Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957On-line version ISSN 2500-7440
Rev. colomb. Gastroenterol. vol.35 no.4 Bogotá Oct./Dec. 2020 Epub July 12, 2021
https://doi.org/10.22516/25007440.521
Review article
Endoscopic ultrasound: Current applications to approach gastrointestinal solid tumors
1Unidad de Gastroenterología y endoscopia digestiva, Hospital Pablo Tobón Uribe, Medellín, Colombia.
2Residente de gastroenterología y endoscopia digestiva, Universidad CES, Medellín, Colombia.
3Gastroenterología y endoscopia digestiva, Hospital Universitario San Ignacio, Bogotá. Director de gastroenterología y endoscopia, Clínica de Marly, Bogotá, Colombia.
Endosonography is a diagnostic method that has revolutionized the way to approach patients with tumors in the gastrointestinal tract and other extra-digestive organs and structures. Currently, it is a method of choice to assess subepithelial lesions of the gastrointestinal tract and to classify gastrointestinal tumors in the esophagus, stomach, rectum, and pancreas. Therefore, this literature review presents evidence on the classical indications of endosonography, as well as current applications to approach gastrointestinal tumors.
Keywords: Endosonography; Neoplasm staging; Gastrointestinal neoplasms; Pancreatic neoplasms
La endosonografía es un método diagnóstico que viene revolucionando el abordaje de los pacientes con tumores del tracto gastrointestinal y de otros órganos y estructuras extradigestivas. En la actualidad, se viene posicionando como un método de elección en la evaluación de lesiones subepiteliales gastrointestinales y en la estadificación de muchos tumores gastrointestinales como de esófago, estómago, recto y páncreas. Por lo anterior pretendemos hacer una revisión de tema mostrando la evidencia de la endosonografía en indicaciones clásicas y aplicaciones actuales en tumores gastrointestinales.
Palabras clave: Endosonografía; estadificación de neoplasias; neoplasias gastrointestinales; neoplasias pancreáticas
Introduction
Endoscopic ultrasound (EUS) is a hybrid method in which endoscopy devices are used as a vehicle with a high-resolution ultrasound transducer at its distal end. With this method, endoscopic and ultrasound images that can be used to explore gastrointestinal wall-dependent or extradigestive lesions, taking advantage of the anatomical relationships of the gastrointestinal tract with intra-abdominal organs, are obtained. This technique was created in three institutions in Japan in 1980, where the first prototype was designed, and was later produced by Olympus Co Ltd. EUS was designed to create a diagnostic tool to study small pancreatic carcinoma1. In Colombia, this technique was introduced in 1994 by Dr. Luis Carlos Sabbagh, who, years later, and in response to the need for training in Central and South American countries, founded the Endoscopic Ultrasound Training Center endorsed by the World Gastroenterology Organisation. The use and applications of EUS have spread across Colombia, and there are now large institutions in the country where this method is used by multidisciplinary groups for diagnostic and therapeutic purposes.
Knowledge on and development of this technique has skyrocketed. Higher definition devices and high-resolution transducers are currently available for diagnostic purposes (radial transducer equipment [Figure 1A and C] and linear transducer equipment [Figure 1B and D]) and they are commonly used for performing cytology tests or fine needle biopsies of solid lesions and therapeutic procedures such as drainage of collections and ablation of solid tumors, among others.
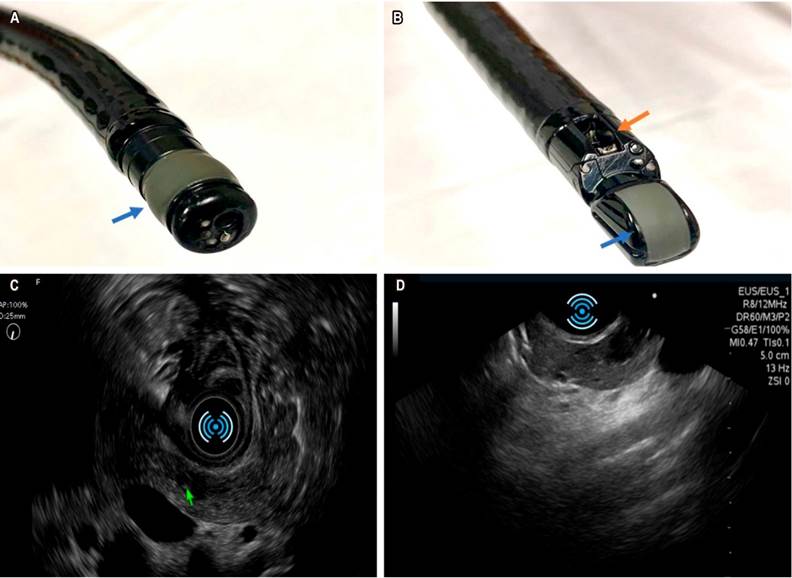
Figure 1 A. Device with radial transducer: endoscopic vision is frontal, sonographic image is radial; blue arrow points to radial transducer. B. Linear transducer: oblique-viewing endoscope, ultrasound sector scanner; the blue arrow points to the transducer and the orange arrow, to the working channel, which is perpendicular to the transducer. C. Radial sonographic vision, documentation of an oval hypoechoic lesion in the neck of the pancreas compatible with a neuroendocrine tumor. D. Linear transducer view of the same lesion in the neck of the pancreas.
The American Gastroenterological Association considers that a gastroenterologist or gastrointestinal surgeon who has completed hands-on training at a teaching facility with a reasonable number of procedures per year, are at an advantage or have better skills to perform this procedure, in addition to having sufficient experience in performing endoscopic retrograde cholangiopancreatography (ERCP). Several scientific societies, such as the American Society for Gastrointestinal Endoscopy (ASGE), the European Society of Gastrointestinal Endoscopy (ESGE), and the British Society of Gastroenterology (BSG), recommend that students carry out between 225-250 procedures under the supervision of a specialist during their training, of which 50 to 75 should be paracentesis2,3.
Endosonography is a safe method in trained hands. When used for diagnostic purposes, this procedure has similar complications to upper gastrointestinal endoscopy. On the other hand, when performed with the linear transducer for therapeutic purposes, there is a 2-fold increased risk of cervical esophageal perforation compared to conventional endoscopy due to increased rigidity, increased diameter of the device, and oblique endoscopic view4,5. In addition, risks are inherent to the target or lesion/collection to be punctured. The most frequently reported adverse events are perforation (0.03 %), bleeding (0.13 %), and acute pancreatitis in paracentesis of the pancreas (between 0 % and 2 %); bile peritonitis occurrence is extremely rare (few case reports)6.
Current uses of EUS
Assessment of subepithelial lesions (SET)
Subepithelial lesions are tumors that emerge from the innermost layers of the muscular layer of the mucosa, the submucosal layer, or the muscular layer of the digestive tract. They are most commonly found in the stomach, where 1 out of every 300 endoscopies may reveal them6. Elevations or protrusions of the mucosa, most of which are less than 2 cm in diameter, are normally discovered by chance during routine endoscopic examinations. In some cases, these lesions may occur in the context of bleeding, obstruction, or metastasis. Studying SETs is important because the endoscopic presentation of these lesions may be similar, and up to 15% of them may be malignant or potentially malignant7. Endosonography is considered the most accurate technique for the assess of subepithelial lesions due to its ability to define histologic layers with certainty and therefore the most likely site of tumor origin (Table 1). Furthermore, it outperforms other imaging modalities such as computed tomography (CT) or magnetic resonance imaging (MRI) in terms of characterization of small lesions (< 2 cm)7 because it allows the accurate differentiation between extrinsic compression of the gastrointestinal tract and intramural growth.
Table 1 Endoscopic and endosonographic characteristics of subepithelial lesions in the digestive tract
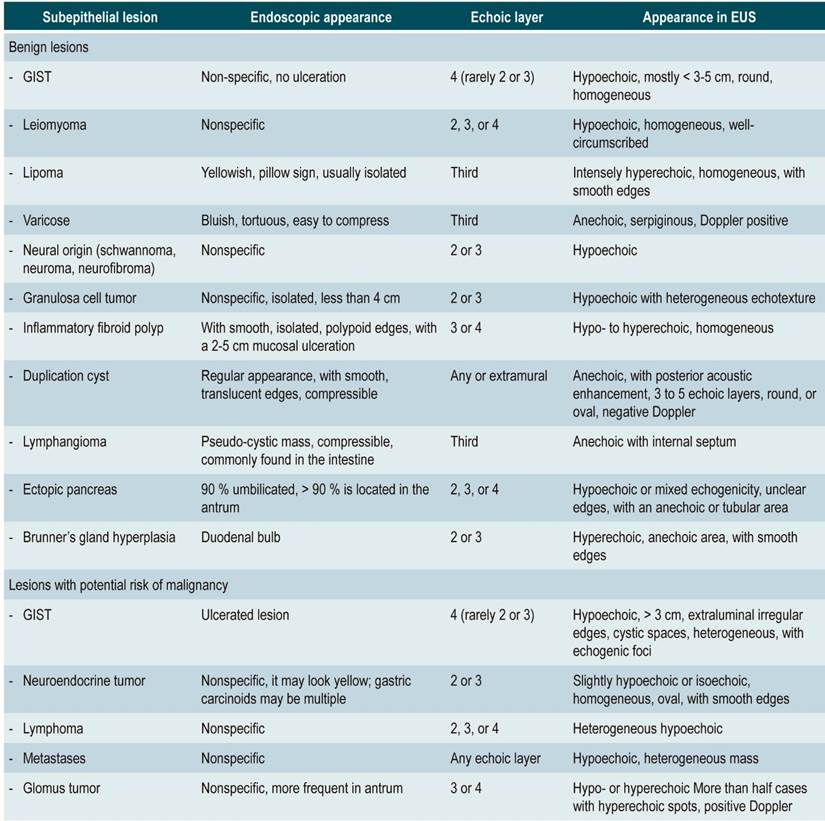
GIST: gastrointestinal stromal tumor. EUS: Endoscopic ultrasound. Adapted from: Standards of Practice Committee et al. The role of endoscopy in subepithelial lesions of the GI tract. Gastrointest Endosc. 2017;85(6):1117-1132.
The five main echoic layers can be identified in gastrointestinal wall lesions: the first and second (mucosa, including the muscular layer of the mucosa), the third (submucosa), the fourth (the muscular layer in muscularis propia) and the fifth (serosa or adventitia) (Figure 2A). Endosonography also allows measuring the extent of the lesion and assessing any underlying lymphadenopathy for staging purposes. Gastrointestinal stromal tumor (GIST) is found in the group of lesions that have the potential to become cancerous. Most of them are gastric (accounting for 60% to 70% of all cases) (Figure 2B), with 20%-30% located in the small intestine and slightly less than 5% in the esophagus. In addition to immunohistochemistry (IHC), the aim of tissue collection in the diagnosis of GIST is to obtain material showing spindle cells. The use of CD117, DOG1, S100, CD34, and PDGFRA stains in IHC is recommended since they allow properly differentiating GIST from other subepithelial lesions8. EUS-guided fine needle aspiration/puncture (EUS-FNA) has been traditionally used for collecting the tissue. Its diagnostic performance is highly variable (between 46% and 93%) and is usually limited because the size of the specimen may be insufficient to perform IHC. A recent multicenter study compared the performance of EUS-guided fine needle biopsy (EUS-FNB) to EUS-FNA regarding cytopathology samples collection, the ability to obtain IHC-based diagnosis, and definitive diagnostic performance. Results in cytopathology samples collection were 92 % vs. 46 % (p = 0.001), 89 % vs. 41 % (p = 0.001) in IHC-based diagnosis, and 89 % vs. 37 % (p = 0.001) in definitive diagnosis between the FNB and the FNA groups, respectively, concluding that EUS-FNB outperforms EUS-FNA in terms of histological diagnostic performance of GIST9.
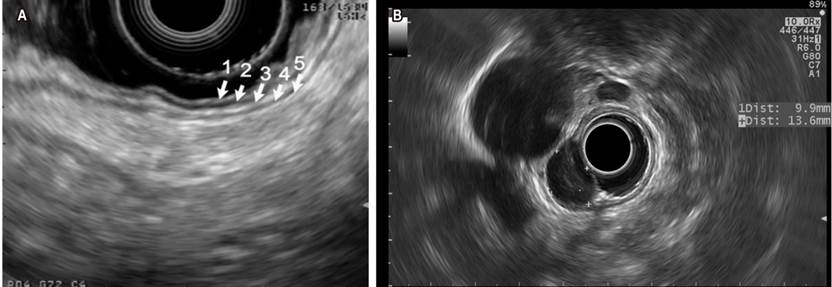
Figure 2 A. Endosonographic view with linear transducer of the echoic layers of the normal gastric wall. B. Homogenous hypoechoic oval lesion corresponding to a GIST of the fourth echoing layer, assessed using a radial transducer. Images taken by Dr. Cañadas, Clínica Marly.
According to a recent study, EUS has an overall accuracy of 64.2% compared to CAT (50.9%). In particular, the accuracy of EUS compared to CAT for diagnosing GIST, leiomyomas, and ectopic pancreas was 83.9% vs. 74.2%, 37.5% vs. 0.0%, and 57.1% vs. 14.3%, respectively. The majority of misdiagnoses in EUS were hypoechoic lesions originating in the fourth echoic layer, and the most common misdiagnosed lesions was GIST, which is mistaken for leiomyoma and vice versa10.
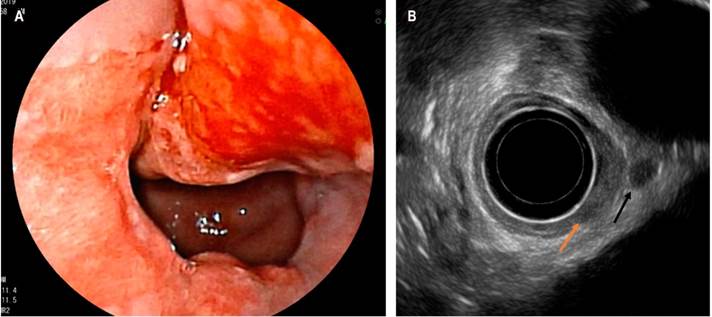
Another current use of EUS is the assessment of depth in subepithelial lesions to determine the need for endoscopic resections. Figure 3 shows a neuroendocrine tumor of the second echo layer that is being treated with a mucocostomy.
Diagnosis and staging of esophageal tumors
Esophageal cancer is the sixth leading cause of cancer worldwide. It is usually diagnosed in patients with long-standing reflux symptoms or dysphagia, who undergo an endoscopy where a biopsy is performed. Sometimes it is diagnosed based on imaging studies such as abdominal CAT scan in the case of abdominal pain or unexplained weight loss or, less commonly, as an incidental finding. For this reason, most esophageal cancer diagnoses are made in advanced stages.
Currently, the Union for International Cancer Control (UICC) classification and the 8th edition of the Tumor, Node, and Metastases (TNM) staging classification are used for the staging of esophageal and gastroesophageal junction tumors. The reclassification of the stages is the most significant difference between the 7th and 8th editions of the TNM staging classification. In the eighth edition, stages IIIB and IIIC (T3-4a N1-3) were reclassified as stage IVA, and M1 disease was reclassified as stage IVB. These patients have a poor prognosis, as poor as that of individuals with metastatic disease. Another change in the 8th edition of the TNM staging was the division of T1 tumors into T1a and T1b tumors. This separation is critical because the risk of nodal metastases increases from 3%-6% for mucosal T1a tumors to 21.24% for submucosal T1b tumors11. This differentiation of T1 tumors is also crucial for guiding decisions about endoscopic vs. surgical treatment12, and the role of EUS is critical in this scenario. Initial staging of esophageal cancer could be done with a contrast-enhanced CAT scan of the chest and abdomen to determine whether the disease is unresectable or detect distant metastases. If the patient is considered to have a potentially curable disease, performing a positron emission tomography (PET scan) is recommended, followed by endosonography. Only patients with an esophageal tumor that spreads to the stomach should undergo diagnostic laparoscopy.
The optimal order in which these imaging studies should be performed is debatable, but most experts agree that a PET scan must be done before an EUS. The detection of distant metastasis in a PET scan would prevent a patient from undergoing an UES, which reduces risks and costs related to the procedure. It has been reported that UES influences treatment decisions in 29% of patients, mainly in the diagnosis of lymph node metastases and in the definition of gross tumor volume during radiation therapy planning12. It has been described that PET scan tumor and disease length measurements vary greatly and tend to produce smaller dimensions13. In conclusion, PET scan is the method of choice for detecting metastases, with a 38 % improvement in diagnostic efficiency over CAT scan14.
In general, EUS is currently regarded as the gold standard for locoregional stage assessment, as it is the method of choice for T and N (Figure 4)12. It also has an advantage over other methods since fine needle punctures or biopsies are possible (thus increasing specificity). It has three drawbacks: it is an operator-dependent procedure that, in most cases, requires sedation, the inherent risks of the technique, and the inability to pass the lesion in the case of stenosing tumors. Regarding the latter aspect, it has been reported that approximately 30% of tumor stenosis cannot be passed; however, this number drops to 3% when using miniprobes15.
Diagnosis and staging of gastric tumors
Gastric cancer is considered the fourth most prevalent cancer in the world and the third in Colombia. It is the cancer with the second highest mortality rate worldwide. Consequently, early diagnosis has a major effect on its prognosis; however, the most common concern is that most people seek medical care when the disease is in an advanced stage. To have a clear idea of the effect of timely diagnosis, 5-year survival rates in European countries range from 10% to 30%, whereas 5-year survival rates in Japan, where mass screening campaigns are performed, can reach 90%16.
EUS is the best method available for evaluating tumors and nodes. In the case of nodes, it performs better than CAT and MRI basically because samples can be collected by puncture or fine needle aspiration biopsy, but it is not very useful for evaluating metastases, so this study is usually ordered as a complement to PET scan, MRI, or CAT scan17.
In a meta-analysis that included 4 397 patients, endosonography was found to be useful in differentiating superficial gastric carcinomas (T1 to T2, with an area under the curve ratio of 0.86) from advanced carcinomas (T3 to T4, with an area under the curve ratio of 0.9). Overall, endosonography has a sensitivity and specificity of 0.85 (95% confidence interval [CI]: 0.78 to 0.91) and 0.90 (95% CI: 0.85 to 0.93) to determine whether the tumor is a carcinoma of the mucosa or an invasive gastric cancer. Somehow, according to this meta-analysis, despite EUS good diagnostic performance, positive and negative likelihood ratio values were insufficient to recommend its use as a single test, and therefore it should be usually performed as a complement to PET scan, MRI, or CAT scan18.
In addition, in a study comparing the diagnostic performance of endosonography versus multidetector computed tomography (MDCT) in locoregional staging of gastric adenocarcinoma and conducted in 77 European surgical patients with gastric adenocarcinoma, of which 42 had complete preoperative staging and were eventually included in the study, the overall accuracy for T staging of endosonography was superior to that of MDCT (62 % vs. 50 %). Likewise, in the subgroup analyses of early (T1-T2) and advanced (T3-T4) stages, accuracy and sensitivity were higher for EUS (83.3% vs. 64.29% and 84.4% vs. 59.5%, respectively), although these differences were not statistically significant. Furthermore, the overall accuracy and sensitivity of EUS for N staging were lower than those of MDCT (57 % vs 64 % and 29 % vs 55 %), although differences were not statistically significant. It was therefore concluded that the diagnostic performance of EUS is comparable to that of the new MDCT technique in terms of preoperative staging of T and N gastric adenocarcinoma; however, both techniques should be considered complementary until randomized studies validating these results in larger sample sizes are conducted19.
Diagnosis of colorectal tumors: high-risk rectal polyps
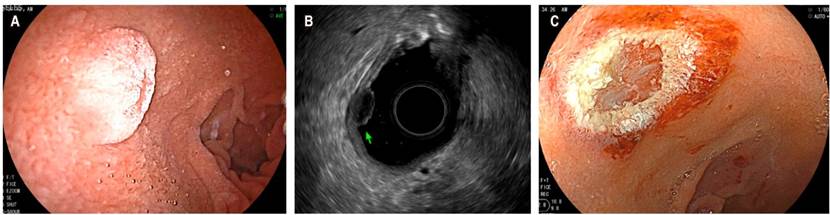
Determining the likelihood of deep-layer invasion in non-pedunculated polypoid rectal lesions larger than 2 cm in diameter is of great importance to choose the best resection technique (endoscopic resection by means of mucosectomy, endoscopic submucosal dissection or minimally invasive surgical techniques). Some experts recommend using complementary imaging studies, such as EUS, to determine the best resection technique in high-risk lesions such as high-grade dysplasia, Paris 0-IIa+c, non-granular lateral extension, Kudo V pit pattern, or in lesions larger than 3 cm20. Lesions with deep submucosal (sm) invasion have an increased risk of nodal metastases (2% for sm1, 8% for sm2, and 23% for sm3), thus, surgical treatment is recommended. In this context, MRI has a variable performance for defining the degree of invasion of polypoid lesions in the rectum, especially in polypoid T1 lesions (pt1), where the accuracy of invasion depth ranges from 25 % to 98 %21. A meta-analysis of 42 studies involving 5 309 patients found that EUS has a sensitivity and specificity of 88% 98% for the staging of T1 rectal lesions, and of 80 % 96 %, respectively for the staging of T2 lesions. Therefore, EUS has been suggested as the best diagnostic technique for early lesions (pt1), while MRI is recommended for assessing pT2 or more advanced lesions22.
The European Society of Gastrointestinal and Abdominal Radiology (ESGAR), in its guidelines for the study of rectal neoplasms, states that EUS is the method of choice for the differentiation and staging of rectal T1 lesions (Figure 5)23. When compared to traditional endoscopy, EUS has been shown to reduce the risk of undiagnosed carcinoma in polypoid rectum lesions from 21% to 3%24. In this context, the use of EUS elastography to measure elastic properties such as tissue hardness has begun to be studied with the aim of distinguishing benign from rectal malignant polyps. In this sense, a study has reported that elastography could allow differentiating benign adenomas from invasive adenocarcinomas with a sensitivity of 96%, a specificity of 86%, and a diagnostic accuracy of 94% when compared with the pathology report25.
Rectal cancer
Proper staging of rectal cancer is crucial to determining the prognosis and the best treatment for the patient. The uT classification is proposed as a staging tool for rectal cancer to identify the T (tumor) (Table 2)26. While EUS might be superior in staging T in early lesions (T1), but its performance is inferior to that of MRI in terms of accuracy in staging T2 and larger lesions22. In this regard, a meta-analysis published in 2004 found that EUS and MRI had comparable diagnostic results in staging N (nodes) in rectal tumors, with a sensitivity and specificity of 67% and 78% and 66% and 76%, respectively27.
Table 2 Rectal cancer staging using EUS (uT Classification)
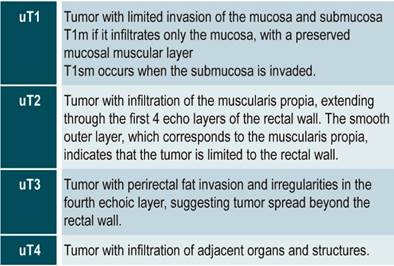
Adapted from: Marone P et al. Role of endoscopic ultrasonography in the loco-regional staging of patients with rectal cancer. World J Gastrointest Endosc. 2015;7(7):688-701.
The revised edition (2020) of the National Comprehensive Cancer Network (NCCN) guidelines suggests that MRI must be performed as the first staging study prior to surgical excision, whether transanal or transabdominal, in polypoid lesions with invasive carcinoma foci undergoing endoscopic resection and whose histopathological sample is fragmented, with undefined edges, or with unfavorable histology. This guideline recommends using EUS if MRI is contraindicated or for assessing superficial lesions28. For initial staging of rectal tumors, pelvic MRI is also recommended as the first staging study and EUS is reserved for patients with MRI contraindications. Regarding follow-up of patients undergoing transanal surgical excision, evaluating anastomosis with EUS or MRI every 3 to 6 months for the first 2 years and then every 6 months for 5 years has been suggested to rule out local relapses28,29.
Diagnosis and staging of biliopancreatic solid tumors
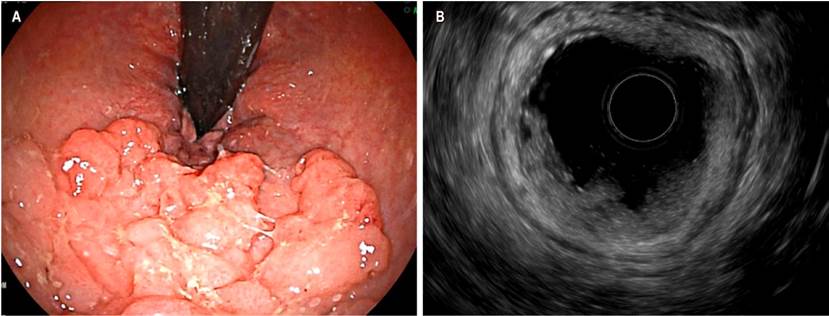
Pancreatic cancer is highly lethal; it is the fourth leading cause of deaths attributed to cancer in the United States. Surgical treatment by duodenopancreatectomy with Whipple technique is a curative option, but only 15% of patients are candidates at the time of clinical presentation. In advanced stages, survival time with chemotherapy is 8.5 months30. EUS has gained ground in the treatment of biliopancreatic disorders, with a diagnostic accuracy ranging from 78 % to 98 %. When FNA or FNB is added to EUS, its diagnostic performance improves. Figure 6 depicts a solid lesion in the head of the pancreas where an FNA was performed.
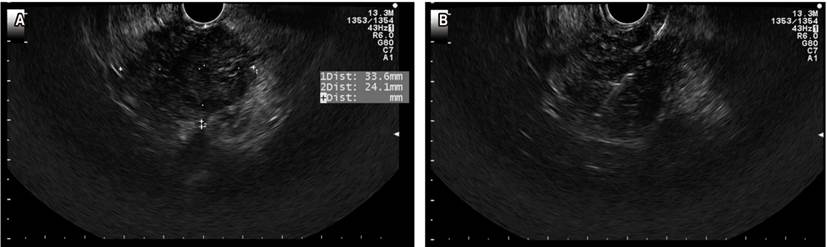
Figure 6 A. Solid lesion in the head of the pancreas. B. FNA of the lesion. Image taken by Dr. Cañadas, Clínica Marly.
Diagnostic accuracy is affected by lesion-specific factors such as location, size, and type; technical aspects such as number of passes; sampling technique (aspiration, slow-pull technique, fanning technique); the endoscopist’s expertise; and the presence of a pathologist in the room where the procedure is performed, which is one of the least studied factors.
EUS-FNA is the method of choice for taking samples from pancreatic solid lesions30-32. It is considered to be more sensitive than abdominal CT and MRI in lesions smaller than 10 mm. It is also a safe and cost-effective method as it provides a high diagnostic performance33. We believe that one of the keys to achieving the best results with EUS-FNA is to obtain sufficient/representative samples of the lesion and properly transfer them to the pathology laboratory. EUS-FNA has been conducted with in-room pathologists in some world reference centers, which has allowed reducing the number of passes and, in general, improving the test diagnostic performance. The HPTU working group in Medellin, Colombia, recently published their preliminary experience with performing biliopancreatic punctures and biopsies with an in-room pathologist (Figure 7). Said study emphasizes that this strategy allowed a high diagnostic performance (close to 90 %) with few false negatives34.
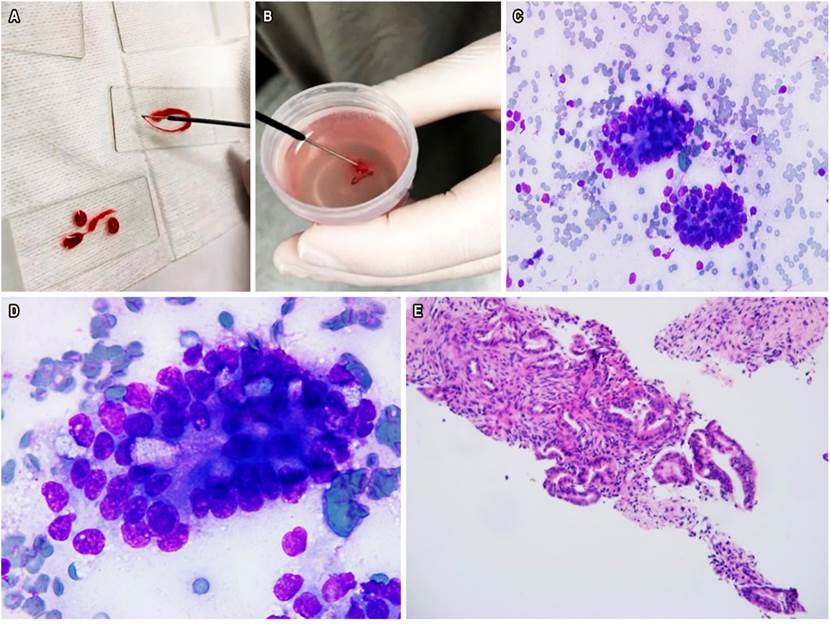
Figure 7 A. Pancreatic tissue in the endoscopy room. B. FNA placed in a jar of formalin. C. Diff-Quik 40x cytology: evidence of pancreatic ductal adenocarcinoma. D. Diff-Quik 100x cytology with ductal adenocarcinoma. E. FNA with evidence of ductal adenocarcinoma. Image taken by Dr. Mosquera-Klinger, HPTU.
Applications under development
Endosonography-guided ablation of neuroendocrine tumors (insulinoma)
Sporadic pancreatic insulinoma is a benign tumor in the β cells of the islets of Langerhans that is normally isolated and less than 20 mm in size. When this type of tumor is functional, patients experience symptoms associated with excessive insulin production. Whipple’s triad (symptoms of hypoglycemia, venous blood hypoglycemia, low plasma glucose with relief of symptoms) is described in these patients. Its clinical diagnosis is supported by biochemical and radiological findings. Treatment is usually surgical, either by duodenopancreatectomy with Whipple technique, distal or total pancreatectomy, or enucleation, depending on size and location.
In 1999, EUS-guided radiofrequency ablation was described in porcine models35; later, cases of treatment in solid pancreatic lesions were reported as a palliative measure in patients with high surgical risk (36-38). EUS-guided ablation is currently being developed as a treatment for pancreatic insulinomas in patients with various comorbidities who are not suitable for surgical treatment or reject undergoing surgery39-43. In Colombia, the first two cases of ethanol ablation were conducted on two women who presented with coma as the first clinical symptom of hyperinsulinemic hypoglycemia and concomitant high level of C-peptide. Abdominal MRI was normal in both cases and was associated with endosonographic findings compatible with a neuroendocrine tumor. In both cases, hypoglycemia resolved immediately after ablation, with no complications and complete resolution of symptoms (at 12 months of follow-up)44.
EUS-guided splenic punctures
The risk of splenic punctures has restricted histological analysis of focal splenic lesions or splenomegaly of unknown origin. The most serious adverse event is bleeding (hemoperitoneum or subcapsular hematoma), but lesions of neighboring structures such as pleura, lung, splenic flexure of colon and vascular lesions in general may also occur45,46. Since laparoscopic splenic biopsies require general anesthesia and are limited to cases with noticeable lesions on the surface of the organ, non-invasive approaches are preferred over surgery. In terms of diagnostic performance, in a study published by Werner et al., diagnosis was achieved in almost 70% of patients in which biopsies were performed via laparoscopy47. Dr. Soderstrom published a paper on percutaneous needle aspiration biopsies that included over 1 000 blind aspiration biopsies and no significant complications were reported48. Subsequently, several manuscripts have described the use of percutaneous fine needle aspiration biopsies guided by ultrasound and CT in benign and malignant splenic diseases, reporting good efficacy and safety results49-51. In this sense, a multicenter study involving 398 patients who underwent percutaneous ultrasound guided FNA in Italy45, reports complications were observed in only 5.2 % of participants. Percutaneous biopsies have limitations in obese patients, in those with a history of abdominal surgery and of ascites; in addition, a highly experienced interventional radiologist is required in all cases where this type of biopsy is performed.
Due to the spleen’s proximity to the gastric wall, use of EUS for fine-needle aspiration biopsies of the spleen is possible, and its benefit is a real-time visualization of the needle and its movements. Recently, the experience of European center using this technique was published; in said study, a definitive histopathological diagnosis was obtained in 66.7 % of all cases, and half of them were associated with splenic lymphoma. Also, one of the most relevant findings of this study was that there were no associated complications, concluding that EUS-guided FNA of the spleen is necessary or the first option in patients with suspected malignancy or lesions of uncertain origin in the context of splenomegaly or suspicious or small-sized space-occupying benign lesions in the spleen that cannot be found with percutaneous biopsies52.
Conclusions
Endosonography has a wide variety of uses in the detection and staging of gastrointestinal tumors. It complements other imaging modalities such as CAT scans, MRIs, and PET scans in the staging of esophageal, stomach, and rectal neoplasms. For these three neoplasms, it performs best in staging T (tumor) and N (ganglion).
In addition, it is the method of choice in the evaluation of subepithelial lesions because it can discriminate, with high specificity, the affected echoing layer and characterize the type, size, and location of the lesions.
It also is the procedure with the best diagnostic performance in pancreatic lesions smaller than 10 mm, increasing its specificity thanks to the possibility of performing a biopsy through needle puncture and aspiration of the lesion.
EUS-guided ablation is a promising treatment option for functional pancreatic insulinoma in patients not suitable for surgical management or who reject undergoing surgery. Finally, a new application of endosonography is EUS-guided punctures and biopsies of the spleen in patients with fever of unknown origin, unexplained weight loss and who have space-occupying lesions or splenomegaly of unknown origin
REFERENCES
1. Yasuda K. The Handbook of endoscopic ultrasonography in digestive tract. 1st edition. New York: Blackwell; 2000. [ Links ]
2. Polkowski M, Larghi A, Weynand B, Boustière C, Giovannini M, Pujol B, Dumonceau JM; European Society of Gastrointestinal Endoscopy (ESGE). Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline. Endoscopy. 2012;44(2):190-205. https://doi.org/10.1055/s-0031-1291543 [ Links ]
3. Wani S, Keswani RN, Petersen B, Edmundowicz SA, Walsh CM, Huang C, Cohen J, Cote G. Training in EUS and ERCP: standardizing methods to assess competence. Gastrointest Endosc. 2018;87(6):1371-1382. https://doi.org/10.1016/j.gie.2018.02.009 [ Links ]
4. Eloubeidi MA, Tamhane A, Lopes TL, Morgan DE, Cerfolio RJ. Cervical esophageal perforations at the time of endoscopic ultrasound: a prospective evaluation of frequency, outcomes, and patient management. Am J Gastroenterol. 2009;104(1):53-6. https://doi.org/10.1038/ajg.2008.21 [ Links ]
5. Mosquera-Klinger G, Torres Rincón R. Perforación iatrogénica faringoesofágica tratada con prótesis esofágica totalmente cubierta: reporte de caso. Gastroenterol Hepatol. 2019;42(7):429-30. https://doi.org/10.1016/j.gastrohep.2018.08.008 [ Links ]
6. ASGE Standards of Practice Committee, Early DS, Acosta RD, Chandrasekhara V, Chathadi KV, Decker GA, Evans JA, Fanelli RD, Fisher DA, Fonkalsrud L, Hwang JH, Jue TL, Khashab MA, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Sharaf RN, Shergill AK, Cash BD. Adverse events associated with EUS and EUS with FNA. Gastrointest Endosc. 2013;77(6):839-43. https://doi.org/10.1016/j.gie.2013.02.018 [ Links ]
7. Standards of Practice Committee, Faulx AL, Kothari S, Acosta RD, Agrawal D, Bruining DH, Chandrasekhara V, Eloubeidi MA, Fanelli RD, Gurudu SR, Khashab MA, Lightdale JR, Muthusamy VR, Shaukat A, Qumseya BJ, Wang A, Wani SB, Yang J, DeWitt JM. The role of endoscopy in subepithelial lesions of the GI tract. Gastrointest Endosc. 2017;85(6):1117-1132. https://doi.org/10.1016/j.gie.2017.02.022 [ Links ]
8. Polkowski M. Endoscopic ultrasound and endoscopic ultrasound- guided fine-needle biopsy for the diagnosis of malignant submucosal tumors. Endoscopy 2005;37(7):635-45. https://doi.org/10.1055/s-2005-861422 [ Links ]
9. Nishida T, Blay JY, Hirota S, Kitagawa Y, Kang YK. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. 2016;19(1):3-14. https://doi.org/10.1007/s10120-015-0526-8 [ Links ]
10. Trindade AJ, Benias PC, Alshelleh M, Bazarbashi AN, Tharian B, Inamdar S, Sharma N, Zelt C, Korrapati P, Barakat M, Sejpal DV, Ryou M. Fine-needle biopsy is superior to fine-needle aspiration of suspected gastrointestinal stromal tumors: a large multicenter study. Endosc Int Open. 2019;7(7):E931-E936. https://doi.org/10.1055/a-0953-1640 [ Links ]
11. Kim SY, Shim KN, Lee JH, Lim JY, Kim TO, Choe AR, Tae CH, Jung HK, Moon CM, Kim SE, Jung SA. Comparison of the Diagnostic Ability of Endoscopic Ultrasonography and Abdominopelvic Computed Tomography in the Diagnosis of Gastric Subepithelial Tumors. Clin Endosc. 2019;52(6):565-573. https://doi.org/10.5946/ce.2019.019 [ Links ]
12. Sabik JF, Rice TW, Goldblum JR, Koka A, Kirby TJ, Medendorp SV, Adelstein DJ. Superficial esophageal carcinoma. Ann Thorac Surg. 1995;60(4):896-901; discussion 902. https://doi.org/10.1016/0003-4975(95)00542-s [ Links ]
13. Hulshoff JB, Mul VEM, de Boer HEM, Noordzij W, Korteweg T, van Dullemen HM, Nagengast WB, Oppedijk V, Pierie JPEN, Plukker JTM. Impact of Endoscopic Ultrasonography on 18F-FDG-PET/CT Upfront Towards Patient Specific Esophageal Cancer Treatment. Ann Surg Oncol. 2017;24(7):1828-1834. https://doi.org/10.1245/s10434-017-5835-1 [ Links ]
14. Foley KG, Morgan C, Roberts SA, Crosby T. Impact of Positron Emission Tomography and Endoscopic Ultrasound Length of Disease Difference on Treatment Planning in Patients with Oesophageal Cancer. Clin Oncol (R Coll Radiol). 2017;29(11):760-766. https://doi.org/10.1016/j.clon.2017.07.014 [ Links ]
15. Foley K, Findlay J, Goh V. Novel imaging techniques in staging oesophageal cancer. Best Pract Res Clin Gastroenterol. 2018;36-37:17-25. https://doi.org/10.1016/j.bpg.2018.11.009 [ Links ]
16. Morgan MA, Twine CP, Lewis WG, Lambe R, Oliphant HE, Robinson M, Crosby TD, Roberts SA. Prognostic significance of failure to cross esophageal tumors by endoluminal ultrasound. Dis Esophagus. 2008;21(6):508-13. https://doi.org/10.1111/j.1442-2050.2008.00809.x [ Links ]
17. Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239-248. https://doi.org/10.2147/CMAR.S149619 [ Links ]
18. Mocellin S, Pasquali S. Diagnostic accuracy of endoscopic ultrasonography (EUS) for the preoperative locoregional staging of primary gastric cancer. Cochrane Database Syst Rev. 2015;2015(2):CD009944. https://doi.org/10.1002/14651858.CD009944.pub2 [ Links ]
19. Cimavilla Román M, de la Serna Higuera C, Loza Vargas LA, Benito Fernández C, Barrio Andrés J, Madrigal Rubiales B, Fernández Pérez G, Pérez-Miranda M. Endoscopic ultrasound versus multidetector computed tomography in preoperative gastric cancer staging. Rev Esp Enferm Dig. 2017;109(11):761-767. https://doi.org/10.17235/reed.2017.4638/2016 [ Links ]
20. Banerjee AK, Longcroft-Wheaton G, Beable R, Conti J, Khan J, Bhandari P. The role of imaging and biopsy in the management and staging of large non-pedunculated rectal polyps. Expert Rev Gastroenterol Hepatol. 2018;12(8):749-755. https://doi.org/10.1080/17474124.2018.1492377 [ Links ]
21. Fuchsjäger MH, Maier AG, Schima W, Zebedin E, Herbst F, Mittlböck M, Wrba F, Lechner GL. Comparison of transrectal sonography and double-contrast MR imaging when staging rectal cancer. AJR Am J Roentgenol. 2003;181(2):421-7. https://doi.org/10.2214/ajr.181.2.1810421 [ Links ]
22. Puli SR, Bechtold ML, Reddy JB, Choudhary A, Antillon MR, Brugge WR. How good is endoscopic ultrasound in differentiating various T stages of rectal cancer? Meta-analysis and systematic review. Ann Surg Oncol. 2009;16(2):254-65. https://doi.org/10.1245/s10434-008-0231-5 [ Links ]
23. Beets-Tan RGH, Lambregts DMJ, Maas M, Bipat S, Barbaro B, Curvo-Semedo L, Fenlon HM, Gollub MJ, Gourtsoyianni S, Halligan S, Hoeffel C, Kim SH, Laghi A, Maier A, Rafaelsen SR, Stoker J, Taylor SA, Torkzad MR, Blomqvist L. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2018;28(4):1465-1475. https://doi.org/10.1007/s00330-017-5026-2 [ Links ]
24. Worrell S, Horvath K, Blakemore T, Flum D. Endorectal ultrasound detection of focal carcinoma within rectal adenomas. Am J Surg. 2004;187(5):625-9; discussion 629. https://doi.org/10.1016/j.amjsurg.2004.01.005 [ Links ]
25. Waage JE, Leh S, Røsler C, Pfeffer F, Bach SP, Havre RF, Haldorsen IS, Ødegaard S, Baatrup G. Endorectal ultrasonography, strain elastography and MRI differentiation of rectal adenomas and adenocarcinomas. Colorectal Dis. 2015;17(2):124-31. https://doi.org/10.1111/codi.12845 [ Links ]
26. Marone P, de Bellis M, D’Angelo V, Delrio P, Passananti V, Di Girolamo E, Rossi GB, Rega D, Tracey MC, Tempesta AM. Role of endoscopic ultrasonography in the loco-regional staging of patients with rectal cancer. World J Gastrointest Endosc. 2015;7(7):688-701. https://doi.org/10.4253/wjge.v7.i7.688 [ Links ]
27. Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging--a meta-analysis. Radiology. 2004;232(3):773-83. https://doi.org/10.1148/radiol.2323031368 [ Links ]
28. Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Garrido-Laguna I, Grem JL, Gunn A, Hoffe S, Hubbard J, Hunt S, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Johnson-Chilla A, Gurski LA. NCCN Guidelines Insights: Rectal Cancer, Version 6.2020. J Natl Compr Canc Netw. 2020 Jul;18(7):806-815. [ Links ]
29. Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Engstrom PF, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wuthrick E, Gregory KM, Gurski L, Freedman-Cass DA. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(7):874-901. https://doi.org/10.6004/jnccn.2018.0061 [ Links ]
30. Bhutani MS, Koduru P, Joshi V, Saxena P, Suzuki R, Irisawa A, Yamao K. The role of endoscopic ultrasound in pancreatic cancer screening. Endosc Ultrasound. 2016;5(1):8-16. https://doi.org/10.4103/2303-9027.175876 [ Links ]
31. Uehara H, Ikezawa K, Kawada N, Fukutake N, Katayama K, Takakura R, Takano Y, Ishikawa O, Takenaka A. Diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration for suspected pancreatic malignancy in relation to the size of lesions. J Gastroenterol Hepatol. 2011;26(8):1256-61. https://doi.org/10.1111/j.1440-1746.2011.06747.x [ Links ]
32. Hewitt MJ, McPhail MJ, Possamai L, Dhar A, Vlavianos P, Monahan KJ. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012;75(2):319-31. https://doi.org/10.1016/j.gie.2011.08.049 [ Links ]
33. Ayres LR, Kmiotek EK, Lam E, Telford JJ. A Comparison of Endoscopic Ultrasound-Guided Fine-Needle Aspiration and Fine-Needle Biopsy in the Diagnosis of Solid Pancreatic Lesions. Can J Gastroenterol Hepatol. 2018;2018:1415062. https://doi.org/10.1155/2018/1415062 [ Links ]
34. Mosquera-Klinger G, Carvajal JJ, Echeverri C, Pérez JC, Cardona R, Valencia-Maturana J, Sánchez-Garrido H. Rendimiento diagnóstico de las punciones de lesiones biliopancreáticas guiadas por ultrasonido endoscopio con patólogo en sala. En prensa. [ Links ]
35. Goldberg SN, Mallery S, Gazelle GS, Brugge WR. EUS-guided radiofrequency ablation in the pancreas: results in a porcine model. Gastrointest Endosc. 1999;50(3):392-401. https://doi.org/10.1053/ge.1999.v50.98847 [ Links ]
36. Wu Y, Tang Z, Fang H, Gao S, Chen J, Wang Y, Yan H. High operative risk of cool-tip radiofrequency ablation for unresectable pancreatic head cancer. J Surg Oncol. 2006;94(5):392-5. https://doi.org/10.1002/jso.20580 [ Links ]
37. Spiliotis JD, Datsis AC, Michalopoulos NV, Kekelos SP, Vaxevanidou A, Rogdakis AG, Christopoulou AN. Radiofrequency ablation combined with palliative surgery may prolong survival of patients with advanced cancer of the pancreas. Langenbecks Arch Surg. 2007;392(1):55-60. https://doi.org/10.1007/s00423-006-0098-5 [ Links ]
38. Elias D, Baton O, Sideris L, Lasser P, Pocard M. Necrotizing pancreatitis after radiofrequency destruction of pancreatic tumours. Eur J Surg Oncol. 2004;30(1):85-7. https://doi.org/10.1016/j.ejso.2003.10.013 [ Links ]
39. Lakhtakia S, Ramchandani M, Galasso D, Gupta R, Venugopal S, Kalpala R, Reddy DN. EUS-guided radiofrequency ablation for management of pancreatic insulinoma by using a novel needle electrode (with videos). Gastrointest Endosc. 2016;83(1):234-9. https://doi.org/10.1016/j.gie.2015.08.085 [ Links ]
40. Waung JA, Todd JF, Keane MG, Pereira SP. Successful management of a sporadic pancreatic insulinoma by endoscopic ultrasound-guided radiofrequency ablation. Endoscopy. 2016;48 Suppl 1:E144-5. https://doi.org/10.1055/s-0042-104650 [ Links ]
41. Limmer S, Huppert PE, Juette V, Lenhart A, Welte M, Wietholtz H. Radiofrequency ablation of solitary pancreatic insulinoma in a patient with episodes of severe hypoglycemia. Eur J Gastroenterol Hepatol. 2009;21(9):1097-101. https://doi.org/10.1097/MEG.0b013e328323d70e [ Links ]
42. Pai M, Habib N, Senturk H, Lakhtakia S, Reddy N, Cicinnati VR, Kaba I, Beckebaum S, Drymousis P, Kahaleh M, Brugge W. Endoscopic ultrasound guided radiofrequency ablation, for pancreatic cystic neoplasms and neuroendocrine tumors. World J Gastrointest Surg. 2015;7(4):52-9. https://doi.org/10.4240/wjgs.v7.i4.52 [ Links ]
43. Rossi S, Viera FT, Ghittoni G, Cobianchi L, Rosa LL, Siciliani L, Bortolotto C, Veronese L, Vercelli A, Gallotti A, Ravetta V. Radiofrequency ablation of pancreatic neuroendocrine tumors: a pilot study of feasibility, efficacy, and safety. Pancreas. 2014;43(6):938-45. https://doi.org/10.1097/MPA.0000000000000133 [ Links ]
44. Mosquera-Klinger G, Carvajal JJ. Insulinoma pancreático sintomático: Tratamiento ablativo mediante etanolización guiado por ultrasonido endoscópico. Rev Esp Enf Dig. En prensa, 2020. https://doi.org/10.17235/reed.2020.7109/2020 [ Links ]
45. Civardi G, Vallisa D, Bertè R, Giorgio A, Filice C, Caremani M, Caturelli E, Pompili M, De Sio I, Buscarini E, Cavanna L. Ultrasound-guided fine needle biopsy of the spleen: high clinical efficacy and low risk in a multicenter Italian study. Am J Hematol. 2001;67(2):93-9. https://doi.org/10.1002/ajh.1085 [ Links ]
46. Quinn SF, vanSonnenberg E, Casola G, Wittich GR, Neff CC. Interventional radiology in the spleen. Radiology. 1986;161(2):289-91. https://doi.org/10.1148/radiology.161.2.3763890 [ Links ]
47. Werner T, Koch J, Frenzel C, Lohse AW, Denzer UW. Effectiveness and safety of minilaparoscopy-guided spleen biopsy: a retrospective series of 57 cases. Surg Endosc. 2012;26(9):2416-22. https://doi.org/10.1007/s00464-012-2190-y [ Links ]
48. Söderström N. How to use cytodiagnostic spleen puncture. Acta Med Scand. 1976;199(1-2):1-5. https://doi.org/10.1111/j.0954-6820.1976.tb06683.x [ Links ]
49. Solbiati L, Bossi MC, Bellotti E, Ravetto C, Montali G. Focal lesions in the spleen: sonographic patterns and guided biopsy. AJR Am J Roentgenol. 1983;140(1):59-65. https://doi.org/10.2214/ajr.140.1.59 [ Links ]
50. Silverman JF, Geisinger KR, Raab SS, Stanley MW. Fine needle aspiration biopsy of the spleen in the evaluation of neoplastic disorders. Acta Cytol. 1993;37(2):158-62. [ Links ]
51. Zeppa P, Vetrani A, Luciano L, Fulciniti F, Troncone G, Rotoli B, Palombini L. Fine needle aspiration biopsy of the spleen. A useful procedure in the diagnosis of splenomegaly. Acta Cytol. 1994;38(3):299-309. [ Links ]
52. Mosquera-Klinger G, de la Serna Higuera C, Bazaga S, García-Alonso FJ, Sánchez Ocaña R, Antolín Melero B, de Benito Sanz M, Madrigal B, Torres Á, Pérez-Miranda M. Endoscopic ultrasound-guided fine-needle aspiration for splenomegaly and focal splenic lesion: is it safe, effective and necessary? Rev Esp Enferm Dig. 2020;112(5):355-359. https://doi.org/10.17235/reed.2020.6667/2019 [ Links ]
Citation: Mosquera-Klinger G, Carvajal-Gutiérrez JJ, Gómez-Venegas AA, Niño-Ramírez S, Cañadas-Garrido R. Endoscopic ultrasound: Current applications to approach gastrointestinal solid tumors. Rev Colomb Gastroenterol. 2020;35(4):506-518. https://doi.org/10.22516/25007440.521
Received: March 16, 2020; Accepted: May 18, 2020











 text in
text in