Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957On-line version ISSN 2500-7440
Rev. colomb. Gastroenterol. vol.35 no.4 Bogotá Oct./Dec. 2020 Epub July 12, 2021
https://doi.org/10.22516/25007440.510
Review article
The role of pharmacists in the comprehensive care of patients with hepatitis C: a systematic review
1Grupo Promoción y Prevención Farmacéutica, Universidad de Antioquia. Medellín, Colombia.
Objective:
To identify and characterize the actions/interventions carried out by pharmaceutical services to approach patients with Hepatitis C and propose a clinical pathway for managing the disease that involves pharmacists.
Methodology:
A systematic review was conducted in PubMed and EMBASE using the terms “Hepatitis C,” “Pharmaceutical Services,” “Community Pharmacy Services,” and “Pharmacies.” Articles published until March 31, 2019, whose central topic was the activities carried out by the pharmaceutical services in the care of patients with Hepatitis C, were included. Information on the activity performed, the person in charge, whether the intervention was individual or collective, and the implementation environment was collected. The activities were grouped into promotion and prevention, administrative management, pharmaceutical care, research, and other support services. Based on the above, a clinical pathway for the management of Hepatitis C involving pharmacists was proposed.
Results:
Sixty articles were included, mainly descriptive studies. Most publications reported interventions in the United States and Spain. Pharmaceutical staff involvement was identified at each stage of the care process, including the provision of harm reduction services, Hepatitis C virus screening, enrolling patients to treatment, medication prescription, and laboratory orders.
Conclusions:
The actions/interventions carried out by the pharmaceutical service for Hepatitis C management were identified and characterized. A clinical pathway has been proposed to integrate professional pharmaceutical services with other patient care activities.
Keywords: Pharmacy Services; Patient Care Management; Hepatitis C; Antivirals; Comprehensive Health Care
Objetivo:
identificar y caracterizar las acciones/intervenciones realizadas desde los servicios farmacéuticos en el abordaje de pacientes con hepatitis C y proponer una vía clínica para la gestión de la enfermedad que incluya la participación del farmacéutico.
Método:
revisión sistemática en PubMed y EMBASE empleando los términos “Hepatitis C”, “Pharmaceutical Services”, “Community Pharmacy Services”, y “Pharmacies”; artículos publicados hasta el 31 de marzo de 2019, cuyo tema central fueran las actividades realizadas por los servicios farmacéuticos en la atención a pacientes con hepatitis C. Se recopiló información sobre la actividad realizada, responsable, si la intervención era individual o colectiva y el entorno de aplicación. Las actividades se agruparon en promoción y prevención, gestión administrativa, atención farmacéutica, investigación y otros servicios de apoyo. De acuerdo con esto, se propuso una vía clínica para el manejo de la hepatitis C con participación del farmacéutico.
Resultados:
se incluyeron 60 artículos, principalmente de estudios descriptivos. La mayoría de las publicaciones reportó intervenciones realizadas en Estados Unidos y España. Se identificó la participación del personal farmacéutico en cada una de las etapas del proceso de atención, que incluye la provisión de servicios de reducción del daño, tamizaje del virus de la hepatitis C, vinculación de los pacientes al tratamiento, prescripción de medicamentos y órdenes de laboratorio.
Conclusiones:
se identifican y caracterizan las acciones/intervenciones realizadas desde el servicio farmacéutico para el manejo de la hepatitis C y se propone una vía clínica en la que se integran los servicios profesionales farmacéuticos a las demás actividades de la atención del paciente.
Palabras clave: Servicios farmacéuticos; manejo de atención al paciente; hepatitis C; antivirales; atención integral de salud
Introduction
In the last decade, significant progress has been made in the treatment of hepatitis C virus (HCV) infection due to the development of direct-acting antivirals (DAD), which allow simpler, shorter, more effective and better tolerated treatments compared to conventional pharmacological therapies (interferon and ribavirin)1,2. DADs have been shown to increase patient adherence and minimize treatment discontinuation. However, there are still barriers to access this therapy and to achieve treatment continuity, especially among vulnerable populations facing access barriers associated with low educational levels, socioeconomic status, health education, and psychoactive substance abuse1.
In this sense, as nonphysician practitioners, pharmacists may assist hepatitis C patients with their health care3,4, considering that they can improve patients’ access to treatment and health care services5,6 by working as part of multidisciplinary teams and by providing close and continuous clinical follow-up to ensure adherence to pharmacotherapy1.
Although several authors have described the activities carried out by pharmaceutical providers in the prevention, screening and management of hepatitis C, they have been published separately and there is no consolidated information on the subject. Therefore, the aim of this study was to identify and describe the activities and procedures performed by pharmaceutical providers in relation to the management of hepatitis C patients and, as a result, suggest a clinical pathway for the comprehensive management of this disease that considers the participation of pharmacists.
Methods
Identification and selection of articles
A review was performed in PubMed/Medline and EMBASE using the following search strategies: (“Hepatitis C”[Mesh]) AND (((((“Pharmaceutical Services”[Mesh]) OR “Pharmaceutical Services, Online”[Mesh]) OR “Pharmacy Service, Hospital”[Mesh]) OR “Community Pharmacy Services”[Mesh]) OR “Pharmacies”[Mesh]), and ‘hepatitis c’/exp AND ‘pharmaceutical care’/exp, respectively. Search filters: Full text access articles describing studies conducted in human beings and published in English or Spanish before March 31, 2019. Editorials, notes, pieces of news, opinions, abstracts presented in congresses, and original papers describing activities and procedures performed by pharmaceutical services aimed at the provision of care to hepatitis C patients were included.
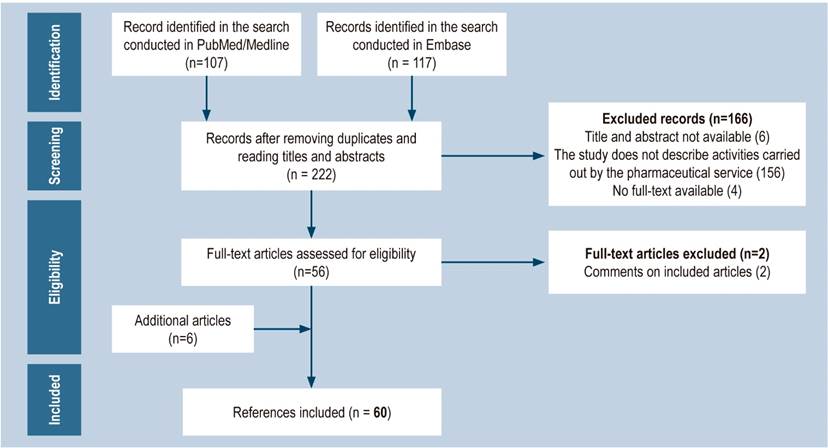
The publications identified in the search were subjected to a paired analysis. Initially, the title and abstract of each record were reviewed for eligibility purposes. Subsequently, the full text of the preselected articles was read and those related to articles previously included or that did not provide new information were excluded. The search was complemented with other studies appearing in the reference list of the articles included for analysis and that were considered relevant for the purposes of the review (Figure 1).
Data extraction and categorization of information
Data gathered from each article were inserted into a database that included the following variables: action/intervention, description of the activity, type of intervention (individual or collective), person in charge (professional pharmacist, technician or other), setting in which the activity was carried out (outpatient service, hospital, community or prison), health care categories (public health management, health promotion, diagnosis, treatment, rehabilitation or palliation) and references of the studies describing the intervention.
Actions and interventions identified were classified into categories based on the functions of the pharmaceutical service: promotion and prevention, administrative management, pharmaceutical care, research, and other support services.
To understand the results, the information retrieved from all articles included in the review was consolidated according to the actions and interventions described by them. Information on the strategies used to assess the quality of the interventions retrieved was also identified.
Results
Identified studies
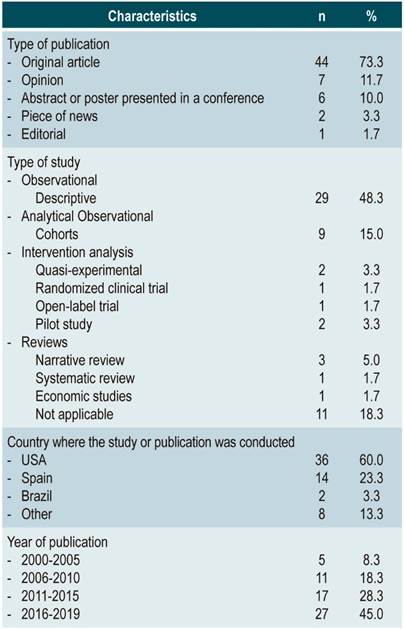
In total, 60 articles were included in the review (Figure 1); of these, 83% reported pharmaceutical activities carried out mainly in the United States (USA) and Spain. In addition, 45% were published between 2016 and 2019 (Table 1).
Actions and interventions carried out by the Pharmaceutical Service for the management of chronic hepatitis C infection
Table 2 lists the actions/interventions, the personnel in charge of them, the settings where they were performed, and the references of the articles describing them.
Four classifications were used to describe the setting:
Community, if the activities were carried out in pharmacies or drugstores, since they are usually located in neighborhoods and offer a service that is not related to other health care facilities.
Outpatient, if the activities were performed by pharmaceutical services and institutions providing outpatient services, that is, providing health services to patients who do not need hospitalization.
In-patient, when activities were developed during the provision of care to in-patients.
Prison, when activities were carried out during the provision of care to patients in the prison health system.
Promotion and prevention
Patient information and education7-11
Educational activities aimed at increasing public and individual awareness on the transmission mechanism of hepatitis B and C and how to prevent their infection.
Provision of educational material on hepatitis C and visible posters in the pharmacy encouraging patients to ask for medical advice about their health problems.
Vaccine supply8
Provision of vaccination service including vaccines against hepatitis A and B and other vaccines that may be necessary in patients with chronic liver disease.
Harm reduction services9,12-16
Key services to prevent sexually transmitted infections in at-risk population groups such as injecting drug users (IDUs). They may include:
Education interventions and delivery of educational materials on human immunodeficiency virus (HIV), HCV, and the provision of sterile syringes, either over-the-counter or in syringe exchange programs.
Provision of sharps containers for needle disposal.
Provision of sterile items such as syringes, needles, water, and metal lids.
Disposal of used syringes.
Initiation of Naloxone administration by the pharmacist (according to the protocols established for the management of opioid overdose).
Training or information on how to inject safely and prevent overdose.
Provision of condoms for free and referral to social services, referral centers or medical care services for treatment.
Provision of rapid HCV tests followed by a counselling or referral to a physician process depending on the test result.
Administrative management
Administrative support1,6,20,22-26
Collecting information on the patient’s baseline clinical parameters (laboratory results, fibrosis staging, clinical notes made by the medical staff) and sociodemographic data to complete pre-authorization records required by insurance companies, the health system or health care providers. This information can be recorded in the patient’s medical record. If a comprehensive program is available, pharmacists can request the delivery of the medication in the patient’s pharmacy of choice and schedule a visit to initiate treatment.
Procurement and distribution of drugs8
Besides the usual medication procurement and dispensing process, pharmacists can identify patients at risk of non-adherence based on their medication refill history. They can also ensure that patients get their prescribed medications on time, speed up the pre-authorization process, and make sure all drugs are updated in the pharmacy system to check for possible drug interactions.
Pharmaceutical care
The drug dispensing process may be carried out during the follow-up consultation with the pharmacist by arranging with the patient whether the medications will be dispensed at the nearest pharmacy, delivered to their home, or directly provided in observed therapy. In some contexts, pharmacy students may be available to check with the patient over the telephone if their prescribed drug has been delivered to the pharmacy.
Pharmacotherapy-related follow-up (face-to-face or by telephone)1,3,5,8,10,20,25,27-30,32-48
This process aims at improving adherence to treatment and, therefore, at having a higher probability of achieving sustained virologic response and preventing the development of resistance. It allows for the early detection and resolution of drug-related issues that may arise during treatment.
It is usually performed monthly in person or via telepharmacy, depending on the needs of the patient needs and available resources. Follow-up can be complemented with periodic phone calls, especially in patients in whom risk factors for non-adherence have been identified.
This type of follow-up involves:
Initial assessment by the pharmacist (a pharmaceutical interview aimed at collecting sociodemographic and social history [alcohol consumption, drug abuse] data), of health conditions, history of allergies, outpatient pharmacotherapy (active ingredients, dosage regimen and route of administration), prescription type and use of alternative therapies.
Pharmacotherapy validation.
Immunization history review.
Verification of laboratory tests orders and drug prescriptions.
Evaluation of adherence to treatment and its effectiveness.
Pharmaceutical intervention (if applicable).
In the event the patient consumes alcohol or psychoactive substances, the pharmacist can provide information on the importance of alcohol withdrawal and recommend places to support the abandonment of such substances.
Patient education and promotion of adherence to treatment8,11,20,23,27,30,32-36,38,40,46,49-51
This is an ongoing process that takes place during the follow-up visits; however, the first visit is key, since this is where basic information about hepatitis C and its treatment is discussed, therapy goals are established, and the importance of adherence to medication and follow-up is stressed to the patient. This process allows for the tailoring of management strategies to the individual needs of the patient, thus optimizing the treatment outcomes.
Topics to be addressed:
Hepatitis C (HCV genotype, its association with treatment duration and likelihood of cure, severity of the disease, measures to reduce the risk of transmission).
Treatment (route of administration and how to store the drug, what to do if a dose is missed, drug interactions).
Adverse drug reactions and how to manage them. The patient may be instructed to take note of any uncomfortable experience during treatment and may be advised to adopt, preferably, non-pharmacological strategies to improve drug tolerance. In this way, pharmacists can dissuade the patient from abandoning treatment.
Lifestyle changes to limit disease progression, such as avoiding alcohol use.
Indications about that, in case of visiting the emergency room or requiring hospital admission, patients should bring antiviral drugs with them since treatment may not be available in those facilities.
Providing written information (physical or digital) and using tools to improve adherence to treatment, such as pill organizers, reminders, and medication taking schedules is recommended. Refill reminders may also be used to remind patients to pick their medications on time or have them delivered to their home. Alarms may also be created on patients’ mobile devices to remind them when to take their medications.
Pharmacovigilance1,8,11,22,29,33,35,36,38,45,47,52,53)
Since DAAs are mainly characterized by symptomatic adverse drug reactions, timely review by pharmacists and consultation with physicians may result in interventions such as monitoring, dose reduction, change or prescription of medications, and recommendation of non-pharmacological measures for symptomatic management, to avoid early treatment discontinuation or lack of patient adherence.
Pharmacists conduct this review usually during follow-up visits or at the time of dispensing the drugs, when patients are asked about the adverse effects commonly associated with antiviral treatment, and lab tests are reviewed to monitor the occurrence of adverse drug reactions. Then, any detected reaction is reported to pharmacovigilance systems with complete information of the drug.
Similarly, the pharmacovigilance process should include the review of safety reports prepared by agencies for drug regulation and the notification of other health professionals.
Research8
This item includes taking part in the development of clinical trials on novel therapies and writing research articles on the use of antiviral drugs in different patient populations. Pharmacists may actively collaborate with other health care providers in several types of research, such as efficacy and safety, pharmacokinetics, and pharmacoeconomics studies.
Other support services
Development of information and clinical decision support systems20,33,44,45,54
This item comprises the development of information and clinical decision support systems that can help promote the quality and efficiency of the health care process. They can be as complex as institutions want to make them. The simplest ones could be clinical guidelines that set standard criteria for the selection of patients that will undergo treatment, treatment protocols for special population groups (elderly, chronic kidney disease, children, among others), details of the health care process and indications for the management of drug interactions, all in compliance with clinical practice guidelines implemented in each country.
More complex systems allow recording patients’ clinical information using their medical records, assessing patients’ adherence to antiviral therapy, and even generating automated pharmaceutical events and warnings regarding the efficacy and safety of the pharmacological therapy. These systems can ease pharmacotherapy-related follow-up by automatically incorporating hematological, biochemical, and microbiological analyzes from laboratory databases, and providing real-time data for statistical processing and analysis.
Participation in multidisciplinary teams33
This involves the creation of working groups with various health professionals responsible for the provision of health care to patients with hepatitis C, including pharmacists, to discuss individual cases in order to reach a consensus on the most suitable and cost-effective treatments.
Support activities during the transition of patients from one level of care to another3,8,33
Clinical pharmacists’ involvement in patient transfers from one level of care to another between health care institutions (for example, from a hospital to an outpatient clinic or vice versa). Clinical pharmacists have the possibility to train medical and health care staff on the importance of the continuity of treatment of hepatitis C, adverse events, and drug interactions. Some of these processes include medications reconciliation, coordination, and procurement.
Support group55
These groups aim at offering support to hepatitis C patients, as well as their friends and relatives. In this context, educational sessions discussing general symptoms of the disease, treatment, adverse drug reactions, diet, liver effects, the emotional impact of being diagnosed with hepatitis C infection, alternative treatments, access to health services, personal experiences, and other topics may be arranged for each month of the year.
Provision of training to health care professionals8,47
This item refers to the provision of training to health care professionals on disease complications, treatment criteria, and proper use of medications in accordance with clinical practice guidelines. Depending on the context, these training sessions can be done either face-to-face or through online conferences. This action may encourage the creation of clinical discussion boards.
Sending motivational letters and emails56
This is a strategy to encourage adherence to treatment by sending motivational letters to patients, as well as responding to their requests for information.
Assessment of the activities carried out by pharmaceutical services in the provision of health care process
Pharmaceutical providers should offer more than just health services; they should also provide quality assurance processes. As a result, metrics must be built to assess the effects of pharmaceutical professionals’ practices and to record information about patients who have been treated3.
Patient compliance rates, usage habits, adherence to treatment recommendations, treatment efficacy, incidence rate and severity of drug-related problems and adverse drug reactions, degree of avoidable drug-related problems, costs, and patient satisfaction are some of the metrics that can be considered29,44,45,47.
Management indicators should be presented periodically to the work team33 and other health professionals, using presentations, live online broadcasts, web publishing or e-mail distribution47.
Cost assessment
The role of pharmacists in the care of hepatitis C patients is critical in cost containment, mainly by preventing unnecessary medical expenses through therapy optimization8,44,57. For this reason, they should participate in health care-related costs54, costs saved due to pharmaceutical interventions28,32, and return on investment32 analyses, since this information contributes to justifying the implementation or expansion of pharmaceutical care services58.
Lavitas et al. analyzed cost saving following a comprehensive HCV drug treatment program in cooperation with the Clinical Pharmacy Services of the University of Massachusetts and the Massachusetts Medicaid program in USA, finding that optimizing treatment regimens avoided spending more than $3.7 million in patients who completed treatment32.
In addition, Radley et al., in a study comparing the costs of conventional HCV care (in hospitals) to pharmacist-led care (in primary care clinics), found that the latter could reduce costs by up to 74.4% (£ 933 vs. £ 238), since this type of care require less expensive facilities and simplified testing and monitoring requirements. Also, a comprehensive care program may increase the number of people who can access treatment and shorten the time it takes for people with HCV infection to be cured10.
Patient satisfaction assessment
Pharmaceutical services have been introduced in a variety of settings; however, there are few studies assessing patient satisfaction with the services provided, especially in the field of hepatology23. Robustillo et al. reported the need to measure patient satisfaction as it allows knowing their opinion on the services and therapies they receive, as they consider it a measure of outcome in direct health care39.
The most common period to assess patient satisfaction is when the care process ends, although some studies, such as the one conducted by Menchen et al., have evaluated the difference in satisfaction levels of patients with HCV with the intervention carried out prior to the implementation of the comprehensive care model for these patients and 6 months later59.
In addition to service satisfaction, clinical satisfaction can also be assessed39. Key aspects to be evaluated may include satisfaction with treatment (effectiveness, adverse reactions, and treatment modifications)39, satisfaction with the information received (lifestyle and vaccination recommendations, and education interventions about hepatitis C management and treatment), and satisfaction with service (waiting times, accessibility to the health care provider, ability of the health care provider to handle questions and complaints, and referral to other services, if required)23,40,59.
Assessment of HCV knowledge
A questionnaire may be administered before and after a training session to determine the impact of the pharmacist’s instruction on the awareness of the patient or health practitioners in topics such as transmission process, treatment, and adverse reactions, in order to find out about the initial and final state of HCV knowledge7,17.
Use of technologies in pharmaceutical practice
The inclusion of information and communication technologies (ICTs) in pharmaceutical practice (telepharmacy) has resulted in shorter treatment times and increased patient access to health care services5 because it facilitates the training and assistance provided by pharmaceutical staff even after hours (hotline 24/7), when the pharmacist is out of the workplace, or when patients reside in rural areas and travel distances must be reduced5,8,22,48,49,56.
Likewise, telepharmacy allows pharmacotherapy-related follow-up in patients living in remote areas and who cannot travel to hospitals for face-to-face visits5,22, monitoring the incidence of adverse events, providing guidance on over-the-counter medications for their management, and, if necessary, referring patients to a doctor60. Grisheau et al. described a model in which telephone contact with the patient was arranged two weeks after starting the treatment. Calls were made every four weeks to assess adverse effects, adherence to the treatment plan, the need for drugs refills, and ensure that patient health details and appropriate tests were followed up on. All of these calls were entered into each patient’s electronic medical record and sent to their attending physician. It is worth noting that follow-up calls were alternated with follow-up visits at the hospital, so that patients had at least some kind of contact with the medical staff every two weeks during treatment27.
Similarly, ICTs can be used to remind patients using emails, mobile messages, or calls, about their paperwork, appointments, or medical check-ups and routine laboratory tests, or to assess the hepatitis C-related quality of life in these patients25,30.
Proposal of a clinical pathway for the management of hepatitis C that considers the participation of the pharmacist
This proposal, which includes the perspective of pharmaceutical services (Figure 2), is based on the guidelines suggested by the Colombian Ministry of Health and Social Protection for the management of hepatitis C61. The findings of this review were incorporated into the Ministry’s proposal.
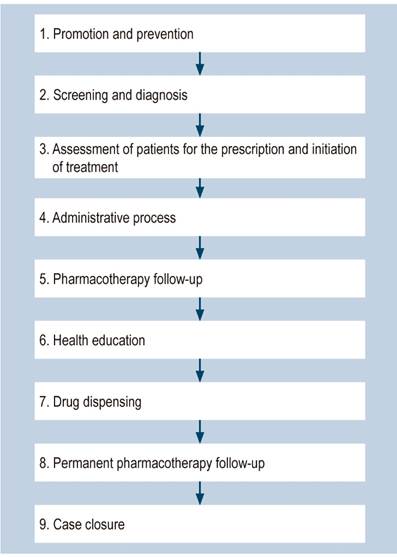
Figure 2 General flow diagram of the proposed clinical pathway for the management of hepatitis C with the involvement of the pharmacist. Adapted from: Colombian Ministry of Health and Social Protection - Instituto de Evaluación Tecnológica en Salud. Vía clínica para el tratamiento de hepatitis C (Clinical pathway for the treatment of hepatitis C). Bogotá, D.C: Ministerio de Salud y Protección Social; 2017.
When applying this model in specific contexts, the regulations that govern the practices of pharmacists, the practices they are authorized to perform, the available services, and the degree of interconnection between health care professionals, health care provision environments, and the different health care provision processes should be considered.
The general treatment flow diagram is shown in Figure 2.
Promotion, prevention, screening, and diagnosis
People infected with HCV can be screened in three settings: community, outpatient, and inpatient, either by pharmaceutical or nursing staff (Figure 3). Initially, carrying out educational talks about the disease and risk factors for infection is suggested. Afterwards, a checklist may be administered to those who agree to identify behaviors or risk factors for infection. People with risk factors may be screened with rapid anti-HCV tests, depending on their availability. Any positive result must be confirmed by performing an HCV RNA test.
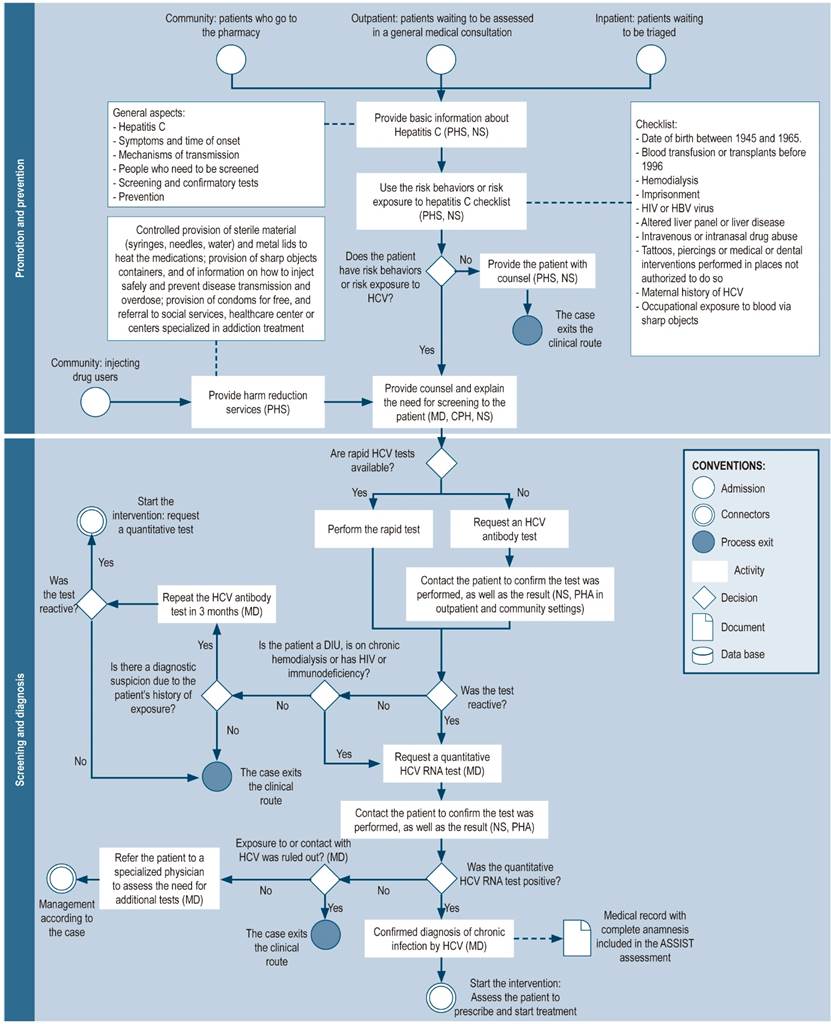
Figure 3 Pharmaceutical promotion and prevention, screening, and diagnosis. NS: nursing staff; CPH: clinical pharmacist; MD: medical doctor; PHS: pharmacy staff (including technicians, professionals, or support students); PHA: pharmacy assistant; IDU: injecting drug user; HCV: hepatitis C virus, HIV: human immunodeficiency virus.
The role of community pharmacists in the detection of HCV patients is crucial because people who are not linked to the health system and who are at risk of HCV infection initially seek assistance from community pharmacies. Therefore, rapid HCV testing, as well as pharmaceutical stall trained to use these tests should be available at this level of care, since this could favor the identification of people with undiagnosed HCV21. In this way, the community pharmacy may serve as an HCV patients recruitment node, in particular the most vulnerable population groups, such as low-income people and IDU, as well as provide support in the prevention of this disease and its transmission and in connecting the patient to health care centers.
Assessment of patients for the prescription and initiation of treatment
Patients with a confirmed diagnosis of chronic hepatitis C must be evaluated by medical staff in order to initiate treatment. Furthermore, the support of a multidisciplinary team53, which could be composed of medical, nursing, social care, psychology, and pharmaceutical professionals, is recommended to assess potential risks of non-adherence to treatment and to timely reduce any access barriers and achieve the therapeutic goals (Figure 4).
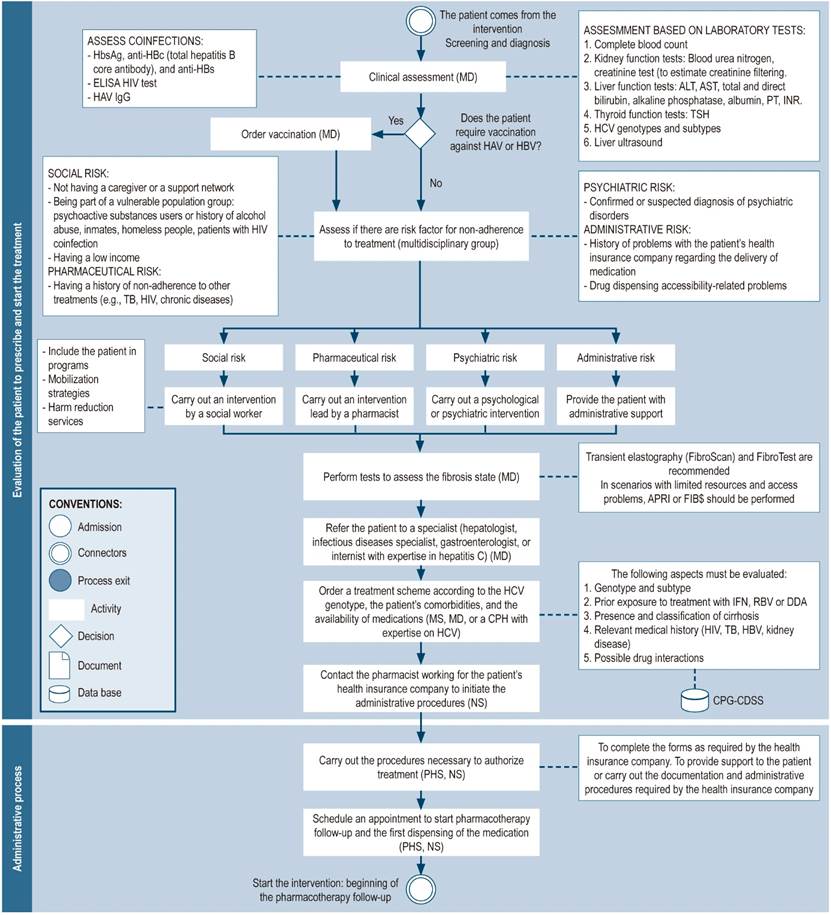
Figure 4 Evaluation of patients for the prescription and initiation of treatment; administrative process. *Depending on the circumstances, general practitioners or clinical pharmacists who have expertise in hepatitis C may prescribe DAAs after having consulted with a specialist and assessing the patient’s clinical parameters, medical record, comorbidities, and potential contraindications to the therapy, in accordance with the country’s clinical practice guidelines. HBsAg: hepatitis B surface antigen; ALT: alanine aminotransferase; AST: aspartate aminotransferase; ELISA: enzyme-linked immunosorbent assay ; NS: nursing staff; CPH: clinical pharmacist; HB: Hepatitis B; Hb: Hemoglobin; IFN: interferon; CPG: clinical practice guideline; INR: international normalized ratio; MD: medical doctor; MS: medical specialist (hepatologist, gastroenterologist, infectologist, or internist with experience in the treatment of hepatitis C); PHS: pharmacy staff (including technicians, professionals, or support students); PT: prothrombin time; RBV: ribavirin; CDSS: clinical decision support system; TB: tuberculosis; TSH: thyrotropin; HBV: hepatitis B virus; HCV: hepatitis C virus.
Administrative process
Due to the high costs of DAAs, strategies such as the pre-authorization process have been required to control spending within health systems. Insurance providers use this procedure to assess whether care is medically necessary and sufficient based on the patient’s diagnosis and condition6. Pharmacists have assisted in the coordination of this process by working in medication acquisition and dispensing2,6,22,23,25-27,36, therapy optimization, and expenditure control (Figure 4)44.
Pharmacotherapy follow-up
Due to the recent commercialization of new DAAs, intensive pharmacotherapy-related follow-up programs are expected to track the safety and efficacy of these drugs in clinical practice28. This is an active process in which the pharmacist works as part of a multidisciplinary team in order to make a comprehensive assessment of the patient, their diseases, and treatments, allowing for the timely identification of potential and actual drug-related problems8.
Pharmacotherapy follow-up begins before treatment is started (Figure 5) and continues throughout the course of treatment. It consists of several activities:
Initial assessment by the pharmacist (pharmaceutical interview): this is the first encounter of the patient with the pharmacist. Its aim is to establish a relationship of trust based on active listening and empathy. The patient’s concerns regarding their condition and care are addressed in this initial meeting, which is focused on recognizing their expectations36.
Validation of pharmacotherapy: the pharmacist reviews the medical prescriptions and verifies their suitability, as well as that of the antiviral scheme selected in compliance with clinical practice guidelines, by using the details obtained during the pharmaceutical interview and the patient’s medical record (considering the genotype, staging of cirrhosis status, and history of previous treatment). Also, the pharmacist considers other conditions resulting in contraindications to the therapy1,3,8,10,25,27,29,30,32,33,35,37,41,42,44-48. It should be noted that in countries where no pangenotypic schemes are available, viral genotype evaluation is necessary to personalize therapy and offer cost-effective treatments57. The validation of pharmacotherapy is a strategy for the prevention, identification and resolution of medication errors28 or drug-related complications8,29, which may include the use of alternative drugs or therapies that may interfere with antiviral treatment, drug-disease interactions, or the need to improve the management of conditions other than hepatitis C in these patients8. In some contexts, the pharmacist must ensure that the prescribed treatments are in accordance with the pharmaceutical benefit plans established by the institution or country, as is the case with the Veterans Health Administration in the United States of America3.
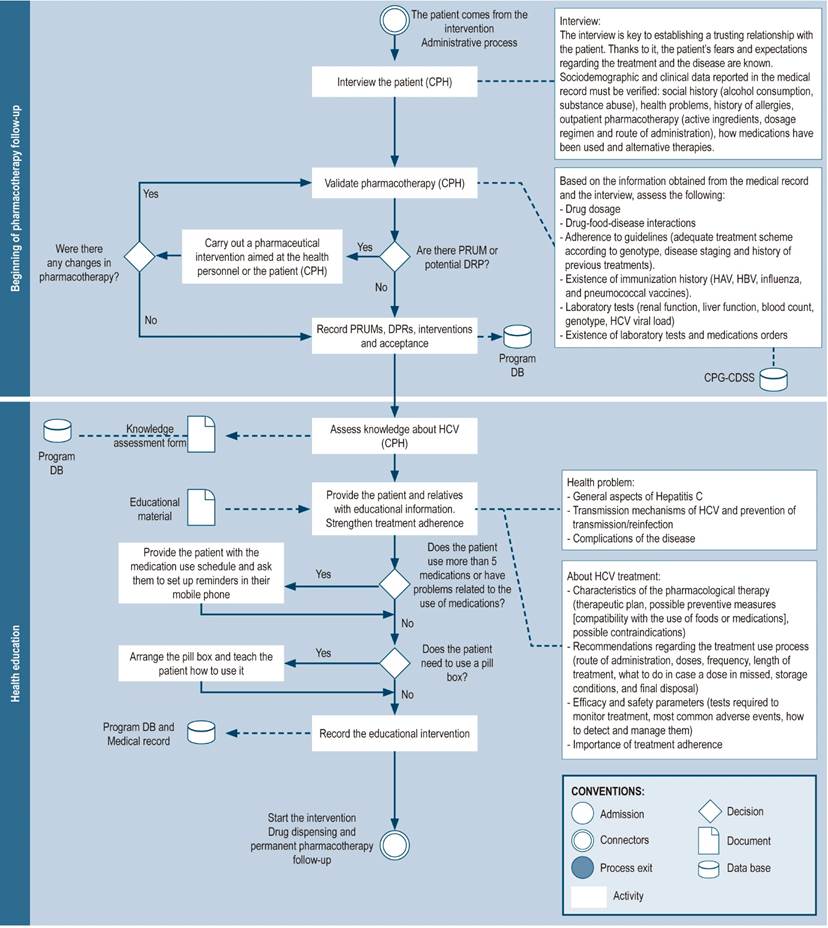
Figure 5 Beginning pharmacotherapy-related follow-up and health education. DB: database; CPH: clinical pharmacist; CPG: clinical practice guideline; PRUM: problems related to the use of medications; DRP: drug-related problems; CDSS: clinical decision support system.
Review of immunization history
It comprises the evaluation of the patient’s immunization history and the need for vaccines against hepatitis A and B viruses, influenza, and pneumococcal disease8,48.
Verification of laboratory tests orders and drug prescriptions
The pharmacist verifies that the medication prescriptions are appropriate for the length of treatment, as well as the laboratory tests orders required for follow-up3. In certain countries, the pharmacist has the authority to prescribe or modify, in accordance with the treatment protocol and with the physician’s signature, the medications and laboratory tests required to determine the safety and efficacy of therapy3,34,48.
Health education
The pharmacist’s participation in health education may solve the limited time availability that medical providers must advise patients on disease status and proper use of medications. This can positively impact the patient’s adherence to hepatitis C treatment and increase cure rates8.
In addition to the individual educational phase, support groups may be formed to provide support to patients with hepatitis C (Table 2), which improves their awareness and perspective on the disease, as well as the visibility and perception of the role of the pharmacist as a community health care provider55.
Drug dispensing
Drug dispensing can occur during the pharmacotherapy-related follow-up consultation with the pharmacist28,29 by coordinating with the patient if medications will be dispensed at the nearest pharmacy25,27, delivered at their home1,22,30, or through directly observed therapy (also known as daily controlled supply), where patients receive daily treatment, and in the case of doses to be used during the weekend (i.e. when the drug administration is in charge of the patient), the pharmacist and the patient can develop an action plan to perform this activity31.
The dispensing modality depends on the context, the available services, and the patient’s needs. Thus, assessing the adherence of the patient adherence, as well as data on sociodemographic factors and clinical conditions is suggested to establish the most appropriate dispensing modality and frequency (Figure 6).
To monitor adherence to treatment, a record of the dispensation of medications should be kept and, if directly observed therapy is not used, checking the drug intake using ICTs is suggested.
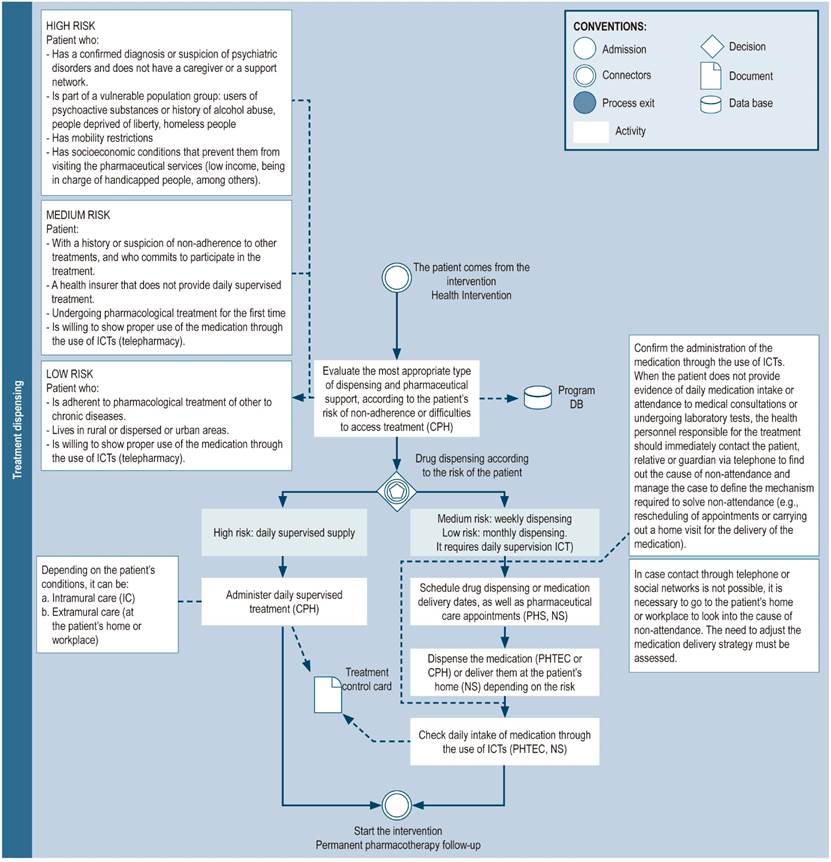
Figure 6 Treatment dispensing. DB: database; NS: nursing staff; CPH: clinical pharmacist; PHS: pharmacy staff (includes technicians, professionals, or support students); PHSE: pharmaceutical service; CDSS: clinical decision support system; PHTEC: pharmacy technician; ICT: information and communication technologies; HCV: hepatitis C virus.
Permanent pharmacotherapy follow-up
In-person pharmacotherapy follow-up visits may be conducted every 28-30 days and include, besides the activities described in the initial phase of pharmacotherapy follow-up (Figure 7), the following actions:
Verification of medications introduced or excluded from the patient’s pharmacotherapy35.
Evaluation of treatment efficacy by reviewing laboratory findings such as viral load and liver function tests.
Detection, evaluation and notification of adverse drug reactions and recommendations for their management: treatment safety is assessed by means of the patient interview and the review of lab test results (blood count, thyroid tests [for ribavirin or interferon therapy] and kidney function)8,19,22,30,35,36,42,47.
Assessment of patient adherence: this assessment can be made by asking questions about medication use36, inspecting the packaging of the tablets dispensed to the patient49, analyzing drug dispensing dates reported in information systems1,8,23,33,41,44,47, and keeping records of medication use10. Adherence measurement questionnaires such as Morisky-Green40,43, Haynes-Sackett38, or a visual analogue scale (VAS) on which the patient assesses compliance40 may also be used.
Support actions during the transition of the patient from one level of care to another: If the patient is to be treated at a hospital, whether for the treatment of an adverse reaction or complications cause by their diseases, the pharmacist must provide support to ensure medication reconciliation and therapy continuity in said situation.
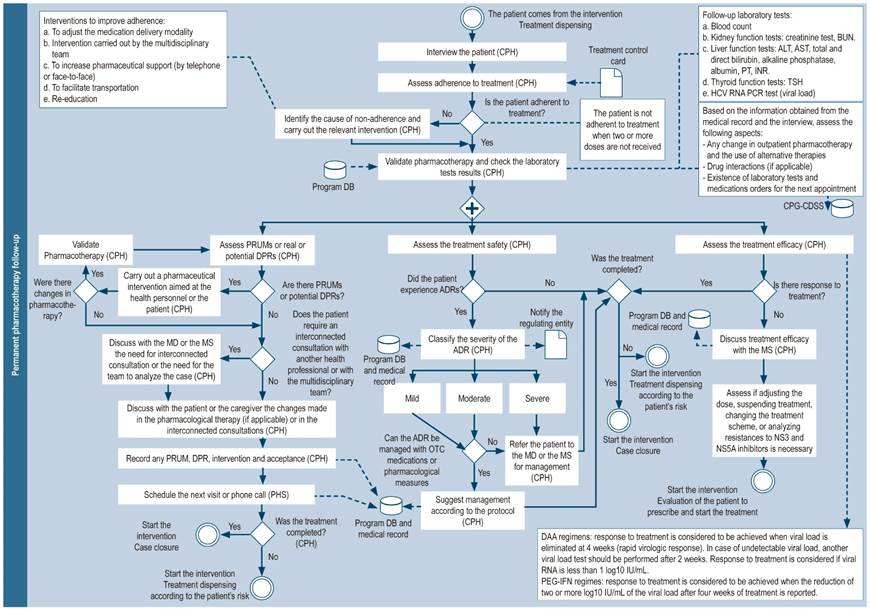
Figure 7 Permanent pharmacotherapy follow-up. DB: database; BUN: blood urea nitrogen; CPH: clinical pharmacist; MD: medical doctor; MS: medical specialist (hepatologist, gastroenterologist, infectologist or internist with experience in hepatitis C); NS3: non-structural protein 3; NS5A: non-structural protein 5A; OTC: over-the-counter drugs; PEG-IFN: Pegylated interferon; PHS: pharmacy staff (includes technicians, professionals or support students); PRUM: problems related to the use of medications; DRP: Drug-related problems; ADR: adverse drug reaction; CDSS: clinical decision support system.
Pharmaceutical care
Medication errors or problems related to actual or potential medications may be detected in pharmaceutical practice, or patient therapy may need to be optimized, leading to interventions to the medical or nursing staff or the patient, depending on the circumstances (Table 3)1,3,8,19,22,23,28-34,37,41,42,45,58,60. All interventions and suggestions made by the pharmacist, including appointments, phone calls, pharmacotherapy evaluations, and all activities related to patient counseling and adherence, should be recorded in the patient’s medical record or in the information systems8,60.
Case closure
The case may be closed when the patient completes treatment and is cured (sustained virological response: undetectable viral load 12 weeks after the end of treatment), when the patient dies, or when the patient wishes to discontinue the treatment (Figure 8). A follow-up consultation is suggested between 6 and 12 months after achieving sustained virological response, especially in patients susceptible to reinfection (IDU, people living with HIV, men who have sex with men, patients on hemodialysis, sex workers). Additionally, patients with advanced stage cirrhosis or hepatocarcinoma should continue to see their specialist physician as usual (hepatologist or gastroenterologist). The quality of the care provided should be measured at the end of the care process using satisfaction questionnaires.
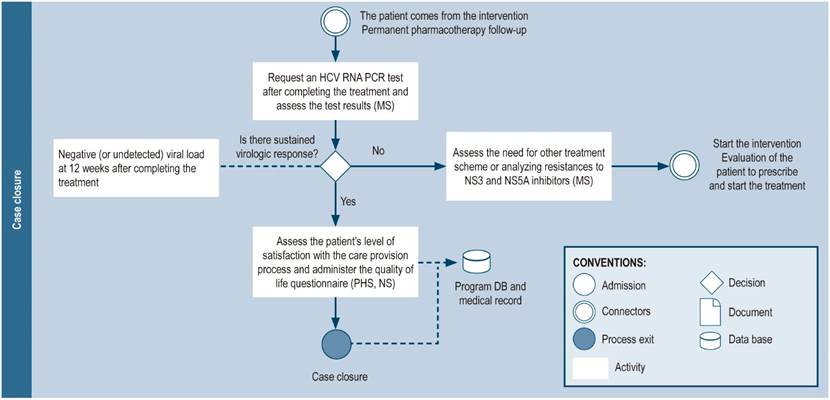
Figure 8 Closing the case. RNA: Ribonucleic acid; DB: database; NS: nursing staff; MS: medical specialist (hepatologist, gastroenterologist, infectologist, or internist with experience in hepatitis C treatment); PHS: pharmacy staff (including technicians, professionals, or support students); HCV: hepatitis C virus.
Discussion
The involvement of pharmaceutical services in the care of patients with hepatitis C, besides promoting adherence and improving clinical outcomes, may minimize treatment costs by promoting therapy optimization44. Their participation in outpatient, community and inpatient settings is not only essential to the population enrolled in health systems but is also critical for individuals who do not usually use the health care system, either due to insecurity or non-affiliation, since they often use this form of pharmaceutical services to receive some kind of assistance. In this sense, implementing the procedures outlined in this review could expand care coverage to the affiliated population, while also promoting prompt diagnosis and treatment in vulnerable population groups and non-users of the health system. In addition, a comprehensive provision of health care services to these patients enhances cooperation between pharmacists and other health professionals, which would allow assessing the safety of DAAs.
Consequently, a clinical pathway for the treatment of patients with hepatitis C complemented by pharmacy service actions/interventions is presented here. Depending on the context of each country, the existing legislation, the available resources, and the degree of interconnection between health professionals, and health care settings, this strategy may be adapted for its implementation. It may also be used to treat other sexually transmitted diseases and other costly chronic diseases.
More relevant findings
This review identified the activities carried out by pharmaceutical services in the management of hepatitis C, which allowed proposing a clinical pathway for the management of hepatitis C management complemented by the actions/interventions of pharmaceutical services. It is important to note the actions of the pharmaceutical staff actions at each point of the care process, in which their role is extended beyond pharmacotherapy follow-up and is given an active participation in key processes such as HCV screening and the linking of patients to treatment. In this regard, Radley et al. and Dong et al. highlighted the importance of community pharmacies and opioid replacement services as potential places for performing HCV screening, particularly in people who are not enrolled in the health care system to promote timely detection of HCV and achieve an early onset of treatment9,10. Similarly, the provision of harm reduction services is of great importance to prevent HCV transmission, especially in high-risk populations such as IDUs9,12-16.
The participation of pharmacists in promotion and prevention activities should be considered, especially in the Colombian and Latin American contexts. To facilitate these services, a formal training plan is needed to enhance acceptability and foster positive attitudes among pharmacy workers9,12. Furthermore, public relations and marketing strategies are needed to increase the visibility of community pharmacists (pharmacies and drug stores) in this expanded role of patient care, as well as the establishment an operational model that allows pharmacist to receiving remuneration for their activity and the services offered9.
Another process to consider is drug prescription, in which pharmacists have not played any significant role to date. However, since DAAs are easier to implement, as Galewitz et al.4 and Radley et al.10 have noted, clinical pharmacists specialized in hepatitis C may be able to prescribe these antivirals (prior the approval by specialists, the review of clinical practice guidelines, the review of the patient’s medical record, and the assessment of clinical parameters, comorbidities, and potential contraindications of antiviral therapy).
Pharmaceutical care can also support primary health care by shortening patients’ waiting times to see a doctor. In the United States, for example, the training of clinical pharmacists in treating patients with hepatitis C has enabled them to prescribe drugs, request laboratory tests, refer patients to specialists, and perform physical examinations4, so specialists are consulted only when necessary. With this type of adjustment, the agenda of specialists could be freed up to provide treatment to more chronic patients or to focus on patients with more specific needs.
Similarly, patient education by the pharmacist or a multidisciplinary team is highlighted as one of the most relevant practices to promote patient and family empowerment, as well as active involvement in health-related decision-making8,34.
Moreover, the use of ICTs in pharmaceutical practice has been shown to increase access to treatment in patients with hepatitis C and other chronic diseases5.
Similarities and differences between publications
During the search, no systemic reviews addressing our objective were found. Editorials such as the one authored by Briggs20 summarize the role of pharmaceutical staff in the management of hepatitis C; however, it only refers to the processes they are involved in, without detailing the activities these processes imply and the times required to carry them out. The Joint Opinion of the GI/Liver/Nutrition and Infectious Diseases Practice and Research Networks of the American College of Clinical Pharmacy8 details all the roles that a clinical pharmacist may have, but it does not describe the activities that can be performed with the support of other pharmaceutical service staff such as pharmacy technicians or support students. This review, in addition to summarizing and describing each of the activities that were identified and the environment in which they are carried out, proposes a clinical approach for the management of HCV in which pharmaceutical staff (professionals, technicians and support personnel) are included within the care process and their interrelationship with other health professionals is considered.
Some of the interventions described in this review are restricted by Colombia’s current regulatory framework, especially those related to treatment decision-making and treatment modifications, or the referral of patients to be vaccinated against other types of hepatitis. Therefore, the generation of evidence of the effectiveness of this type of intervention in cooperation with the health care team in the treatment of these of patients, especially with treating physicians, is essential. In this regard, Briggs20 states that the role of pharmaceutical workers as health care providers must be made clear, particularly for public policy makers, so that the latter consider these health workers as key players within the health care team, since they can contribute to meeting the health needs of patients and the community.
Limitations of the review
The assessment of the quality of the studies was not considered as a criterion for the inclusion for full analysis of the articles, since the main objective of the review was to classify all the actions and procedures carried out by pharmaceutical services in the treatment of patients with hepatitis C setting and the contexts in which they were implemented.
Indications and guidelines for future research
Real-life studies are needed to generate objective evidence of the impact that the clinical pathway proposed in this review might have on the improvement of access to treatment, on clinical outcomes, and on the costs associated with the provision of health care to these patients.
Acknowledgments
To the Pharmaceutical Promotion and Prevention Research Group of the Universidad de Antioquia (Medellín, Colombia) for their scientific and methodological assistance in the production of this study.
REFERENCES
1. Tran AN, Sachdev R, Fricker ZP, Leber M, Zahorian T, Shah B, Nunes DP, Long MT. Intensive Pharmacy Care Improves Outcomes of Hepatitis C Treatment in a Vulnerable Patient Population at a Safety-Net Hospital. Dig Dis Sci. 2018;63(12):3241-9. https://doi.org/10.1007/s10620-018-5231-0 [ Links ]
2. Andres J, Lott S, Qureshi K. Eight-Week Outcomes of Ledipasvir/Sofosbuvir in Noncirrhotic Treatment-Naive Patients with Hepatitis C: Analysis of Pharmacy-Based Data. J Manag Care Spec Pharm. 2018;24(1):23-8. https://doi.org/10.18553/jmcp.2018.24.1.23 [ Links ]
3. Gauthier TP, Moreira E, Chan C, Cabrera A, Toro M, Carrasquillo MZ, Corentin M, Sherman EM. Pharmacist engagement within a hepatitis C ambulatory care clinic in the era of a treatment revolution. J Am Pharm Assoc JAPhA. 2016;56(6):670-6. https://doi.org/10.1016/j.japh.2016.06.013 [ Links ]
4. Galewitz P. VA Shifts To Clinical Pharmacists To Help Ease Patients’ Long Waits [Internet]. Kaiser Health News; 2016 [citado el 28 de abril de 2018]. Disponible en: Disponible en: https://khn.org/news/va-treats-patients-impatience-with-clinical-pharmacists/ [ Links ]
5. You A, Kawamoto J, Smith JP. A pharmacist-managed telemedicine clinic for hepatitis C care: a descriptive analysis. J Telemed Telecare. 2014;20(2):99-101. https://doi.org/10.1177/1357633X13519043 [ Links ]
6. Dunn EE, Vranek K, Hynicka LM, Gripshover J, Potosky D, Mattingly TJ. Evaluating a Collaborative Approach to Improve Prior Authorization Efficiency in the Treatment of Hepatitis C Virus. Qual Manag Health Care. 2017;26(3):136-9. https://doi.org/10.1097/QMH.0000000000000137 [ Links ]
7. Calderon Y, Cowan E, Schramm C, Stern S, Brusalis C, Iscoe M, Rahman S, Verma R, Leider J. HCV and HBV testing acceptability and knowledge among urban emergency department patients and pharmacy clients. Prev Med. 2014;61:29-33. https://doi.org/10.1016/j.ypmed.2013.12.026 [ Links ]
8. Mohammad RA, Bulloch MN, Chan J, Deming P, Love B, Smith L, Dong BJ, GI Liver Nutrition and Infectious Diseases Practice and Research Networks of the American College of Clinical Pharmacy. Provision of clinical pharmacist services for individuals with chronic hepatitis C viral infection: Joint Opinion of the GI/Liver/Nutrition and Infectious Diseases Practice and Research Networks of the American College of Clinical Pharmacy. Pharmacotherapy. 2014;34(12):1341-54. https://doi.org/10.1002/phar.1512 [ Links ]
9. Dong BJ, Lopez M, Cocohoba J. Pharmacists performing hepatitis C antibody point-of-care screening in a community pharmacy: A pilot project. J Am Pharm Assoc JAPhA. 2017;57(4):510-515.e2. https://doi.org/10.1016/j.japh.2017.04.463 [ Links ]
10. Radley A, Tait J, Dillon JF. DOT-C: A cluster randomised feasibility trial evaluating directly observed anti-HCV therapy in a population receiving opioid substitute therapy from community pharmacy. Int J Drug Policy. 2017;47:126-36. https://doi.org/10.1016/j.drugpo.2017.05.042 [ Links ]
11. Iizuka T, Eguchi Y, Akase T, Ishizuka H, Yoshiyama Y. Primary Care Enlightenment to Local Inhabitants -Cooperation of Medical Institution and Community Pharmacy in Treatment of Chronic Hepatitis C. Yakugaku Zasshi. 2010;130(12):1633-9. https://doi.org/10.1248/yakushi.130.1633 [ Links ]
12. Cooper EN, Dodson C, Stopka TJ, Riley ED, Garfein RS, Bluthenthal RN. Pharmacy participation in non-prescription syringe sales in Los Angeles and San Francisco counties, 2007. J Urban Health Bull N Y Acad Med. 2010;87(4):543-52. https://doi.org/10.1007/s11524-010-9483-z [ Links ]
13. Garfein RS, Stopka TJ, Pavlinac PB, Ross A, Haye BK, Riley ED, Bluthenthal RN. Three years after legalization of nonprescription pharmacy syringe sales in California: where are we now? J Urban Health Bull N Y Acad Med. 2010;87(4):576-85. https://doi.org/10.1007/s11524-010-9463-3 [ Links ]
14. Stancliff S, Agins B, Rich JD, Burris S. Syringe access for the prevention of blood borne infections among injection drug users. BMC Public Health. 2003;3:37. https://doi.org/10.1186/1471-2458-3-37 [ Links ]
15. Yang Y, Latkin CA, Luan R, Yang C. A cross-sectional study of the feasibility of pharmacy-delivered harm reduction services among people who inject drugs in Xichang, China. BMC Public Health. 2015;15:885. https://doi.org/10.1186/s12889-015-2236-x [ Links ]
16. Oramasionwu CU, Johnson TL, Zule WA, Carda-Auten J, Golin CE. Using Pharmacies in a Structural Intervention to Distribute Low Dead Space Syringes to Reduce HIV and HCV Transmission in People Who Inject Drugs. Am J Public Health. 2015;105(6):1066-71. https://doi.org/10.2105/AJPH.2015.302581 [ Links ]
17. Isho NY, Kachlic MD, Marcelo JC, Martin MT. Pharmacist-initiated hepatitis C virus screening in a community pharmacy to increase awareness and link to care at the medical center. J Am Pharm Assoc JAPhA. 2017;57(3S):S259-64. https://doi.org/10.1016/j.japh.2017.03.006 [ Links ]
18. Pharmacy hepatitis project results positive. The Pharmaceutical Journal. 15 de enero de 2010; News & analysis: 35. [ Links ]
19. Showande S, Olaifa A. Community pharmacists ‘ involvement in the ordering and interpretation of laboratory tests. Int J Pharm Sci Res. 2013;4(3):988-96. [ Links ]
20. Briggs AL. Pharmacists’ increasing involvement in hepatitis C management and prevention. J Am Pharm Assoc JAPhA. 2018;58(1):5-6. https://doi.org/10.1016/j.japh.2017.12.008 [ Links ]
21. D’Angelo RG, Klepser M, Woodfield R, Patel H. Hepatitis C Virus Screening: A Review of the OraQuick Hepatitis C Virus Rapid Antibody Test. J Pharm Technol. 2015;31(1):13-9. https://doi.org/10.1177/8755122514548228 [ Links ]
22. Cohen SM, Kwasny MJ, Ahn J. Use of specialty care versus standard retail pharmacies for treatment of hepatitis C. Ann Pharmacother. 2009;43(2):202-9. https://doi.org/10.1345/aph.1L227 [ Links ]
23. Martin MT, Faber DM. Patient satisfaction with the clinical pharmacist and prescribers during hepatitis C virus management. J Clin Pharm Ther. 2016;41(6):645-9. https://doi.org/10.1111/jcpt.12436 [ Links ]
24. Pham TT, Keast SL, Farmer KC, Thompson DM, Rathbun RC, Nesser NJ, Holderread BP, Skrepnek GH. Sustained Virologic Response and Costs Associated with Direct-Acting Antivirals for Chronic Hepatitis C Infection in Oklahoma Medicaid. J Manag Care Spec Pharm. 2018;24(7):664-76. https://doi.org/10.18553/jmcp.2018.24.7.664 [ Links ]
25. Martin MT, Telebak E, Taylor PA, Volozhina O. Development of a specialty medication prior-authorization service at an urban academic medical center. Am J Health Syst Pharm. 2016;73(15):1174-9. https://doi.org/10.2146/ajhp160059 [ Links ]
26. Vu TM, Toribio W, Riazi F, Ciprian G, Gibbs N, Giardina M, Camacho JA, Parrella K, Cambe J, Amory C, Chasan R, Sigel KM, Weiss JJ. Increasing Access to Hepatitis C Virus Medications: A Program Model Using Patient Navigators and Specialty Pharmacy to Obtain Prior Authorization Approval. J Manag Care Spec Pharm. 2018;24(4):329-33. https://doi.org/10.18553/jmcp.2018.24.4.329 [ Links ]
27. Grischeau M, Zenner J. Optimizing workflow at a multidisciplinary clinic for management of hepatitis C virus infection. Am J Health-Syst Pharm AJHP Off J Am Soc Health-Syst Pharm. 2012;69(24):2131-3. https://doi.org/10.2146/ajhp120264 [ Links ]
28. Ribed A, Rodriguez-González C, Collado-Borrell R, Camino S, Elena L, Alvaro G, Ibañez-García S, Tovar-Pozo M, Herranz-Alonso AM, Sanjurjo-Sáez M. CP-102 Pharmaceutical care monitoring of hepatitis C outpatients: Guaranteeing safety and efficiency. Eur J Hosp Pharm. 2016;23(Suppl 1):A44.3-A45. https://doi.org/10.1136/ejhpharm-2016-000875.102 [ Links ]
29. Márquez-Peiró JF, Pérez-Peiró C, Carmena-Carmena J, Jiménez-Torres NV. Identifying improvement opportunities in the management of hepatitis C. Farm Hosp. 2006;30(3):154-60. https://doi.org/10.1016/S1130-6343(06)73966-6 [ Links ]
30. Henderson RR, Visaria J, Bridges GG, Dorholt M, Levin RJ, Frazee SG. Impact of specialty pharmacy on telaprevir-containing 3-drug hepatitis C regimen persistence. J Manag Care Spec Pharm. 2014;20(12):1227-34. https://doi.org/10.18553/jmcp.2014.20.12.1227 [ Links ]
31. Foisy MM, Akai PS. Pharmaceutical care for HIV patients on directly observed therapy. Ann Pharmacother. 2004;38(4):550-6. https://doi.org/10.1345/aph.1D444 [ Links ]
32. Lavitas P, Tesell M, Hydery T, Greenwood BC, Price M, Lenz K, Jeffrey P. Overview of Comprehensive Hepatitis C Virus Medication Management in a State Medicaid Program. J Manag Care Spec Pharm. 2016;22(10):1161-6. https://doi.org/10.18553/jmcp.2016.22.10.1161 [ Links ]
33. Chamorro-de-Vega E, Rodriguez-Gonzalez CG, Gimenez-Manzorro A, de Lorenzo-Pinto A, Iglesias-Peinado I, Herranz A, Sanjurjo M; GRUviC Study Group. Improving pharmacotherapy outcomes in patients with hepatitis C virus infection treated with direct-acting antivirals: The GRUviC project. Int J Clin Pract. 2017;71(8):e12988. https://doi.org/10.1111/ijcp.12988 [ Links ]
34. Kolor B. Patient education and treatment strategies implemented at a pharmacist-managed hepatitis C virus clinic. Pharmacotherapy. 2005;25(9):1230-41. https://doi.org/10.1592/phco.2005.25.9.1230 [ Links ]
35. Zaepfel M, Cristofaro L, Trawinski A, McCarthy K, Rightmier E, Khadem T. Evaluation of a Hepatitis C Patient Management Program at a University Specialty Pharmacy. Ann Pharmacother. 2017;51(4):307-14. https://doi.org/10.1177/1060028016683495 [ Links ]
36. Gomes LO, Teixeira MR, Rosa JAD, Foppa AA, Rover MRM, Farias MR. The benefits of a public pharmacist service in chronic hepattis C treatment: The real-life results of sofosbuvir-based therapy. Res Social Adm Pharm. 2020;16(1):48-53. https://doi.org/10.1016/j.sapharm.2019.02.008 [ Links ]
37. Collado-Borrell R, Lallana-Sainz E, Gimenez-Manzorro A, Ribed-Sanchez A, Lorenzo-Pinto AD, Chamorro E, Romero-Jimenez R, Tovar-Pozo M, Herranz-Alonso A, Sanjurjo-Saez M. PS-020 Drug interactions of new direct acting antiviral agents detected in an intensive pharmaceutical care programme of hepatits C patients. Eur J Hosp Pharm. 2016;23(Suppl 1):A222-3. https://doi.org/10.1136/ejhpharm-2016-000875.505 [ Links ]
38. Masip M, Tuneu L, Pagès N, Torras X, Gallego A, Guardiola JM, Faus MJ, Mangues MA. Prevalence and detection of neuropsychiatric adverse effects during hepatitis C treatment. Int J Clin Pharm. 2015;37(6):1143-51. https://doi.org/10.1007/s11096-015-0177-1 [ Links ]
39. Robustillo M de las A, Almeida-Gonzalez CV, Morillo-Verdugo R. Relación entre la complejidad farmacoterapéutica y la satisfacción del paciente con el tratamiento. Farm Hosp. 2017;(4):470-8. [ Links ]
40. Cañamares Orbis I, Saez de la Fuente J, Izquierdo García E, Esteban Alba C, Such Díaz A, Escobar Rodriguez I. Experiencia autorreferida en pacientes tratados con antivirales directos frente al virus de la hepatitis C. Farm Hosp. 2016;40(6):569-78. [ Links ]
41. Rodríguez-Camacho JM, Vela VV, Fernández MJH, Martín MVM, Bautista MJM, Gil LO. Monitoring of Pharmaceutical Care Hepatitis C program (2007-2011). Eur J Hosp Pharm. 2012;19(2):133.1-133. https://doi.org/10.1136/ejhpharm-2012-000074.127 [ Links ]
42. García-Pelayo M, Montilla E, Fuentes B. CP-079 Pharmaceutical Care for patients with hepatitis C treated with telaprevir. Role in the Regional Hospital. Eur J Hosp Pharm. 2014;21(Suppl 1):A32.3-A33. https://doi.org/10.1136/ejhpharm-2013-000436.78 [ Links ]
43. 44th ESCP International Symposium on Clinical Pharmacy Medicines Information: Making Better Decisions: Lisbon, Portugal, 28-30 October 2015. Int J Clin Pharm. 2016;38(2):470-598. https://doi.org/10.1007/s11096-015-0240-y [ Links ]
44. Yang S, Britt RB, Hashem MG, Brown JN. Outcomes of Pharmacy-Led Hepatitis C Direct-Acting Antiviral Utilization Management at a Veterans Affairs Medical Center. J Manag Care Spec Pharm. 2017;23(3):364-9. https://doi.org/10.18553/jmcp.2017.23.3.364 [ Links ]
45. Margusino-Framiñán L, Cid-Silva P, Mena-de-Cea Á, Sanclaudio-Luhía AI, Castro-Castro JA, Vázquez-González G, Martín-Herranz I. Intelligent MONitoring System for antiviral pharmacotherapy in patients with chronic hepatitis C (SiMON-VC). Farm Hosp. 2017;41(n01):68-88. https://doi.org/10.7399/fh.2017.41.1.10590 [ Links ]
46. Sebhatu P, Martin MT. Genotype 1 hepatitis C virus and the pharmacist’s role in treatment. Am J Health Syst Pharm. 2016;73(11):764-74. https://doi.org/10.2146/ajhp150704 [ Links ]
47. Belperio PS, Backus LI, Ross D, Neuhauser MM, Mole LA. A Population Approach to Disease Management: Hepatitis C Direct-Acting Antiviral Use in a Large Health Care System. J Manag Care Pharm. 2014;20(6):533-40. https://doi.org/10.18553/jmcp.2014.20.6.533 [ Links ]
48. Walters-Smith N, Marshall SM. Opportunities and considerations for pharmacist intervention in the management of the chronic hepatitis C patient. J Manag Care Pharm JMCP. 2009;15(5):417-9. https://doi.org/10.18553/jmcp.2009.15.5.417 [ Links ]
49. Marino EL, Alvarez-Rubio L, Miro S, Modamio P, Banos F, Lastra CF, Alberdi-Leniz A. Pharmacist intervention in treatment of patients with genotype 1 chronic hepatitis C. J Manag Care Pharm JMCP. 2009;15(2):147-50. https://doi.org/10.18553/jmcp.2009.15.2.147 [ Links ]
50. Yeste-Gómez I, Rodríguez-González CG, Giménez-Manzorro Á, Ais-Larisgoitia A, Sanjurjo-Saez M. Information leaflets for patients with hepatitis C receiving treatment with triple therapy. Eur J Hosp Pharm Sci Pract. 2013;20(1):13-9. https://doi.org/10.1136/ejhpharm-2012-000176 [ Links ]
51. Asavakarn S, Sirivatanauksorn Y, Promraj R, Ruenrom A, Limsrichamrern S, Kositamongkol P, Mahawithitwong P, Tovikkai C, Dumronggittigule W. Systematic Pharmaceutical Educational Approach to Enhance Drug Adherence in Liver Transplant Recipients. Transplant Proc. 2016;48(4):1202-7. https://doi.org/10.1016/j.transproceed.2015.12.100 [ Links ]
52. Smith JP, Dong MH, Kaunitz JD. Evaluation of a pharmacist-managed hepatitis C care clinic. Am J Health-Syst Pharm AJHP Off J Am Soc Health-Syst Pharm. 2007;64(6):632-6. https://doi.org/10.2146/ajhp060153 [ Links ]
53. Morillo-Verdugo R, Romero-Gómez M. Un nuevo escenario terapéutico en el tratamiento de la hepatitis crónica por virus C. Farm Hosp. 2012;36(6):466-8. [ Links ]
54. Marcoux RM, Simeone JC, Colavita M, Larrat EP. An Innovative Approach to Pharmacy Management in a State Correctional System. J Correct Health Care. 2012;18(1):53-61. https://doi.org/10.1177/1078345811421732 [ Links ]
55. Rodis JL, Kibbe P. Development of a hepatitis C support group. Am J Health Syst Pharm. 2006;63(17):1594-6. https://doi.org/10.2146/ajhp050481 [ Links ]
56. Hussein M, Benner JS, Lee D, Sesti A-M, Battleman DS, Brock-Wood C. Propensity score matching in the evaluation of drug therapy management programs: an illustrative analysis of a program for patients with hepatitis C virus. Qual Manag Health Care. 2010;19(1):25-33. https://doi.org/10.1097/QMH.0b013e3181ccbc7a [ Links ]
57. Veenstra DL, Higashi MK, Phillips KA. Assessing the cost-effectiveness of pharmacogenomics. AAPS PharmSci. 2000;2(3):80-90. https://doi.org/10.1208/ps020329 [ Links ]
58. Carnevale RC, de Godoi Rezende Costa Molino C, Visacri MB, Mazzola PG, Moriel P. Cost analysis of pharmaceutical care provided to HIV-infected patients: an ambispective controlled study. Daru J Fac Pharm Tehran Univ Med Sci. 2015;23(1):13. https://doi.org/10.1186/s40199-014-0074-5 [ Links ]
59. Menchen B, Folguera C, De rivas A, Saavedra V, Sanchez A. CP-149 Redesign of the management model and pharmaceutical care of patients with hepatitis C virus infection. Eur J Hosp Pharm. 2016;23(Suppl 1):A66.1-A66. https://doi.org/10.1136/ejhpharm-2016-000875.149 [ Links ]
60. Mehta BH, Rodis JL, Nahata MC, Bennett MS. Advancing patient care through innovative practice: The Clinical Partners Program. Am J Health Syst Pharm. 2005;62(23):2501-7. https://doi.org/10.2146/ajhp050017 [ Links ]
61. Ministerio de Salud y Protección Social, Instituto de Evaluación Tecnológica en Salud. Vía clínica para el tratamiento de hepatitis C. Bogotá, D.C: Ministerio de Salud y Protección Social; 2017. [ Links ]
Citation: Ledezma-Morales M, Salazar-Ospina A, Amariles P, Hincapié-García JA. The role of pharmacists in the comprehensive care of patients with hepatitis C: a systematic review. Rev Colomb Gastroenterol. 2020;35(4):485-505. https://doi.org/10.22516/25007440.510
Received: February 07, 2020; Accepted: July 08, 2020











 text in
text in