Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957On-line version ISSN 2500-7440
Rev. colomb. Gastroenterol. vol.35 no.4 Bogotá Oct./Dec. 2020 Epub July 12, 2021
https://doi.org/10.22516/25007440.545
Original article
Non-inferiority between two low-volume agents (sodium picosulfate/magnesium citrate vs. sodium sulfate/potassium/magnesium) to prepare the bowel for diagnostic procedures: an observational study
1MD. Subdirección de Estudios Clínicos y Epidemiología Clínica. Fundación Santa Fe de Bogotá. Bogotá, Colombia.
2MD. Internista, Gastroenteróloga. Sección de endoscopia digestiva. Fundación Santa Fe de Bogotá. Bogotá, Colombia.
3MD. Internista. Sección de endoscopia digestiva. Fundación Santa Fe de Bogotá. Bogotá, Colombia
4MD. Internista, Gastroenterólogo. Sección de endoscopia digestiva. Fundación Santa Fe de Bogotá. Bogotá, Colombia.
5MD. Cirujano General, Gastroenterólogo. Sección de endoscopia digestiva. Fundación Santa Fe de Bogotá. Bogotá, Colombia.
6MD. Cirujano General, Coloproctología. Sección de endoscopia digestiva. Fundación Santa Fe de Bogotá. Bogotá, Colombia.
7MD. Internista, Gastroenterólogo. Sección de endoscopia digestiva. Fundación Santa Fe de Bogotá. Bogotá, Colombia.
8MD. Internista, Gastroenterólogo. Sección de endoscopia digestiva. Fundación Santa Fe de Bogotá. Bogotá, Colombia.
9Matemático y Estadístico. Subdirección de Estudios Clínicos y Epidemiología Clínica. Fundación Santa Fe de Bogotá. Bogotá, Colombia.
10MD. MSc. Epidemiología. Jefe de la Subdirección de Estudios Clínicos y Epidemiología Clínica. Fundación Santa Fe de Bogotá. Bogotá, Colombia.
11MD. Internista, Gastroenterólogo. MSc Epidemiología. Director de la Subdirección de Estudios Clínicos y Epidemiología Clínica. Jefe de la Sección de endoscopia digestiva. Fundación Santa Fe de Bogotá. Bogotá, Colombia.
Introduction:
Colorectal cancer is a public health problem; however, early detection reduces morbidity and mortality. Colonoscopy is the procedure of choice for detecting precancerous lesions, and success depends on proper bowel cleansing.
Materials and methods:
Prospective study in adults who underwent colonoscopy at the Fundación Santa Fe in Bogotá, Colombia. Preparations were evaluated using the Boston Bowel Preparation Scale. A score ≥6 points indicated adequate preparation. A logistic regression analysis was carried out to establish the effectiveness of the medicines with a non-inferiority ratio of 3-5%.
Results:
598 patients were evaluated. 49% (293) received sodium picosulfate/magnesium citrate and 51% (305) received sodium sulfate/potassium/magnesium, with an average Boston score of 6.98±1.86 (78% Boston ≥6) and 7.39±1.83 (83%), respectively (p=0.649). According to the analysis of the presence and frequency of unwanted symptoms, picosulfate was better tolerated (p < 0.001).
Conclusions:
Bowel preparation studies in patients from a real-life scenario are scarce. Low-volume agents had similar overall and segmental effectiveness in the colon, confirming non-inferiority; sodium picosulfate/magnesium citrate was better tolerated. A cost-effectiveness study could establish the best option according to the needs of the study population.
Keywords: Colorectal neoplasms; Sodium picosulfate; Intestinal preparation; Real-llife evidence.
Introducción:
el cáncer colorrectal es un problema de salud pública; sin embargo, la detección temprana reduce su morbimortalidad. La colonoscopia es el procedimiento de elección para detectar lesiones premalignas y el éxito depende de una limpieza adecuada. El objetivo es evaluar el desempeño de dos preparaciones de bajo volumen empleados en un hospital de alto nivel.
Materiales y métodos:
estudio prospectivo en adultos que asistieran a colonoscopia en la Fundación Santa Fe de Bogotá, Colombia. Las preparaciones se evaluaron con la escala de Boston, con puntaje ≥ 6 puntos para una limpieza adecuada. Se realizó un análisis de regresión logística para establecer la efectividad de los medicamentos con un cálculo de no inferioridad del 3 %-5 %.
Resultados:
598 pacientes fueron evaluados. El 49 % (293) fue expuesto al picosulfato de sodio/citrato de magnesio y el 51 % (305) fue expuesto al sulfato de sodio/potasio/magnesio. Con un promedio de Boston de 6,98 ± 1,86 (78 % con puntaje de Boston ≥ 6) y 7,39 ± 1,83 (83 %), respectivamente (p = 0,649). Según el análisis de la presencia y frecuencia de síntomas no deseados, el picosulfato fue mejor tolerado (p < 0,001).
Conclusiones:
los estudios de preparación intestinal en pacientes de un escenario real son muy escasos. Los medicamentos de bajo volumen obtuvieron una efectividad global y por segmento de colon similar, confirmando la no-inferioridad; el picosulfato de sodio/citrato de magnesio fue mejor tolerado. Un estudio de costo-efectividad podría definir esto según las necesidades de la población de estudio.
Palabras clave: Neoplasias colorrectales; picosulfato de sodio; preparación intestinal; evidencia en la vida real
INTRODUCTION
Colorectal cancer (CRC) is a public health concern1. According to the World Health Organization (WHO), more than 1.8 million new cases of CRC and 881 000 deaths attributed to it were reported in 2018 worldwide2. It has been estimated that 12 out of 100 000 people are diagnosed with CRC in Colombia every year (50% of them die)3, a significant figure due to the impact this disease has on the country’s health system. Early detection of CRC reduces morbidity and mortality4, and colonoscopy is the recommended and most widely performed procedure for this purpose5-7.
Multiple diseases of the terminal ileum and colon (infectious, inflammatory, hemorrhagic, and neoplastic conditions) can be diagnosed through colonoscopy8-10. In turn, CRC screening allows immediate management of benign or premalignant lesions11. 90% of CRC cases are diagnosed in people over 50 years of age12 since CRC screening must begin at this age for low-risk patients and at 40 years of age for high-risk patients13. The success of this procedure depends on proper bowel preparation for better visualization14,15. In this sense, rescheduling the procedure due to inadequate preparation causes a delayed diagnosis of premalignant lesions in up to 46% patients16,17.
Worldwide, there are currently more than ten drugs available for bowel preparation18. Multiple studies have compared different methods and combinations of available bowel preparation medications19,20. Polyethylene glycol (PEG) is one of the most widely used drugs due to its high efficacy and low incidence of hydroelectrolytic alterations, particularly in patients with multiple comorbidities21,22. However, it has poor tolerability (requiring high fluid intake and low palatability), which may lead to a high probability of incomplete and inadequate preparation23.
Sodium picosulfate/magnesium citrate (SPMC) is a low-volume bowel preparation drug that acts as a stimulant and osmotic laxative. Favorable results have been described regarding its efficacy, even superior in terms of tolerability and safety24. However, several studies have reported an elevated risk of hydroelectrolytic disorders 25,26; therefore, the use of hyperosmolar drugs for bowel preparation in patients older than 65 years is not recommended27,28.
There are different scales that determine the effectiveness of bowel preparation. The Boston Bowel Preparation Scale (BBPS) is an internationally validated 9-point scale (0 = no preparation; 9 = optimal preparation) that divides the colon into 3 individual segments. A special feature of this scale is that assessment takes place after washing/suctioning by the endoscopist29,30. Each segment (right, transverse, and left) has a score of 0 to 3 (0 = unprepared colon with mucosa not seen due to solid stool that cannot be cleared; 1 = some areas are seen, residual stool; 2 = visible mucosa, small fragments of stool and/or opaque liquid; 3 = entire mucosa of colon segment clearly seen, completely free of stool or opaque liquid)31.
In Colombia, few studies have assessed adequate bowel preparation of patients in routine clinical practice. The aim of this study is to compare the efficacy of two low-volume bowel preparation medications (sodium picosulfate/magnesium citrate-SPMC and sodium/potassium/magnesium sulfate) used in adult patients in a high-level of care hospital, a real-world setting, in order to show that there is non-inferiority between both medications.
Materials and methods
Study design
A prospective cohort study was conducted using a study group (SPMC) and a comparator group (sodium/potassium/magnesium sulfate). The study was by the Corporate Research Ethics Committee of the Fundación Santa Fe de Bogotá, and patients included voluntarily agreed to participate after being briefed about the study’s characteristics and its potential benefits and after signing an informed consent form. Likewise, it followed the guidelines for conducting medical research involving human being set forth in the Declaration of Helsinki32 and Resolution No. 008430 of 1993 issued by the Colombian Ministry of Health33. Since this is an observational study, it does not pose any risk to participants, provided that the bowel preparation agents used to develop the study are the same as those used in everyday clinical practice. The use of the drugs evaluated here has been approved by the Superintendency of Industry and Commerce of Colombia and the National Institute of Drug and Food Surveillance (Superintendencia de Industria y Comercio y el Instituto Nacional de Vigilancia de Medicamentos y Alimentos - INVIMA)34.
Study population
Patients between 18 and 95 years old who were scheduled for an outpatient or inpatient colonoscopy for any reason at a high-level of care hospital in Bogotá, Colombia, between May 2019 and December 2019, were recruited. Bowel preparation products routinely used in the hospital are SPMC or sodium/potassium/magnesium sulfate. There was no randomization of drug intake as an observational study assessing patients in a real-life situation was proposed. As a result, the method of bowel preparation was established at the treating physician’s discretion based on the characteristics of each patient so that the best clinical benefit would be achieved in each case, as it is generally done in everyday practice, to prevent intervention. This could be considered a possible limitation of the study; however, a preliminary analysis was performed to minimize selection bias.
Patients with partial or complete colectomy or those in which the procedure was suspended (due to technical problems, patient pain or instability, or anatomical alteration) were excluded. Patients who, besides any of the two agents considered in this study, took other drug orally for bowel preparation, those in whom bowel preparation lasted more than two days, and pregnant or breastfeeding women were also excluded.
Data systematization and analysis
All patients underwent a strict dietary regimen to favor the visualization of the colon mucosa. Additionally, they had to prepare for the procedure with one of the bowel preparation regimes used in this institution: 1. SPMC, single dose; 2. SPMC, split dose; 3. Sodium/potassium/magnesium sulfate, single dose; 4. Sodium/potassium/magnesium sulfate, split dose. Demographic and clinical data were collected upon admission. During the procedure, the behavior of the different colon cleansing products was analyzed using the BBPS. Multiple studies consider a score between 5 and 7 as an adequate bowel preparation20,29,35; in our case, the efficacy of the drug was measured with a ≥ 6 points reference score for adequate preparation.
After the procedure, patients were questioned about how the preparation agent was administered, whether it was completely or incompletely administered, and whether it was a single or split dose. The tolerability variables of the colon cleansing product used were identified by means of a survey asking about possible unwanted symptoms, and by assessing the presence of adverse events within 24 hours after the administration of the bowel preparation drugs. Patients who did not complete appropriately the drug administration scheme completed a survey to identify the main factors related to their non-adherence to the different types of bowel preparation used.
Statistical analysis
The sample size was calculated using the OpenEpi web tool 36 by estimating the percentage of patients with adequate bowel preparation, 86 % and 81 % in each arm37,38, with a standard deviation of 0.5, a significance level of 5 %, a statistical power of 80 %, and a sample loss percentage of 10%. Since this is a non-inferiority analysis, a difference of 3% to 5% is considered to obtain a minimum score of 6 on the scale used. A sample size of 520 patients was calculated, for a total of 260 patients for each group.
An exploratory analysis of the demographic and clinical variables was carried out. Qualitative variables were described using absolute and relative frequencies, and quantitative variables using means averages, standard deviations, medians, and interquartile ranges. The Shapiro-Wilk test was used to determine whether the distribution of data was normal or not. To minimize sample selection bias due to the non-randomization of the agent used as a result of the type of study proposed, a preliminary analysis was conducted, and the comparability of the groups was assessed based on the baseline characteristics of both of them. An exploratory analysis concluded that both groups were comparable since there were no major variations in the characteristics of the population collected per group.
A bivariate analysis was performed to evaluate the possible positive or negative influencing factors in each intestinal preparation. The distribution of variables was described according to the outcome “efficacy”. Crosstabs were made for qualitative variables and quantitative variables using the chi-square (χ2) test and the Student’s t or Mann-Whitney U test, respectively.
Simple logistic regression analyses were performed to determine the significant variables to construct a multivariate regression analysis model. The effectiveness of each drug was assessed individually using a logistic regression model for causality. Also, a series of logistic regression modeling was performed for the assessing the variables that were considered to influence intestinal cleansing of each drug. Subsequently, a comparative analysis of drug efficacy according to adequate cleansing per colon segment (significance level of p-values < 0.05) was performed to evaluate the null hypothesis of non-inferiority between both drugs. Finally, in order to assess tolerability, safety and reasons for non-adherence, a descriptive analysis of the variables related to these outcomes was carried out.
Results
Study population
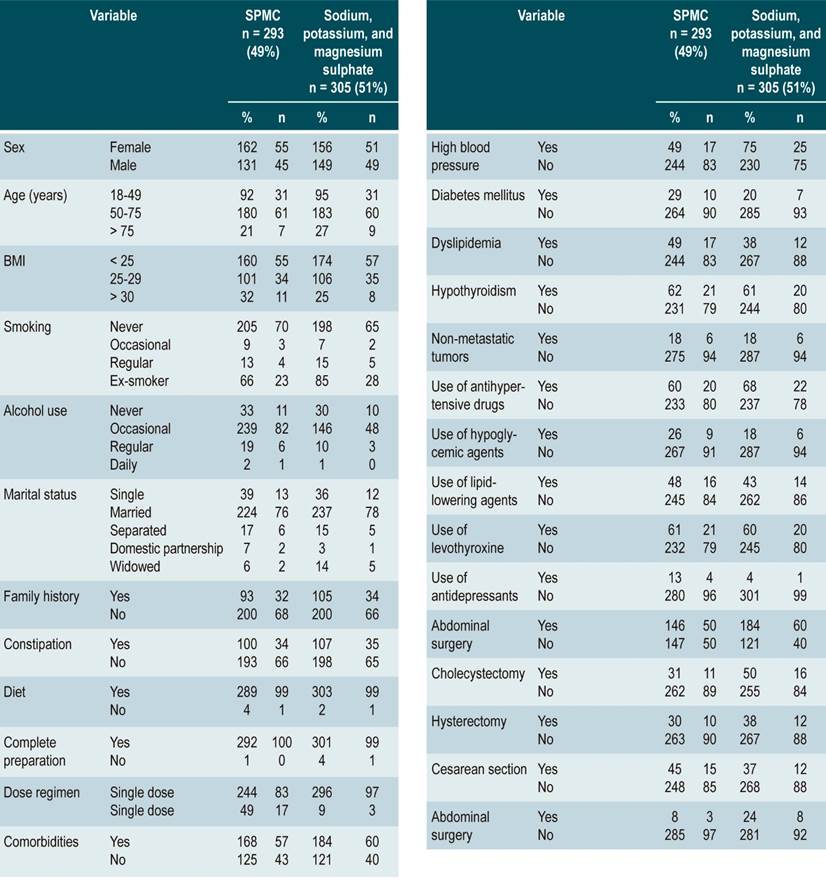
A total of 598 patients who met the eligibility criteria were recruited over a 7-month period. SPMC was administered to 49 % (n = 293), of which 90.3 % (n = 540) received a single dose the day before the procedure was performed (Table 1).
Complete bowel cleansing outcome
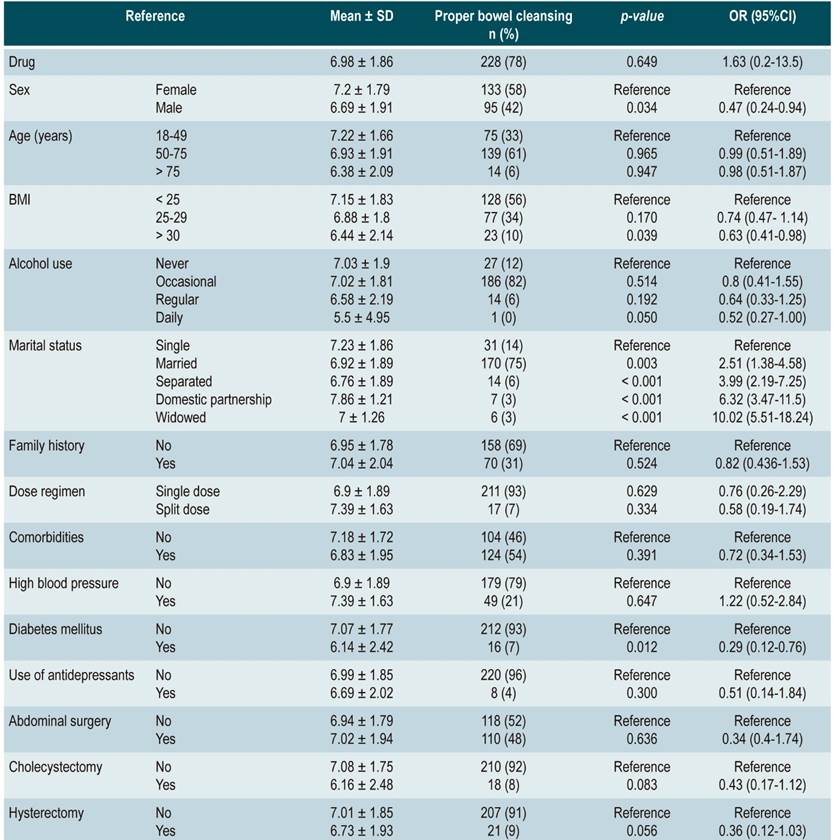
The mean score in the SPMC group was 6.98 ± 1.86, with adequate bowel cleansing (BBPS ≥ 6 points) in 78 % (n = 228) participants, while in the sodium/potassium/magnesium sulfate group the mean score was 7.39 ± 1.83 and adequate intestinal cleansing was observed in 83% (n =254) patients; the different between groups was not significant (p = 0,649) and was found to be within the margin defined as non-inferiority.
In the SPMC group, men had a significantly lower mean BBPS score and adequate cleansing than women (6.69 ± 1.91; 95 [42 %]; odds ratio [OR]: 0.47 [0.24-0.94]; p = 0.034). Likewise, patients with a higher body mass index (BMI > 30) showed a worse bowel cleansing performance (6.44 ± 2.14; OR 0.63 [0.41-0.98]; p = 0.039). In general, there were no significant differences with patients with comorbidities; however, patients with diabetes mellitus performed worse in terms of bowel cleansing (6.14 ± 2.42; 16 [7 %]; OR: 0.29 [0.12-0.76]; p = 0.012) (Table 2).
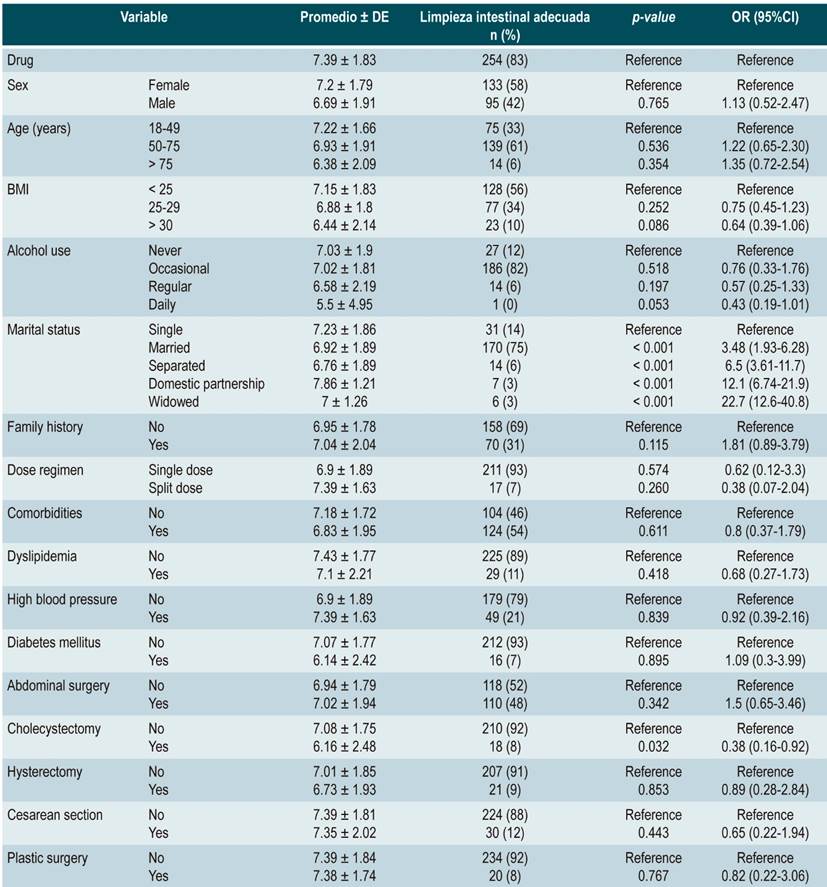
Table 2 Multivariate logistic regression evaluating the Boston Bowel Preparation Scale mean score and the association of variables with adequate bowel preparation in the SPMC group (n = 293)
SD: standard deviation; CI: confidence interval.
In the sodium/potassium/magnesium sulfate group, single patients had an adequate BBPS mean score and appropriate cleansing (7.23 ± 1.86, 31 [14 %]; OR: 1.87 [1.04-3.36]; p = 0.038). No significant differences were found in patients with abdominal surgeries; however, patients who had undergone cholecystectomy showed a worse performance regarding bowel cleansing (6.16 ± 2.48; 18 [8 %]; OR: 0.38 [0.16-0.92]; p = 0.032). Other variables did not show a significant association with proper bowel cleansing (Table 3).
Bowel cleansing per colon segment outcome
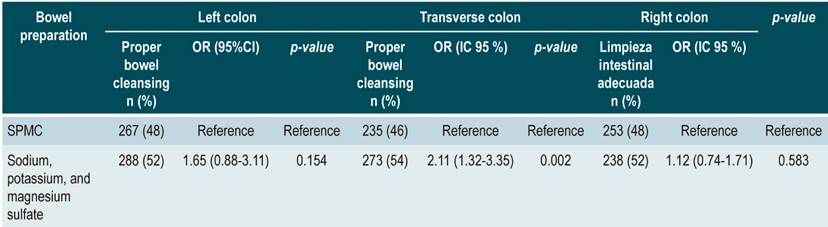
Regarding the left colon segment, 48% (n = 267) of patients who used SPMC had adequate cleansing in relation to those using sodium/potassium/magnesium sulfate (n = 288 [52]; OR: 1.65 [0.88- 3.11]; p = 0.154); in the transverse colon segment, a better outcome was observed when using SPMC (n = 273 [54 %]; OR: 2.11 [1.32- 3.35]; p = 0.002); finally, regarding the right colon segment, results were similar in both groups (n = 238 [52 %]; OR: 1.12 [0.74-1.71]; p = 0.583) (Table 4). Furthermore, a comparison of the number of patients who scored 0-1 in any segment of the colon, despite having an overall BBPS score ≥ 6 points, was made, finding that 0.44% patients who used SPMC for bowel preparation had a score of 0-1 with an overall score of ≥ 6 versus 0.39% in the sodium/potassium/magnesium sulphate group (p = 0.469). In the transverse colon segment, results were 3.07 % vs.1.97 % (p = 0.222), respectively, and in the right colon, 7.02 % vs. 4.33 % (p = 0.1025), respectively.
Table 4 Multivariate logistic regression assessing adequate bowel cleansing and bowel preparation medications per colon segment.
Bowel preparation tolerability
Of the 598 patients, 1% (n = 6) did not complete bowel preparation. 99.3% (n = 291) of patients using SPMC completed preparation, while 98.7% (n = 301) using sodium/potassium/magnesium sulfate completed it. When assessing the presence and frequency of unwanted symptoms and adverse events, a significant difference was observed (p < 0.001), in which SPMC had better tolerability (Figure 1). A correspondence analysis was performed, showing the most frequent symptoms per group. Patients who used SPMC had headache, dry mouth, and tachycardia more frequently; in contrast, a higher rate of neurological disorders and drowsiness was observed in the sodium/potassium/magnesium sulfate group. Abdominal pain and bloating were common symptoms in both groups (Figure 2). One of the reasons for suspending the procedure was patient intolerance due to pain or other anatomical alterations (Figure 3).
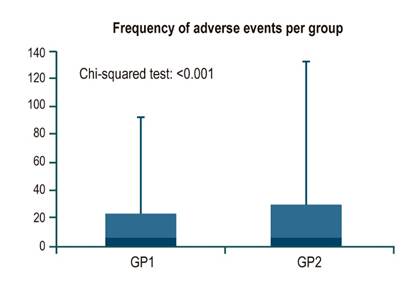
Figure 1 Frequency of adverse events (unwanted symptoms) identified in each group. GP1: Group 1 (SPMC, Travad PIK). GP2: Group 2 (sodium, potassium, and magnesium sulfate, Izinova).

Figure 2 Pooled correspondence analysis of adverse events (unwanted symptoms) per group. GP1: Group 1 (SPMC, Travad PIK). GP2: Group 2 (sodium, potassium, and magnesium sulfate, Izinova).

Figure 3 Reasons for discontinuation of patients. *Significant association at 10 %. GP1: Group 1 (SPMC, Travad PIK). GP2: Group 2 (sodium, potassium, and magnesium sulfate, Izinova).
Average age was assessed according to the bowel preparation drug used and the occurrence of adverse events, finding that the average age of patients who experienced adverse events in the SPMC group was 54.6 years versus 53.3 years in the sodium/potassium/magnesium sulfate group. When assessing each type of adverse event per the type of cleansing drug used, all age averages were below 65 years, except for a 68-year-old patient who used SPMC and experienced a neurological disorder. The proportion of patients ≥ 65 years with adverse effects caused by SPMC (n = 25 [27 %]) and sodium/potassium/magnesium sulfate (n = 33 [25 %]) was also assessed (p = 0.309).
Discussion
Bowel preparation is of vital importance for the proper performance of colonoscopy, which allows the early detection of colonic diseases. Many experts on the topic have made the assessment of the various products used for this purpose a top priority. Schreiber et al.13 assessed the efficacy and safety of NER1006 (PEG) and demonstrated its non-inferiority in relation to SPMC. Patients exposed to NER1006 experienced more adverse events and showed less adherence to treatment. Although they compared high volume agents to low volume agents, their findings were close to those reported in the present study.
Gu et al.37 conducted a comparative observational study on the efficacy and tolerability of bowel preparation medications available in real-life patients from Los Angeles, California. All results were compared with GoLYTELY (PEG 3350), a standard preparation according to the American Society for Gastrointestinal Endoscopy (ASGE). The overall BBPS score was significantly higher (≥ 7) for Miralax (p = 0.001), Suprep (p = 0.001), and MoviPrep (p = 0.004); these drugs have SPMC as an active component and their BBPS scores are similar to those obtained in our study. Prepopik (99.1 %) and magnesium citrate (98.1 %) were better tolerated than Golytely (82.9 %), although no significant differences in bowel cleansing were observed. This occurred in a real-world scenario, with results similar to those of the present study. Real-life evidence aims to validate clinical trials39 with studies conducted to evaluate the behavior of a given drug in routine care.
A recent study compared patient satisfaction with two low-volume agents: oral sulfate solution (OSS) and SPMC. Participants in both groups stated they were willing to undergo repeated colonoscopy using the same laxative in 91% and 93% of cases, respectively. However, the SPMC group significantly outperformed the OSS group (p = 0.006). The most common complaints were bloating and abdominal pain (16.7% vs. 10.2% for the OSS vs. SPMC groups)40. This study also found a significant difference (p < 0.001) in the tolerability of patients regarding both preparations. Similar results were shown for both drugs in relation to the type and frequency of symptoms; nausea and emesis are not common symptoms in individuals using SPMC.
So far, no studies have been conducted in Colombia on the effectiveness of post-market SPMC. Such a study could strengthen and enrich the existing medical literature contributing to make a decision regarding what type of bowel preparation use among the several available options. Our study did not evaluate costs associated with the use of the two agents; however, performing a cost-effectiveness study in the future would have a more significant impact on determining which drug is better to use in some situations based on cost-related factors. This could be an interesting assessment in some regions of Latin America and, especially in countries such as Colombia, where the current health system tends to obstruct access to certain drugs, hindering disease prevention and forcing physicians to provide care to patients when their diseases are already fully developed, and which often are in an advanced stage.
Since this is a single-center research, results described here may differ from those reported by other studies. However, the sample size is thought to be important and meaningful considering the nature of the study; studies with a larger sample size are not considered to have significant variations in their results.
This study has a substantial impact on public health and provides an overall benefit in terms of CRC since, despite a significant difference in the efficacy of both bowel preparation medications assessed was not found, a higher adherence to SPMC was observed. This finding will help make patients more willing to perform complete preparation, increasing the probability of effective bowel cleansing and determining the best method of bowel cleansing to improve polyp detection rates and timely management. Cost-effectiveness studies could help establish the best bowel cleansing drug according to the needs of the population.
Acknowledgments
Not applicable.
REFERENCES
1. Voiosu T, Tanţău A, Voiosu A, Benguş A, Mocanu C, Smarandache B, Baicuş C, Vişovan I, Mateescu B. Preparation regimen is more important than patient-related factors: a randomized trial comparing a standard bowel preparation before colonoscopy with an individualized approach. Rom J Intern Med. 2017;55(1):36-43. https://doi.org/10.1515/rjim-2016-0047 [ Links ]
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. https://doi.org/10.3322/caac.21492 [ Links ]
3. Galán E, Puerto D, Salazar L, Oliveros R, Arredondo L. Manual para la detección temprana del cáncer de colon y recto. Bogotá: Instituto Nacional de Cancerología; 2015. [ Links ]
4. Maratt JK, Calderwood AH. Colorectal Cancer Screening and Surveillance Colonoscopy in Older Adults. Curr Treat Options Gastroenterol. 2019;17(2):292-302. https://doi.org/10.1007/s11938-019-00230-9 [ Links ]
5. Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JM, Parkin DM, Wardle J, Duffy SW, Cuzick J; UK Flexible Sigmoidoscopy Trial Investigators. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. The Lancet. 2010;375(9726):1624-33. https://doi.org/10.1016/S0140-6736(10)60551-X [ Links ]
6. Baxter NN, Warren JL, Barrett MJ, Stukel TA, Doria-Rose VP. Association Between Colonoscopy and Colorectal Cancer Mortality in a US Cohort According to Site of Cancer and Colonoscopist Specialty. J Clin Oncol. 2012;30(21):2664-9. https://doi.org/10.1200/JCO.2011.40.4772 [ Links ]
7. Segnan N, Armaroli P, Bonelli L, Risio M, Sciallero S, Zappa M, Andreoni B, Arrigoni A, Bisanti L, Casella C, Crosta C, Falcini F, Ferrero F, Giacomin A, Giuliani O, Santarelli A, Visioli CB, Zanetti R, Atkin WS, Senore C; SCORE Working Group. Once-Only Sigmoidoscopy in Colorectal Cancer Screening: Follow-up Findings of the Italian Randomized Controlled Trial--SCORE. JNCI J Natl Cancer Inst. 2011;103(17):1310-22. https://doi.org/10.1093/jnci/djr284 [ Links ]
8. Flemming JA, Vanner SJ, Hookey LC. Split-dose picosulfate, magnesium oxide, and citric acid solution markedly enhances colon cleansing before colonoscopy: a randomized, controlled trial. Gastrointest Endosc. 2012;75(3):537-544.e1. https://doi.org/10.1016/j.gie.2011.09.018 [ Links ]
9. le Clercq CMC, Bouwens MWE, Rondagh EJA, Bakker CM, Keulen ETP, de Ridder RJ, Winkens B, Masclee AA, Sanduleanu S. Postcolonoscopy colorectal cancers are preventable: a population-based study. Gut. 2014;63(6):957-63. https://doi.org/10.1136/gutjnl-2013-304880 [ Links ]
10. Cortes BGW, Cabral RM, Carmo GAA do, Queiroz FL de, Leite SM de O, Andrade AC de S, Ferreira A, Alcici MA, Cortes M. Double blinded randomized clinical trial to assess the effectiveness of several preparations for colonoscopy. J Coloproctology. 2018;38(4):302-8. https://doi.org/10.1016/j.jcol.2018.07.001 [ Links ]
11. Hassan C, Rossi PG, Camilloni L, Rex DK, Jimenez-Cendales B, Ferroni E, Borgia P, Zullo A, Guasticchi G; HTA Group. Meta-analysis: adherence to colorectal cancer screening and the detection rate for advanced neoplasia, according to the type of screening test. Aliment Pharmacol Ther. 2012;36(10):929-40. https://doi.org/10.1111/apt.12071 [ Links ]
12. Castells A, Marzo M, Bellas B, et al. Guía de práctica clínica sobre la prevención del cáncer colorrectal. Gastroentorol Hepatol. 2004;10:579-634. https://doi.org/10.1016/S0210-5705(03)70535-4 [ Links ]
13. Schreiber S, Baumgart D, Drenth J, Filip R, Clayton L, Hylands K, Berton A, Salvatore G, Denaro V. Colon cleansing efficacy and safety with 1 L NER1006 versus sodium picosulfate with magnesium citrate: a randomized phase 3 trial. Endoscopy. 2019;51(01):73-84. https://doi.org/10.1055/a-0639-5070 [ Links ]
14. Oh CH, Lee CK, Kim J-W, Shim J-J, Jang JY. Suboptimal Bowel Preparation Significantly Impairs Colonoscopic Detection of Non-polypoid Colorectal Neoplasms. Dig Dis Sci. 2015;60(8):2294-303. https://doi.org/10.1007/s10620-015-3628-6 [ Links ]
15. Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for Colonoscopy Surveillance After Screening and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844-57. https://doi.org/10.1053/j.gastro.2012.06.001 [ Links ]
16. Chokshi RV, Hovis CE, Hollander T, Early DS, Wang JS. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointest Endosc. 2012;75(6):1197-203. https://doi.org/10.1016/j.gie.2012.01.005 [ Links ]
17. Krajicek E, Fischer M, Allegretti JR, Kelly CR. Nuts and Bolts of Fecal Microbiota Transplantation. Clin Gastroenterol Hepatol. 2019;17(2):345-52. https://doi.org/10.1016/j.cgh.2018.09.029 [ Links ]
18. Rutherford CC, Calderwood AH. Update on Bowel Preparation for Colonoscopy. Curr Treat Options Gastroenterol. 2018;16(1):165-81. https://doi.org/10.1007/s11938-018-0165-3 [ Links ]
19. Chaussade S, Schmöcker C, Toulemonde P, Muñoz-Navas M, O’Mahony V, Henri F. Phosphate tablets or polyethylene glycol for preparation to colonoscopy? A multicentre non-inferiority randomized controlled trial. Surg Endosc. 2017;31(5):2166-73. https://doi.org/10.1007/s00464-016-5214-1 [ Links ]
20. Munsterman ID, Cleeren E, van der Ploeg T, Brohet R, van der Hulst R. ‘Pico-Bello-Klean study’: effectiveness and patient tolerability of bowel preparation agents sodium picosulphate-magnesium citrate and polyethylene glycol before colonoscopy. A single-blinded randomized trial. Eur J Gastroenterol Hepatol. 2015;27(1):29-38. https://doi.org/10.1097/MEG.0000000000000192 [ Links ]
21. Gweon T-G, Kim SW, Noh Y-S, Hwang S, Kim N-Y, Lee Y, Lee SW, Lee SW, Lee JY, Lim CH, Hun Kim H, Kim JS, Kyung Cho Y, Myung Park J, Seok Lee I, Myung-Gyu Choi. Prospective, Randomized Comparison of Same-Day Dose of 2 Different Bowel Cleanser for Afternoon Colonoscopy: Picosulfate, Magnesium Oxide, and Citric Acid Versus Polyethylene Glycol. Medicine (Baltimore). 2015;94(13):e628. https://doi.org/10.1097/MD.0000000000000628 [ Links ]
22. Jeon SR, Kim HG, Lee JS, Kim J-O, Lee TH, Cho J-H, Kim YH, Cho JY, Lee JS. Randomized controlled trial of low-volume bowel preparation agents for colonic bowel preparation: 2-L polyethylene glycol with ascorbic acid versus sodium picosulfate with magnesium citrate. Int J Colorectal Dis. 2015;30(2):251-8. https://doi.org/10.1007/s00384-014-2066-9 [ Links ]
23. Martel M, Barkun AN, Menard C, Restellini S, Kherad O, Vanasse A. Split-Dose Preparations Are Superior to Day-Before Bowel Cleansing Regimens: A Meta-analysis. Gastroenterology. 2015;149(1):79-88. https://doi.org/10.1053/j.gastro.2015.04.004 [ Links ]
24. Hosoe N, Nakashita M, Imaeda H, Sujino T, Bessho R, Ichikawa R, Inoue N, Kanai T, Hibi T, Ogata H. Comparison of patient acceptance of sodium phosphate versus polyethylene glycol plus sodium picosulfate for colon cleansing in Japanese: Patient acceptance for laxative. J Gastroenterol Hepatol. 2012;27(10):1617-22. https://doi.org/10.1111/j.1440-1746.2012.07190.x [ Links ]
25. Wexner SD, Force T, Beck DE, Baron TH, Fanelli RD, Hyman N, Wasco KE; American Society of Colon and Rectal Surgeons; American Society for Gastrointestinal Endoscopy; Society of American Gastrointestinal and Endoscopic Surgeons. A consensus document on bowel preparation before colonoscopy: Prepared by a Task Force From The American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Gastrointest Endosc. 2006;63(7):894-909. https://doi.org/10.1016/j.gie.2006.03.918 [ Links ]
26. Landreneau SW, Di Palma JA. Update on Preparation for Colonoscopy. Curr Gastroenterol Rep. 2010;12(5):366-73. https://doi.org/10.1007/s11894-010-0121-4 [ Links ]
27. Weir MA, Fleet JL, Vinden C, Shariff SZ, Liu K, Song H, Jain AK, Gandhi S, Clark WF, Garg AX. Hyponatremia and Sodium Picosulfate Bowel Preparations in Older Adults. Am J Gastroenterol. 2014;109(5):686-94. https://doi.org/10.1038/ajg.2014.20 [ Links ]
28. Gandhi S, Shariff SZ, Fleet JL, Weir MA, Jain AK, Garg AX. Validity of the International Classification of Diseases 10th revision code for hospitalisation with hyponatraemia in elderly patients. BMJ Open. 2012;2(6):e001727. https://doi.org/10.1136/bmjopen-2012-001727 [ Links ]
29. Calderwood AH, Jacobson BC. Comprehensive validation of the Boston Bowel Preparation Scale. Gastrointest Endosc. 2010;72(4):686-92. https://doi.org/10.1016/j.gie.2010.06.068 [ Links ]
30. Chaves Marques S. The Boston Bowel Preparation Scale: Is It Already Being Used? GE - Port J Gastroenterol. 2018;25(5):219-21. https://doi.org/10.1159/000486805 [ Links ]
31. Massinha P, Almeida N, Cunha I, Tomé L. Clinical Practice Impact of the Boston Bowel Preparation Scale in a European Country. GE - Port J Gastroenterol. 2018;25(5):230-5. https://doi.org/10.1159/000485567 [ Links ]
32. Declaración de Helsinki de la AMM - Principios éticos para las investigaciones médicas en seres humanos. Asociación Médica Mundial; 2017. [ Links ]
33. Resolución número 8430 de 1993. Por la cual se establecen las normas científicas, técnicas y administrativas para la investigación en salud. Ministerio de Salud (4 de octubre de 1993). [ Links ]
34. Resolución No. 2018013725 DE 5 de abril de 2018. Por la cual se concede un Registro Sanitario. Ministerio de Salud, INVIMA (5 de abril de 2018). [ Links ]
35. Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69(3):620-5. https://doi.org/10.1016/j.gie.2008.05.057 [ Links ]
36. Sullivan KM, Dean AG. OPENEPI [Internet] [acceso el 15 de junio de 2019]. Disponible en: Disponible en: https://www.openepi.com/SampleSize/SSCohort.htm [ Links ]
37. Gu P, Lew D, Oh SJ, Vipani A, Ko J, Hsu K, Mirakhor E, Pattisapu V, Bullen T, Fuller G, Spiegel BMR, Almario CV. Comparing the Real-World Effectiveness of Competing Colonoscopy Preparations: Results of a Prospective Trial. Am J Gastroenterol. 2019;114(2):305-14. https://doi.org/10.14309/ajg.0000000000000057 [ Links ]
38. Kim ES, Lee WJ, Jeen YT, Choi HS, Keum B, Seo YS, Chun HJ, Lee HS, Um SH, Kim CD, Ryu HS. A randomized, endoscopist-blinded, prospective trial to compare the preference and efficacy of four bowel-cleansing regimens for colonoscopy. Scand J Gastroenterol. 2014;49(7):871-7. https://doi.org/10.3109/00365521.2014.910543 [ Links ]
39. Basch E, Schrag D. The Evolving Uses of “Real-World” Data. JAMA. 2019;321(14):1359-1360. https://doi.org/10.1001/jama.2019.4064 [ Links ]
40. Kim J, Kim HG, Kim KO, Kim HW, Park J, Byeon JS, Hwang SW, Shin HD, Shin JE, Yang HJ, Lee HS, Jung Y, Cho YS, Joo YE, Myung DS, Huh KC, Ahn EM. Clinical comparison of low-volume agents (oral sulfate solution and sodium picosulfate with magnesium citrate) for bowel preparation: the EASE study. Intest Res. 2019;17(3):413-418. https://doi.org/10.5217/ir.2018.00156 [ Links ]
Citation: Pérez-Riveros ED, Rey M, Mendoza B, Robayo JC, Solano-Mariño J, García-Duperly R, Gómez A, Pinto-Carta R, Ardila G, De la Hoz-Valle J, Sierra-Arango F. Non-inferiority between two low-volume agents (sodium picosulfate/magnesium citrate vs. sodium sulfate/potassium/magnesium) to prepare the bowel for diagnostic procedures: an observational study. Rev colomb Gastroenterol. 2020;35(4):436-446. https://doi.org/10.22516/25007440.545
Received: April 23, 2020; Accepted: October 05, 2020











 text in
text in