Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957On-line version ISSN 2500-7440
Rev. colomb. Gastroenterol. vol.35 no.4 Bogotá Oct./Dec. 2020 Epub July 12, 2021
https://doi.org/10.22516/25007440.447
Review article
Nutrition in acute pancreatitis: new concepts for an old problem
1Gastroenterólogo, Hospital General Docente Ambato. Ambato, Ecuador
2Profesor titular de Medicina, Coordinador de Gastroenterología, Universidad Nacional de Colombia, Hospital Universitario Nacional. Bogotá, Colombia
3Gastroenterólogo, Universidad Nacional de Colombia. Bogotá, Colombia
Nutrition management in acute pancreatitis has been a matter of debate worldwide. For many years, the concept of pancreatic rest was widespread and accepted to treat acute pancreatitis. However, current knowledge of early nutrition allows maintaining the intestinal barrier’s integrity, preventing the occurrence of infectious complications, which is associated with a shorter hospital stay, fewer complications, and better prognosis. This review presents the main advantages of early nutrition in acute pancreatitis, its safety, and the route of administration.
Keywords: Nutrition; Pancreatic rest; Nasogastric tube; Nasojejunal tube
El manejo de la nutrición en pancreatitis aguda ha sido cuestión de debate. Durante muchos años el concepto de reposo pancreático fue generalizado y aceptado en el manejo de la pancreatitis aguda. Actualmente se conoce que la nutrición temprana permite mantener la integridad de la barrera intestinal, que previene la aparición de complicaciones infeccionas y se asocia con una menor estancia hospitalaria, menos complicaciones y un mejor pronóstico. En esta revisión se discuten las principales ventajas de la nutrición temprana en pancreatitis aguda, la seguridad de la misma y la vía de administración.
Palabras clave: Nutrición; reposo pancreático; sonda nasogástrica; sonda nasoyeyunal
Introduction
Acute pancreatitis (AP) is one of the most frequent gastrointestinal disorders; it is mainly caused by gallstones and alcohol consumption1,2.
According to the Atlanta classification, two or more of the following criteria must be met to reach an AP diagnosis: abdominal pain suggestive of AP, serum amylase or lipase level greater than three times the upper normal value, and characteristic imaging findings3,4.
80% of AP cases have a mild course of the disease, while 20% are moderately severe and severe AP cases. Of these, 33 % will present with infected necrosis, reaching a mortality rate of approximately 15 %-35 %, with sepsis being its main determinant5.
Consequences of food restriction
Ivan Pavlov, a Russian scientist, was awarded the Nobel Prize in Medicine in 1904 for his work on animals regarding the physiology of digestion and the response to stimuli on the secretion of various glands. This work was extrapolated by other researchers, assuming that suppressing food stimulus would avoid the different phases of pancreatic secretion and, therefore, enzyme release and increased tissue damage. This theory was valid for several years, and many physicians are still careful when it comes to the administration of food in AP6.
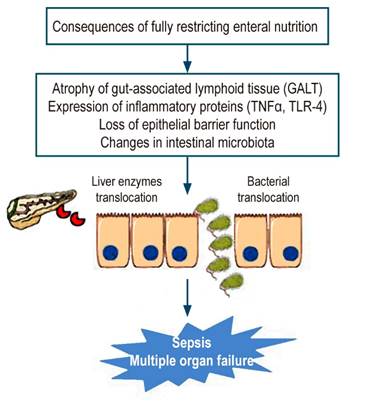
The current understanding of the pathophysiology of AP has shown that the production of different proinflammatory substances, combined with food restriction, alters intestinal motility and the saprobic microbiota, causing bacterial overgrowth and alteration of the intestinal barrier. This allows bacteria to enter the systemic circulation through the lymph nodes, where bacterial endotoxins cause sepsis and multiple organ failure7,8. This is shown in more detail in Figure 1.
Early nutrition
For several years, one of the basic pillars in the treatment of AP was food restriction, or the so-called digestive reset, while AP symptoms “improved” because it was thought that the stimuli produced by food would favor the release and activation of pancreatic enzymes and cause greater tissue damage9. At present, there is no evidence to support this belief and several studies have shown that early initiation of enteral nutrition (i.e., within 24 to 48 hours of symptoms onset) improves nitrogen balance and reduces the incidence of infections, mortality, and hospital stay times, and exocrine pancreatic stimulation is minimal10-12.
The high metabolic cost caused by AP, especially in severe pancreatitis cases, increases the need for nutrients responsible for maintaining immune system homeostasis and tissue regeneration13. According to the guidelines published by the European Society for Clinical Nutrition and Metabolism (ESPEN), up to 80% of severe AP cases have a protein loss of 40 g/day, resulting in a negative nitrogen balance14.
Enteral nutrition versus parenteral nutrition
Parenteral nutrition (PN) has been considered to be the best feeding route in AP patients for approximately 3 decades, mainly in severe cases, despite disadvantages such as increased risk of catheter infection, electrolyte imbalance, multiple organ failure, cost, and difficult placement15,16. In addition, hyperglycemia, which occurs in more than half of patients undergoing PN, should be considered because it is an additional risk factor for infection and mortality17,18.
Current knowledge of the role of the intestine in the pathophysiology of AP, as well as the safety and tolerability of enteral nutrition (EN) have caused PN to be replaced by EN19. In the meta-analysis conducted by Al-Omran et al., which included 348 patients, it was found that EN was substantially superior to PN in terms of mortality, infection, multiple organ failure, and need for surgery20. Two recent meta-analyses confirmed these findings. The first study included 348 people and reported that EN resulted in a substantial reduction in mortality and multiple organ failure rates21. The second was conducted using data of 562 people and found that EN reduced the risk of infection and the need for surgery22.
Early feeding
Recently, the American Association of Gastroenterology recommended starting the diet within 24 hours after the onset of symptoms since it promotes intestinal integrity and function, maintains intercellular bonds, and stimulates brush border enzymes, thus avoiding bacterial translocation23.
In the meta-analysis by Feng et al., which included 1007 patients, the benefit of EN was determined in the first 48 hours in terms of organ failure and development of systemic inflammatory response syndrome (SIRS), finding no difference regarding mortality and pancreatic necrosis24.
A recent systematic review found that early feeding with EN in the first 48 hours reduced the risk of infected necrosis, organ failure, need for surgery, and mortality, compared with late EN and PN25.
In turn, the meta-analysis conducted by Qi et al. found that EN within the first 24 hours was associated with a reduction of infectious complications and multiple organ failure in severe acute pancreatitis patients; however, no benefit was observed in mild and moderately severe pancreatitis cases26.
Finally, a systematic study conducted in Spain found that the safest time to start food administration is when bowel sounds are present; additionally, they used a full caloric diet that was well tolerated, so the duration of hospital stay was shortened, and the course of AP was improved27.
Nasogastric tube (NGT) versus nasojejunal tube (NJT)
A recent Cochrane review showed that there are no significant differences between the use of NGT and NJT because both can maintain intestinal barrier integrity, but the first has a better tolerability28. The meta-analysis by Chang et al. found no differences between the use of NGT and NJT in terms of efficacy and safety29.
NJT is reserved for use in cases of intolerance to NGT, gastric outlet obstruction, duodenal obstruction, and NGT-induced negative energy balance30. In addition, it has been described that using NGT offers advantages such as reduced pain, reduced need for opioids and decreased oral intolerance31.
Dietary composition
There is no general consensus on the ideal composition and type of diet, as current evidence is limited. Numerous studies have shown that patients who tolerate the oral route benefit from solid or liquid low-fat diets; this would represent an advantage because the process of gradually changing the consistency of the diet results in unnecessary increased of hospital stay32-35.
In the study carried out by Endo et al. in 2018, no difference was found between using elemental, semi-elemental and polymeric formulas36. Larino-Noia et al., in a study carried out in Spain, found no differences in diet tolerance, when starting feeding with a solid diet or progressive diet37.
Use of dietary supplements
The use of different dietary supplements such as probiotics, prebiotics or synbiotics has been proposed due to their effect on the microbiota, as well as their protective, trophic, and metabolic role. Although their use may be associated with a decrease in hospital stay times, there is not enough evidence to prescribe them on a regular basis38,39.
Regarding the use of amino acids such as arginine and glutamine, which in theory could offer some benefit in maintaining intestinal integrity, the American Society for Parenteral and Enteral Nutrition (ASPEN) guidelines do not recommend their routine use40.
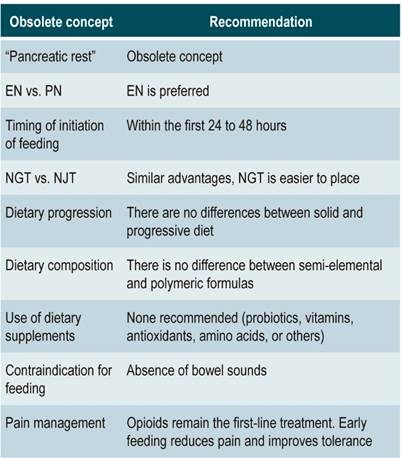
The routine use of Omega-3 fatty acids, antioxidants, and vitamins, all of which could offer benefits in AP patients due to their anti-inflammatory properties, has not been recommended since further research is required to support their use41,42. Table 1 summarizes the key dietary guidelines in the management of AP.
Conclusions
The concept of “pancreatic rest” is now obsolete. Early feeding does not cause additional pancreatic stimulation or damage. The beneficial effect of early feeding on the intestinal barrier is associated with lower bacterial translocation, lower risk of SIRS, and lower mortality. Enteral nutrition is far superior to Parenteral nutrition. There are no differences between the use of NGT and NJT, and if indicated, NGT is preferred because it is easier to place. Pain is not an indication for stopping oral intake of food, so it is necessary to optimize analgesia in order to continue feeding since it is associated with a reduced need for opioids and decreased pain intensity
REFERENCES
1. Peery AF, Crockett SD, Murphy CC, Lund JL, Dellon ES, Williams JL, Jensen ET, Shaheen NJ, Barritt AS, Lieber SR, Kochar B, Barnes EL, Fan YC, Pate V, Galanko J, Baron TH, Sandler RS. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology. 2019;156(1): 254-272.e11. https://doi.org/10.1053/j.gastro.2018.08.063 [ Links ]
2. Olson E, Perelman A, Birk JW. Acute management of pancreatitis: the key to best outcomes. Postgrad Med J. 2019;95(1124):328-333. https://doi.org/10.1136/postgradmedj-2018-136034 [ Links ]
3. Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108(9):1400-15;1416. https://doi.org/10.1038/ajg.2013.218 [ Links ]
4. Bollen TL. Acute pancreatitis: international classification and nomenclature. Clin Radiol. 2016;71(2):121-33. https://doi.org/10.1016/j.crad.2015.09.013 [ Links ]
5. van Dijk SM, Hallensleben NDL, van Santvoort HC, Fockens P, van Goor H, Bruno MJ, Besselink MG; Dutch Pancreatitis Study Group. Acute pancreatitis: recent advances through randomised trials. Gut. 2017;66(11):2024-2032. https://doi.org/10.1136/gutjnl-2016-313595 [ Links ]
6. Petrov MS, Pylypchuk RD, Emelyanov NV. Systematic review: nutritional support in acute pancreatitis. Alim Pharmacol Ther. 2008;28(6):704-712. https://doi.org/10.1111/j.1365-2036.2008.03786.x [ Links ]
7. Singh P, Garg PK. Pathophysiological mechanisms in acute pancreatitis: Current understanding. Indian J Gastroenterol. 2016;35(3):153-66. https://doi.org/10.1007/s12664-016-0647-y [ Links ]
8. Schietroma M, Pessia B, Carlei F, Mariani P, Sista F, Amicucci G. Intestinal permeability and systemic endotoxemia in patients with acute pancreatitis. Ann Ital Chir. 2016;87:138-44. [ Links ]
9. Gupta R, Patel K, Calder PC, Yaqoob P, Primrose JN, Johnson CD. A randomised clinical trial to assess the effect of total enteral and total parenteral nutritional support on metabolic, inflammatory and oxidative markers in patients with predicted severe acute pancreatitis (APACHE II > or =6). Pancreatology. 2003;3(5):406-13. https://doi.org/10.1159/000073657 [ Links ]
10. Ramanathan M, Aadam AA. Nutrition Management in Acute Pancreatitis. Nutr Clin Pract. 2019;34 Suppl 1:S7-S12. https://doi.org/10.1002/ncp.10386 [ Links ]
11. Vaughn VM, Shuster D, Rogers MAM, Mann J, Conte ML, Saint S, Chopra V. Early Versus Delayed Feeding in Patients With Acute Pancreatitis: A Systematic Review. Ann Intern Med. 2017;166(12):883-892. https://doi.org/10.7326/M16-2533 [ Links ]
12. Zhang D, Li H, Li Y, Qu L. Gut rest strategy and trophic feeding in the acute phase of critical illness with acute gastrointestinal injury. Nutr Res Rev. 2019;32(2):176-182.https://doi.org/10.1017/S0954422419000027 [ Links ]
13. Pan LL, Li J, Shamoon M, Bhatia M, Sun J. Recent advances on nutrition in treatment of acute pancreatitis. Front Immunol. 2017;8:762. https://doi.org/10.3389/fimmu.2017.00762 [ Links ]
14. Gianotti L, Meier R, Lobo DN, Bassi C, Dejong CH, Ockenga J, Irtun O, MacFie J; ESPEN. ESPEN guidelines on parenteral nutrition: pancreas. Clin Nutr. 2009;28(4):428-35. https://doi.org/10.1016/j.clnu.2009.04.003 [ Links ]
15. Ziegler TR. Parenteral nutrition in the critically ill patient. N Engl J Med. 2009;361(11):1088-97. https://doi.org/10.1056/NEJMct0806956 [ Links ]
16. Storck LJ, Imoberdorf R, Ballmer PE. Nutrition in Gastrointestinal Disease: Liver, Pancreatic, and Inflammatory Bowel Disease. J Clin Med. 2019;8(8). pii: E1098. https://doi.org/10.3390/jcm8081098 [ Links ]
17. Pasquel FJ, Spiegelman R, McCauley M, Smiley D, Umpierrez D, Johnson R, Rhee M, Gatcliffe C, Lin E, Umpierrez E, Peng L, Umpierrez GE. Hyperglycemia during total parenteral nutrition: an important marker of poor outcome and mortality in hospitalized patients. Diabetes Care. 2010;33(4):739-741. https://doi.org/10.2337/dc09-1748 [ Links ]
18. Petrov MS, Whelan K. Comparison of complications attributable to enteral and parenteral nutrition in predicted severe acute pancreatitis: a systematic review and meta-analysis. Br J Nutr. 2010;103(9):1287-95. https://doi.org/10.1017/S0007114510000887 [ Links ]
19. Petrov MS. Gastric feeding and “gut rousing” in acute pancreatitis. Nutr Clin Pract. 2014;29(3):287-90. https://doi.org/10.1177/0884533614528986 [ Links ]
20. Al-Omran M, Albalawi ZH, Tashkandi MF, Al-Ansary LA. Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database Syst Rev. 2010;(1):Cd002837. https://doi.org/10.1002/14651858.CD002837.pub2 [ Links ]
21. Yao H, He C, Deng L, Liao G. Enteral versus parenteral nutrition in critically ill patients with severe pancreatitis: a meta-analysis. Eur J Clin Nutr. 2018;72(1):66-68. https://doi.org/10.1038/ejcn.2017.139 [ Links ]
22. Wu P, Li L, Sun W. Efficacy comparisons of enteral nutrition and parenteral nutrition in patients with severe acute pancreatitis: a meta- analysis from randomized controlled trials. Biosci Rep. 2018;38(6):pii: BSR20181515. https://doi.org/10.1042/BSR20181515 [ Links ]
23. Crockett SD, Wani S, Gardner TB, Falck-Ytter Y, Barkun AN; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology. 2018;154(4):1096-1101. https://doi.org/10.1053/j.gastro.2018.01.032 [ Links ]
24. Feng P, He C, Liao G, Chen Y. Early enteral nutrition versus delayed enteral nutrition in acute pancreatitis: A PRISMA compliant systematic review and meta-analysis. Medicine (Baltimore). 2017;96(46):e8648. https://doi.org/10.1097/MD.0000000000008648 [ Links ]
25. Song J, Zhong Y, Lu X, Kang X, Wang Y, Guo W, Liu J, Yang Y, Pei L. Enteral nutrition provided within 48 hours after admission in severe acute pancreatitis: a systematic review and meta-analysis. Medicine (Baltimore). 2018;97(34):e11871. https://doi.org/10.1097/MD.0000000000011871 [ Links ]
26. Qi D, Yu B, Huang J, Peng M. Meta-Analysis of Early Enteral Nutrition Provided Within 24 Hours of Admission on Clinical Outcomes in Acute Pancreatitis. JPEN J Parenter Enteral Nutr. 2018;42(7):1139-1147. https://doi.org/10.1002/jpen.1139 [ Links ]
27. Valverde-López F, Wilcox CM, Redondo-Cerezo E. Evaluation and management of acute pancreatitis in Spain. Gastroenterol Hepatol. 2018;41(10):618-628. https://doi.org/10.1016/j.gastrohep.2018.06.012 [ Links ]
28. Moggia E, Koti R, Belgaumkar AP, Fazio F, Pereira SP, Davidson BR, Gurusamy KS. Pharmacological interventions for acute pancreatitis. Cochrane Database Syst Rev. 2017;4(4):CD011384. https://doi.org/10.1002/14651858.CD011384.pub2 [ Links ]
29. Chang YS, Fu HQ, Xiao YM, Liu JC. Nasogastric or nasojejunal feeding in predicted severe acute pancreatitis: a meta-analysis. Crit Care. 2013;17(3):R118. https://doi.org/10.1186/cc12790 [ Links ]
30. Faghih M, Fan C, Singh VK. New Advances in the Treatment of Acute Pancreatitis. Curr Treat Options Gastroenterol. 2019;17(1):146-160. https://doi.org/10.1007/s11938-019-00223-8 [ Links ]
31. Petrov MS, McIlroy K, Grayson L, Phillips AR, Windsor JA. Early nasogastric tube feeding versus nil per os in mild to moderate acute pancreatitis: a randomized controlled trial. Clin Nutr. 2013;32(5): 697-703. https://doi.org/10.1016/j.clnu.2012.12.011 [ Links ]
32. Li J, Chen J, Tang W. The consensus of integrative diagnosis and treatment of acute pancreatitis-2017. J Evid Based Med. 2019;12(1):76-88. https://doi.org/10.1111/jebm.12342 [ Links ]
33. Vege SS, DiMagno MJ, Forsmark CE, Martel M, Barkun AN. Initial medical treatment of acute pancreatitis: American Gastroenterological Association Institute technical review. Gastroenterology. 2018;154(4):1103-1139. https://doi.org/10.1053/j.gastro.2018.01.031 [ Links ]
34. Lodewijkx PJ, Besselink MG, Witteman BJ, Schepers NJ, Gooszen HG, van Santvoort HC, Bakker OJ; Dutch Pancreatitis Study Group. Nutrition in acute pancreatitis: a critical review. Expert Rev Gastroenterol Hepatol. 2016;10(5):571-80. https://doi.org/10.1586/17474124.2016.1141048 [ Links ]
35. Roberts KM, Nahikian-Nelms M, Ukleja A, Lara LF. Nutritional Aspects of Acute Pancreatitis. Gastroenterol Clin North Am. 2018;47(1):77-94. https://doi.org/10.1016/j.gtc.2017.10.002 [ Links ]
36. Endo A, Shiraishi A, Fushimi K, Murata K, Otomo Y. Comparative effectiveness of elemental formula in the early enteral nutrition manage- ment of acute pancreatitis: a retrospective cohort study. Ann Intensive Care. 2018;8(1):69. https://doi.org/10.1186/s13613-018-0414-6 [ Links ]
37. Lariño-Noia J, Lindkvist B, Iglesias-García J, Seijo-Ríos S, Iglesias-Canle J, Domínguez-Muñoz JE. Early and/or immediately full caloric diet versus standard refeeding in mild acute pancreatitis: a randomized open-label trial. Pancreatology. 2014;14(3):167-73. https://doi.org/10.1016/j.pan.2014.02.008 [ Links ]
38. Tian X, Pi YP, Liu XL, Chen H, Chen WQ. Supplemented Use of Pre-, Pro-, and Synbiotics in Severe Acute Pancreatitis: An Updated Systematic Review and Meta-Analysis of 13 Randomized Controlled Trials. Front Pharmacol. 2018;9:690. https://doi.org/10.3389/fphar.2018.00690 [ Links ]
39. Ballesteros Pomar MD, González Arnaiz E. Role of prebiotics and probiotics in the functionality of the microbiota in the patients receiving enteral nutrition. Nutr Hosp. 2018;35(Spec no2):18-26. https://doi.org/10.20960/nh.1956 [ Links ]
40. McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, McCarthy MS, Davanos E, Rice TW, Cresci GA, Gervasio JM, Sacks GS, Roberts PR, Compher C; Society of Critical Care Medicine; American Society for Parenteral and Enteral Nutrition. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2016;40(2):159-211. https://doi.org/10.1177/0148607115621863 [ Links ]
41. Lei QC, Wang XY, Xia XF, Zheng HZ, Bi JC, Tian F, Li N. The role of omega-3 fatty acids in acute pancreatitis: a meta-analysis of randomized controlled trials. Nutrients. 2015;7(4):2261-73. https://doi.org/10.3390/nu7042261 [ Links ]
42. Jeurnink SM, Nijs MM, Prins HA, Greving JP, Siersema PD. Antioxidants as a treatment for acute pancreatitis: A meta-analysis. Pancreatology. 2015;15(3):203-8. https://doi.org/10.1016/j.pan.2015.03.009 [ Links ]
Citation: Mayorga-Garcés A, Otero-Regino W, Parga-Bermúdez J. Nutrition in acute pancreatitis: new concepts for an old problem. Rev Colomb Gastroenterol. 2020;35(4):465-470. https://doi.org/10.22516/25007440.447
Received: July 10, 2019; Accepted: October 15, 2019











 text in
text in