumoral. Rev Esp Enferm Dig. 2018;110(8):485-492. https://doi.org/10.17235/reed.2018.5435/2017 [ Links ]
9. Pina L, Lagos H, Quiche G, Alle L, Sarotto LE. Carcinoma incidental de vesícula biliar en un hospital universitario. Acta Gastroenterol Latinoam. 2017;47(3):190-193. [ Links ]
10. Wu T, Sun Z, Jiang Y, Yu J, Chang C, Dong X, Yan S. Strategy for discriminating cholesterol and premalignancy in polypoid lesions of the gallbladder: a single-centre, retrospective cohort study. ANZ J Surg. 2019;89(4):388-392. https://doi.org/10.1111/ans.14961 [ Links ]
11. Sarici IS, Duzgun O. Gallbladder polypoid lesions >15mm as indicators of T1b gallbladder cancer risk. Arab J Gastroenterol. 2017;18(3):156-158. https://doi.org/10.1016/j.ajg.2017.09.003 [ Links ]
12. Shin SR, Lee JK, Lee KH, Lee KT, Rhee JC, Jang K-T, Kim SH, Choi DW. Can the growth rate of a gallbladder polyp predict a neoplastic polyp? J Clin Gastroenterol. 2009;43(9):865-8. https://doi.org/10.1097/MCG.0b013e31819359aa [ Links ]
13. Lee KF, Wong J, Li JCM, Lai PBS. Polypoid lesions of the gallbladder. Am J Surg. 2004;188(2):186-90. https://doi.org/10.1016/j.amjsurg.2003.11.043 [ Links ]
14. Shah J. Postoperative Histopathology Findings of Ultrasonographically diagnosed Gallbladder Polyp In 32 Patients. The Internet Journal of Third World Medicine. 2010;9(1):1-5. https://doi.org/10.5580/5be [ Links ]
15. Lai HC, Chang SN, Lin CC, Chen CC, Chou JW, Peng CY, Lai SW, Sung FC, Li YF. Does diabetes mellitus with or without gallstones increase the risk of gallbladder cancer? Results from a population-based cohort study. J Gastroenterol. 2013; 47(8); 856-65. https://doi.org/10.1007/s00535-012-0683-z [ Links ]
16. Tannous MB, Arróspide MT, Huerta-Mercado TJ, Levy YS. Gallbladder polyps: Clinical and pathological features in Cholecystectomy patients in the Anglo American clinic in the period of 1999-2007. Rev Gastroenterol Peru. 2011;31(1):32-7. [ Links ]
17. Gurusamy KS, Abu-Amara M, Farouk M, Davidson BR. Cholecystectomy for gallbladder polyp. Cochrane Database Syst Rev. 2009;(1):CD007052. https://doi.org/10.1002/14651858.CD007052.pub2 [ Links ]
18. Myers RP, Shaffer EA, Beck PL. Gallbladder polyps: epidemiology, natural history and management. Can J Gastroenterol. 2002;16(3):187-94. https://doi.org/10.1155/2002/787598 [ Links ]
19. Matos ASB de, Baptista HN, Pinheiro C, Martinho F. Gallbladder polyps: how should they be treated and when?. Rev Assoc Med Bras. 2010;56(3):318-21. https://doi.org/10.1590/S0104-42302010000300017 [ Links ]
20. Sarkut P, Kilicturgay S, Ozer A, Ozturk E, Yilmazlar T. Gallbladder polyps: factors affecting surgical decision. World J Gastroenterol. 2013;19(28):4526-30. https://doi.org/10.3748/wjg.v19.i28.4526 [ Links ]
21. Chattopadhyay D, Lochan R, Balupuri S, Gopinath B-R, Wynne K-S. Outcome of gall bladder polypoidal lesions detected by transabdominal ultrasound scanning: a nine year experience. World J Gastroenterol. 2005;11(14):2171-3. https://doi.org/10.3748/wjg.v11.i14.2171 [ Links ]
22. Yeh CN, Jan YY, Chao TC, Chen MF. Laparoscopic cholecystectomy for polypoid lesions of the gallbladder: a clinicopathologic study. Surgical Laparoscopy Endoscopy & Percutaneous Techniques. 2001;11(3):176-81. https://doi.org/ 10.1097/00019509-200106000-00005 [ Links ]
23. Mainprize KS, Gould SW, Gilbert JM. Surgical management of polypoid lesions of the gallbladder. British Journal of Surgery. 2000;87(4):414-7. https://doi.org/10.1046/j.1365-2168.2000.01363.x [ Links ]
24. Csendes A, Burgos AM, Csendes P, Smok G, Rojas J. Late Follow-Up of Polypoid Lesions of the Gallbladder Smaller Than 10 mm. Ann Surg. 2001;234(5):657-60. https://doi.org/10.1097/00000658-200111000-00011 [ Links ]
25. Zielinski MD, Atwell TD, Davis PW, Kendrick ML, Que FG. Comparison of surgically resected polypoid lesions of the gallbladder to their pre-operative ultrasound characteristics. J Gastrointest Surg. 2009;13(1):19-25. https://doi.org/10.1007/s11605-008-0725-2 [ Links ]
26. Nagata K, Endo S, Honda T, Yasuda T, Hirayama M, Takahashi S, Kato T, Horita S, Furuya K, Kasai K, Matsumoto H, Kimura Y, Utano K, Sugimoto H, Kato H, Yamada R, Yamamichi J, Shimamoto T, Ryu Y, Matsui O, Kondo H, Doi A, Abe T, Yamano HO, Takeuchi K, Hanai H, Saida Y, Fukuda K, Näppi J, Yoshida H. Accuracy of CT Colonography for Detection of Polypoid and Nonpolypoid Neoplasia by Gastroenterologists and Radiologists: A Nationwide Multicenter Study in Japan. Am J Gastroenterol. 2017;112(1):163-171. https://doi.org/10.1038/ajg.2016.478 [ Links ]
27. Kim HJ, Park JH, Park DI, Cho YK, Sohn CI, Jeon WK, Kim BI, Choi SH. Clinical usefulness of endoscopic ultrasonography in the differential diagnosis of gallbladder wall thickening. Dig Dis Sci. 2012;57(2):508-15. https://doi.org/10.1007/s10620-011-1870-0 [ Links ]
28. Aldouri AQ, Malik HZ, Waytt J, Khan S, Ranganathan K, Kummaraganti S, Hamilton W, Dexter S, Menon K, Lodge JP, Prasad KR, Toogood GJ. The risk of gallbladder cancer from polyps in a large multiethnic series. Eur J Surg Oncol. 2009;35(1):48-51. https://doi.org/10.1016/j.ejso.2008.01.036 [ Links ]
29. Sun X-J, Shi J-S, Han Y, Wang J-S, Ren H. Diagnosis and treatment of polypoid lesions of the gallbladder: report of 194 cases. HBPD INT. 2004;3(4):591-4. [ Links ]
30. Manrique RRG, Camapaza YIC, Torres FS, Apaza YMO. Cáncer de vesícula biliar según tipo histológico y clasificación TNM en Arequipa, Perú. Acta Médica Peruana. 2012;29(1):23-7. [ Links ]
31. Segawa K, Arisawa T, Niwa Y, Suzuki T, Tsukamoto Y, Goto H, Hamajima E, Shimodaira M, Ohmiya N. Prevalence of gallbladder polyps among apparently healthy Japanese: ultrasonographic study. Am J Gastroenterol. 1992;87(5):630-3. [ Links ]
32. Yang H-L, Kong L, Hou L-L, Shen H-F, Wang Y, Gu X-G, Qin JM, Yin PH, Li Q. Analysis of risk factors for polypoid lesions of gallbladder among health examinees. World J Gastroenterol. 2012;18(23):3015-9. https://doi.org/10.3748/wjg.v18.i23.3015 [ Links ]
33. Xu Q, Tao L, Wu Q, Gao F, Zhang F, Yuan L, He XD. Prevalences of and risk factors for biliary stones and gallbladder polyps in a large Chinese population. HPB (Oxford). 2012;14(6):373-81. https://doi.org/10.1111/j.1477-2574.2012.00457.x [ Links ]
34. Collett JA, Allan RB, Chisholm RJ, Wilson IR, Burt MJ, Chapman BA. Gallbladder polyps: prospective study. JUM. 1998;17(4):207-11. https://doi.org/10.7863/jum.1998.17.4.207 [ Links ]
35. Escalona PA, León GF, Bellolio RF, Pimentel MF, Guajardo BM, Gennero R, Cruz JP, Viviani P, Ibáñez L. Pólipos vesiculares: correlación entre hallazgos ecográficos e histopatológicos. Rev Méd Chile. 2006;134(10):1237-42. https://doi.org/10.4067/S0034-98872006001000004 [ Links ]
Citation: Mejía CR, Mayta K, Cárdenas M, Verástegui-Díaz A, Quiñones-Laveriano DM, Maravi-Coronado J, Monge E, Vera CA. Risk factors for gallbladder polyp malignancy in two public hospitals of Peru. Rev Colomb Gastroenterol. 2020;35(4):414-420. https://doi.org/10.22516/25007440.478
Received: November 03, 2019; Accepted: September 01, 2020











 text in
text in