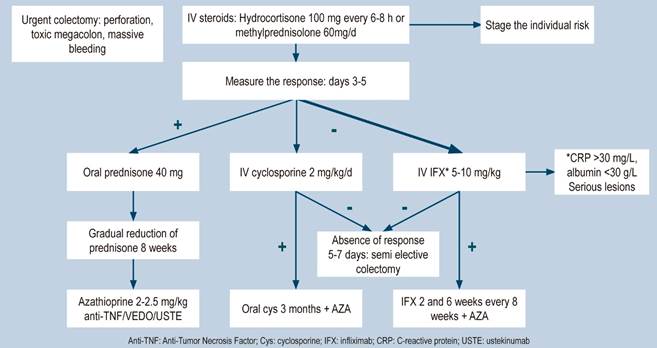
Rationale
Ulcerative colitis (UC) is an incurable chronic multifactorial disease of unknown etiology characterized by the presence of diffuse inflammation in the colonic mucosa in the absence of granulomas. UC affects the rectum and extends proximally in a variable, symmetrical and circumferential fashion along the colon. According to the relevant literature, 75% of the patients with left-sided UC may develop peri-appendiceal inflammation, also known as cecal patch, and 20% of those with UC that has extended to the cecum may develop inflammation of the distal ileum or “backwash ileitis”. The clinical course of UC is intermittent, as it is characterized by remission and relapse periods; usual symptoms of UC include bloody diarrhea, frequently associated with urgency to defecate, and rectal tenesmus1; in the case of extensive colitis, systemic symptoms are also present1,2.
Since it was first described at the end of the 19th century, its prevalence and incidence have constantly changed, as it has been the case of other immunological diseases3. Historically, the highest incidence and prevalence rates of UC have been reported in studies conducted in Scandinavian countries, the United Kingdom, and North America4. Its incidence ranges from 1.2 to 20.3 cases per 100,000 people/year, and its prevalence, from 156 to 291 cases per 100,000 people. According to a systematic review that included 147 studies, the countries with the highest prevalence of UC are found in Europe (up to 505 per 100,000 people in Norway) and North America (286 per 100,000 people)5. In the case of Colombia, a prevalence of 67.07 per 100,000 people and an annual incidence of 15.22 per 100,000 people were reported for 20176.
Regarding age, a bimodal model of UC onset has been described, with a first peak of onset between the ages of 15 and 30 years, and a second peak between the ages of 50 and 70 years. Having a family history of inflammatory bowel disease is the most important independent risk factor, since 5.7% to 15.5% of patients with UC have a first-degree relative with this disease7. In addition, it has been consistently found that smoking has a negative association with UC (“protective factor”), with an odds ratio (OR) of 0.58 (95% confidence interval [CI]: 0.45-0.75), as shown in a meta-analysis8. UC patients who smoke have milder symptoms compared to nonsmokers. Appendectomy is also a protective factor for the development of UC: according to a meta-analysis, a 69% risk reduction (OR: 0.31; 95% CI: 0.25-0.38) was found in people undergoing this procedure9. Helicobacter pylori (H. pylori) has also been found to be negatively associated with UC, with an OR of 0.59 (95% CI: 0.39-0.84) and, conversely, the absence of H. pylori has a risk of 11.06 (95% CI: 7.98-15.02) for developing it. The cause of this negative association is unknown, but the higher number of regulatory T lymphocytes in patients with H. pylori might play an important role10.
All studies about UC have found that it has a negative impact on the quality of life of those who develop it, as it seriously affects their work performance and health conditions. In most patients, UC is not timely diagnosed, and in up to half of the cases, diagnosis is made 1 year after the onset of symptoms11. In the case of Colombia, an observational study conducted in the city of Medellin reported that UC diagnosis was reached, on average, 9.2 months after the onset of symptoms12.
UC is diagnosed based on the patient’s medical record, physical examination findings, endoscopic or radiological alterations, laboratory tests and histopathological findings characteristic of the disease. Diagnosis is confirmed through a biopsy when the other manifestations of UC are present and, depending on the case, the presence of infectious (bacteria, viruses, parasites and fungi) and non-infectious causes of diarrhea (microscopic colitis, bile acid malabsorption, bacterial overgrowth, neoplasms or drug-induced causes, among others) have been ruled out. It should be noted that the diagnosis of UC cannot be reached based only on the biopsy results, that is, in the absence of other manifestations1,13.
The Montreal Classification for Inflammatory Bowel Disease, which was created in 2005 through a consensus of experts, allows classifying UC according to its extent and severity14. Recently, the American College of Gastroenterology proposed a new classification of UC activity, modifying the traditional classification by Truelove and Witts15 by adding biomarkers such as C-reactive protein, calprotectin and endoscopic severity16.
It has been reported that 40% of patients with a de novo diagnosis of UC only develop proctitis, in 30%-40% the disease affects the left colon, and 20%-30% develop pancolitis, being the latter those with a worse prognosis. In about 80% of patients UC activity is mild to moderate at the time of onset17. A recent systematic review that included 30 eligible studies reported that the pooled frequency of colonic extension of UC was 17.8% and 31% at 5 years and 10 years of follow-up, respectively18. Furthermore, a recent systematic review and meta-analysis found that the risk of surgery after being diagnosed with UC was 4.9%, 11.6%, and 15.6%, at 1, 5, and 10 years, respectively; likewise, according to this review, the risk of surgery in UC patients has decreased over the last years19.
UC is treated with pharmacological interventions and, in specific cases, with surgery. However, and despite there are multiple randomized studies, some of the clinical settings taking place in patients with this disease continue to be managed according to clinical judgment and expert opinion, which is reflected in the conceptual differences regarding the treatment of these patients1,2,15,20,21.
In 2015, the Colombian Association of Gastroenterology, with the support of the Clinical Research Institute of the Universidad Nacional de Colombia, developed the Evidence-based clinical practice guideline for the diagnosis and treatment of ulcerative colitis in adults. Since the publication of said guideline, new therapeutic alternatives and new concepts about UC treatment targets have emerged, so updating it by including new recommendations for the management of UC and surveillance colonoscopy in adult patients with this disease in the Colombian context was deemed necessary.
OBJECTIVES
This evidence-based clinical practice guideline (GPC) was developed taking into account the following objectives:
To decrease the unjustified variability that takes place in UC treatment, thus contributing to the rational and relevant use of the resources allocated for the provision of care to patients with UC.
To guide the management of UC at different stages of the disease and in different levels of care.
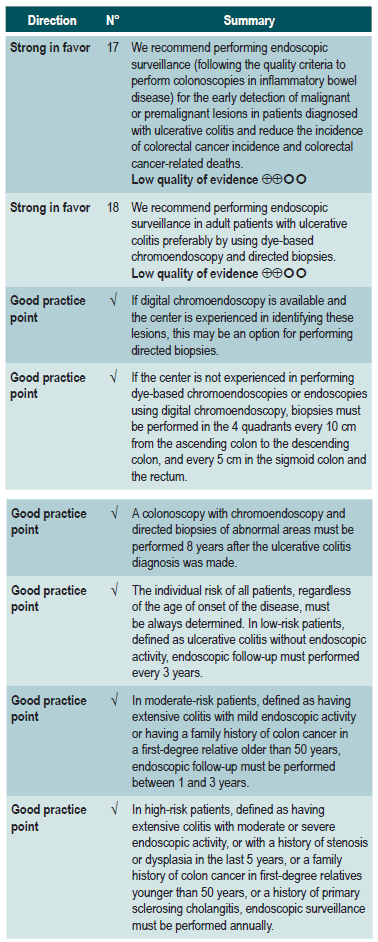
To establish recommendations for the colonoscopic surveillance of colorectal cancer in adults with UC.
POPULATION
Patients to be considered in this clinical practice guideline
Patients older than 16 years diagnosed with UC, regardless of the time of progression and the clinical stage of the disease.
Patients that are not considered in this clinical practice guideline
Patients with Crohn’s disease.
Patients with indeterminate inflammatory bowel disease.
Patients with extraintestinal manifestations of UC.
Patients with side and/or adverse effects resulting from UC treatment.
Pregnant women or nursing mothers with UC.
Patients with infectious colitis.
Patients with a non-confirmed or an uncertain diagnosis of UC.
Pediatric patients under 16 years of age. These patients should be included in a pediatric-to-adult transition care program one year before they turn 16 years old.
HEALTH CARE PROVISION SETTING
This CPG aims to help medical care providers treating patients older than 16 years diagnosed with UC in any level of care. It should be noted that the management of very specific conditions by health care professionals involved in the care of patients with UC requires specific recommendations, which are beyond the scope of this guideline.
USERS OF THE CLINICAL PRACTICE GUIDELINE
This CPG is intended for gastroenterologists, colorectal surgeons (coloproctologists), gastrointestinal surgeons, internal medicine specialists, family medicine specialists, general practitioners, as well as for patients and other health care professionals interested in the management of UC. It can also be used by health care decision makers both in the context of health care provision and health insurance companies, health care payers, and health care policy makers.
FUNDING OF THE CLINICAL PRACTICE GUIDELINE
The development of this CPG was funded by the Colombian Association of Gastroenterology.
MAIN CLINICAL ASPECTS
This CPG will address the medical treatment and endoscopic surveillance of UC. Aspects related to the prognosis or rehabilitation of UC patients will not be addressed.
METHODOLOGY
The GRADE methods for the rapid development of CPGs proposed by the Pan American Health Organization (PAHO) in the Strengthening national evidence-informed guideline programs. A tool for adapting and implementing guidelines in the Americas document22 were followed for the development of this CPG.
COMPOSITION OF THE GROUP IN CHARGE OF THE DEVELOPMENT OF THE CLINICAL PRACTICE GUIDELINE
The group in charge of the development of the clinical practice guideline (GDG) was composed by experts in gastroenterology, colorectal surgery, gastrointestinal surgery, internal medicine, as well as by general practitioners and patients.
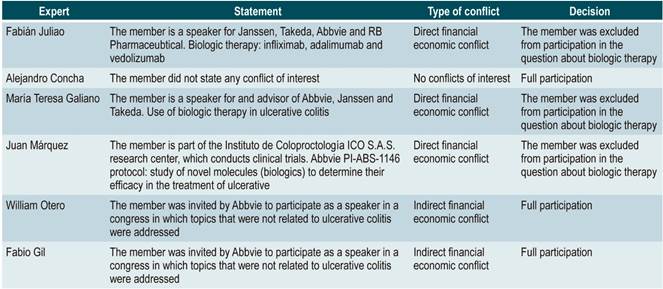
CONFLICTS OF INTEREST STATEMENT
All members of the GDG, of the expert panel, as well as any people involved in the external review of the clinical practice guideline, signed a conflicts of interest form. An analysis of the conflicts of interest was carried out and, based on the conflict or conflicts stated, partial or full participation in the development of the guideline was decided. This analysis is available in Annex 1.
EDITORIAL INDEPENDENCE STATEMENT
It is hereby stated that the Fundación Hospital Pediátrico La Misericordia (HOMI) did not have any influence on the contents of this CPG.
DECISION ABOUT UPDATING THE CLINICAL PRACTICE GUIDELINE
In 2015, the Colombian Association of Gastroenterology, Cochrane STI and the Universidad Nacional de Colombia developed the Evidence-based guideline for the management of ulcerative colitis in adults. The GDG decided by consensus that the recommendations regarding the diagnosis and surgical management of UC made in the said guideline are still valid and do not need to be updated. Therefore, 3 questions were updated:
What is the efficacy and safety of therapeutic interventions for the induction and maintenance of remission in adult patients with UC?
What is the efficacy and safety of biologic therapy for the treatment of patients with moderate to severe UC?
What is the efficacy of colorectal cancer screening and endoscopic surveillance in adult patients with UC?
UPDATING THE CLINICAL QUESTIONS OF THE CLINICAL PRACTICE GUIDELINE
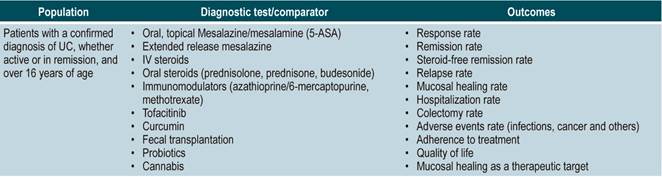
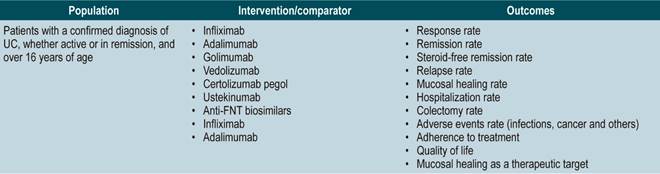
The GDG formulated the questions to be updated according to the PICO (population, intervention, comparison, and outcome) framework. The PICO questions can be found in Annex 2. The GDG conducted an outcome prioritization analysis in order to identify key outcomes that should be included. Clinical outcomes regarding safety, efficacy, quality of life, and all those important for patients were identified and prioritized. Each outcome was classified as critical, important, non-critical, and not important for patients based on a 9-unit scale proposed by the GRADE group23. Then, we proceeded to search for evidence in different databases and to fill out this template.
The GDG reviewed the relevant clinical aspects to be included in the CPG and, based on them, formulated basic questions which were then restructured according to the PICO (population, intervention, comparison, and outcome) framework. The resulting questions can be found in Annex 2.
LITERATURE SEARCH
As a first step, a search of systematic reviews was conducted in the following databases: Pubmed, Econlit, EMBASE, LILACS, Google Scholar, Cochrane Database of Systematic Reviews (CDRS), Center for Reviews and Dissemination, which in turn includes the Health Technology Assessment (HTA) database, the Database of Abstracts of Reviews of Effects (DARE) and the NHS Economic Evaluation Database (NHS EED).
Search strategies were developed and performed by the information specialist of the Cochrane STI Group; it should be noted that the GDG also contributed to this process. Identification forms of words related to the clinical questions, which in turn allowed the selection of MeSH terms and keywords, were used to define the search strategies. The search was limited to studies published in English or Spanish. Search strategies are available in Annex 3 of this document, together with the evidence selection PRISMA flow diagram. The search was conducted until July 2020.
GRADING OF THE EVIDENCE
The systematic reviews (SR) that were identified in relation to the different clinical aspects were assessed using the AMSTAR checklist24; besides, the contents, quality and clinical relevance of each SR were evaluated to identify those with the highest methodological quality and that should be included in the CPG. When there were no high-quality systematic reviews, primary studies were assessed using the risk of bias tool recommended by Cochrane25. In the case no evidence was found, consensus guidelines were identified. Evidence profiles were created using the tools available at https://gradepro.org to synthetize the information of the selected studies, and the levels of evidence were graded according to the GRADE classification. The GRADE evidence profiles can be found in Annex 4.
To achieve transparency and simplicity, the GRADE system grades the quality of evidence in four levels: high, moderate, low and very low. See the How to use this guide section for more information.
FORMULATION OF THE RECOMMENDATIONS
Recommendations were formulated in two steps. First, the GDG made the preliminary recommendations considering the risk-benefit balance, the preferences of patients, and the context in which they would be implemented. Then, the recommendations were discussed and adjusted in an expert panel with the representatives of scientific associations, government agencies, and patients, who helped determine the strength of each recommendation.
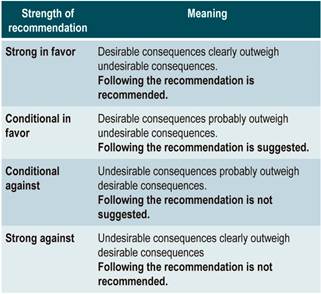
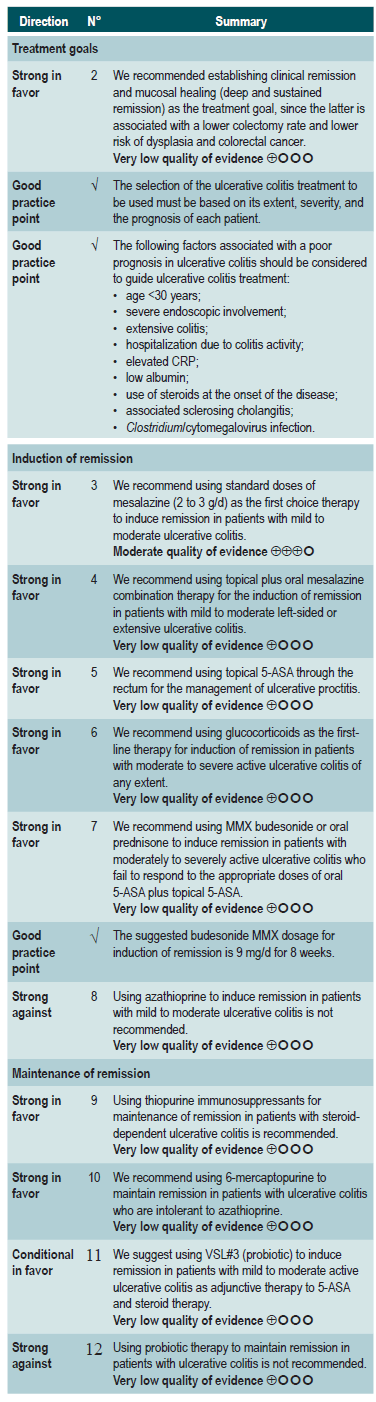
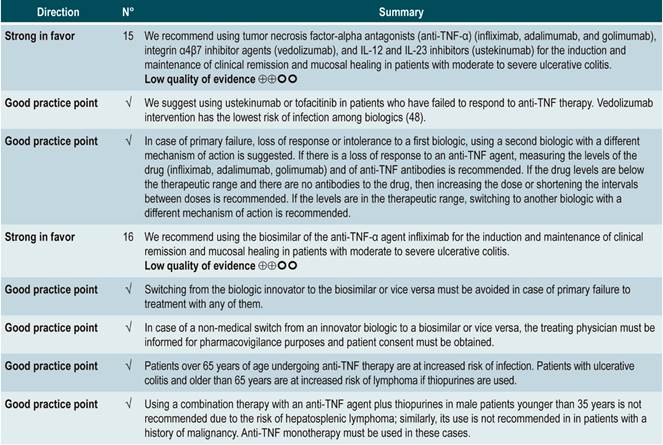
The GRADE methodology grades the strength of a recommendation as “Strong” or as “Conditional”. Once the risk-benefit balance, the quality of evidence, the values and preferences of patients, and the context of implementation were considered, the strength of each recommendation was determined using the following structure:
Finally, both expert panel agreement with the recommendations that were suggested and the inclusion of the participants’ perspective in them were verified. All recommendations and their grading were voted on electronically. When the majority of votes (greater than 70%) was not obtained in the first round, another round was held. A majority was obtained in all the voting held in the first round in the expert panel meeting.
Contextualization of the evidence
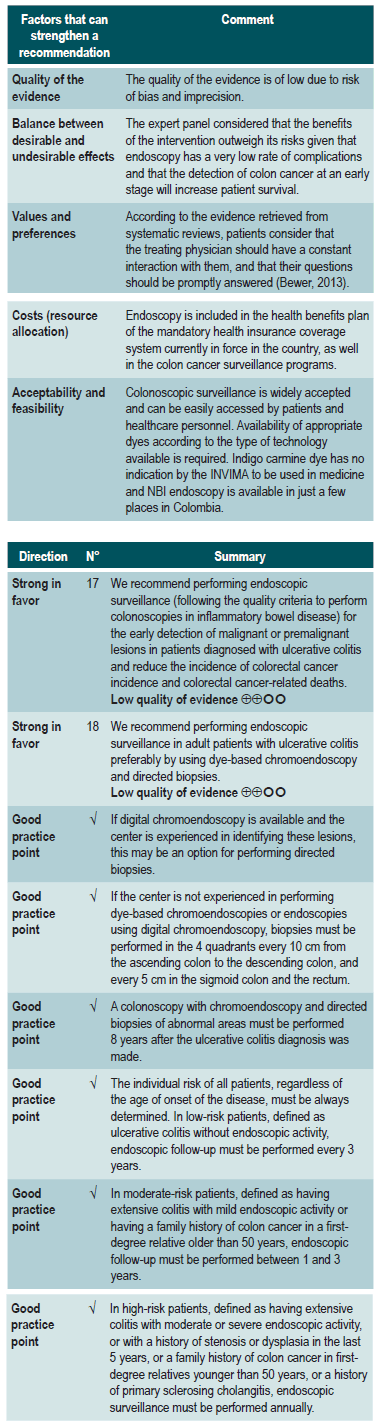
Based on the synthesis of the evidence, the GDG meetings and the expert panel meetings, relevant aspects of the implementation context of the recommendations were identified, which in turn helped in the process of making the recommendations by considering their applicability. In addition, for each group of recommendations, value judgment matrices containing assessments of the impact of the problem, the desirable effects, the undesirable effects, the confidence in the evidence, the consistency of the evidence, the balance of the effects, the resources required, the overall quality of the evidence, and the equity, acceptability and feasibility of their implementation were created.
Inclusion of the preferences of patients
Values and preferences of patients found in the relevant literature and those informed by a representative of the patients to the expert panel were included in this CPG.
Inclusion of costs and access aspects
Global aspects related to costs and access to health services in remote areas were considered in this CPG in order to formulate recommendations that could be implemented in the Colombian context.
Updating the clinical practice guideline
This guideline will be updated in three years following the methodology used in this update.
WHAT IS THE MOST USEFUL SCALE TO DETERMINE THE DISEASE ACTIVITY OF ULCERATIVE COLITIS IN PATIENTS DIAGNOSED WITH IT?
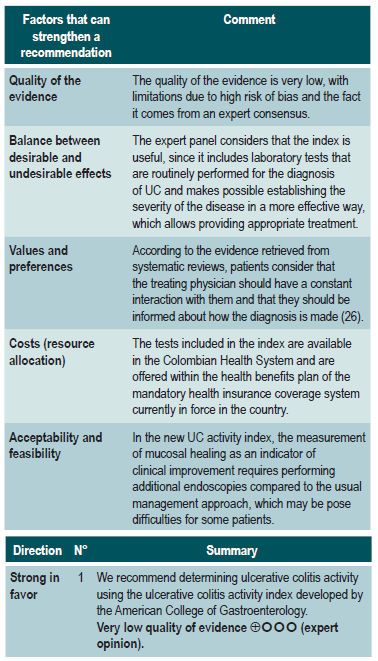
A clinical practice guideline for the management of UC (AGREE II score 12/23) developed by the American College of Gastroenterology in 2019 was identified. Said CPG recommends using an activity index with new definitions: active, moderate/severe, in remission, and fulminant UC. Additionally, it includes patient-reported outcomes and endoscopic and laboratory findings. This was determined by means of the UC activity index26.
What is the most effective and safe treatment for the induction and maintenance of remission in ulcerative colitis according to its extent and severity in patients older than 16 years?
General aspects of ulcerative colitis treatment
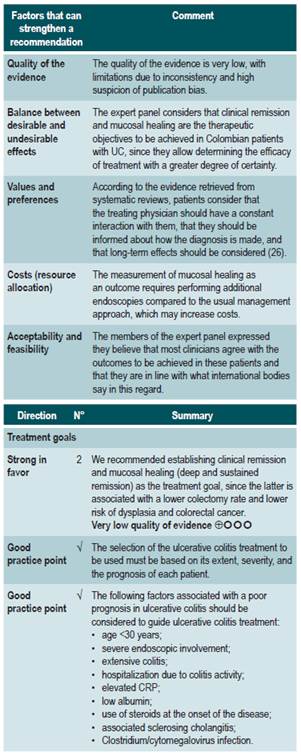
A systematic review (AMSTAR score 2: critically low quality) evaluated long-term clinical outcomes associated with mucosal healing in patients with active UC. Studies conducted in patients with active UC who had not undergone colectomy and in which clinical or endoscopic remission had not been reported prior to starting treatment were included. Likewise, in order to grade the status of mucosal healing, studies in which at least one endoscopic assessment was performed between 1 to 6 months after starting treatment were included. The main outcome assessed in the review was long-term clinical remission, defined as clinical remission at ≥52 weeks and at least 6 months after the first endoscopic assessment performed during follow-up. In addition, the following secondary outcomes were also evaluated: colectomy-free rate, mucosal healing rate, and corticosteroid-free clinical remission time for at least 52 weeks and at least 6 months after the first endoscopic follow-up. It should be noted that the follow-up time of the studies included in the review ranged from 12 to 56.4 months.
This SR retrieved 11 prospective cohort studies and two clinical trials with post hoc analysis for a total of 2073 patients with moderate to severe UC who received non-biologic (prednisolone, immunosuppressants, antibiotics and leukocytopheresis) and biologic therapy (infliximab: six studies; adalimumab: one study). According to the results of this SR, achieving mucosal healing in the first endoscopic evaluation was associated with greater long-term clinical remission (OR: 4.5, 95% CI: 2.12-9.52; 11 studies, 1381 patients) better colectomy-free rate (OR: 4.15, 95% CI: 2.53-6.81; 8 studies, 1480 patients), higher long-term mucosal healing rate (OR: 8.4; 95% CI: 3.13-22.53; 6 studies, 823 patients); yet not differences were found regarding the frequency of corticosteroid-free clinical remission time (OR: 9.7; 95% CI: 0.94-99.67; 3 studies, 576 patients)27.
Therapies in patients with active ulcerative colitis
Therapies for the induction of remission (updated from the clinical practice guideline for the management of ulcerative colitis developed in 2015).
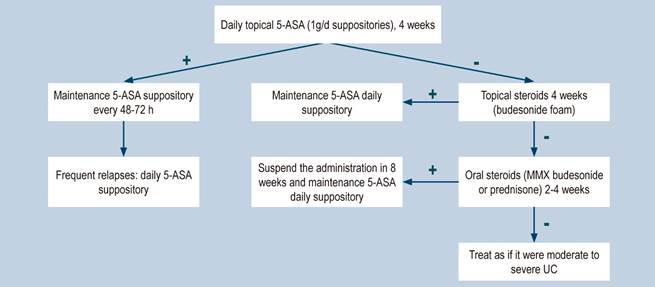
Topical aminosalicylates vs. placebo
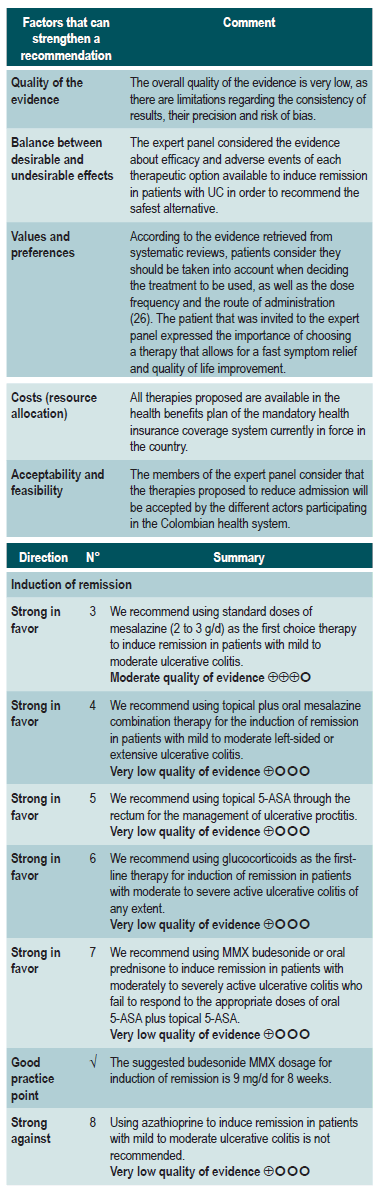
A systematic review identified by the group in charge of the development of the NICE guideline (AMSTAR score 2, low quality) assessed the safety and efficacy of topical aminosalicylates, compared to placebo, for the induction of remission in patients with proctitis and mild to moderate UC extending to the rectum up to 20 cm from the anal verge, and in the colon up to the splenic flexure. The outcomes evaluated in the review were the frequency of resolution of symptoms, the proportion of patients with endoscopic improvement (according to the Baron criteria), the endoscopic and clinical improvement rate, and the occurrence of both serious and non-serious adverse events resulting from therapy (facial erythema or mild fever, among others). Seven controlled clinical trials were retrieved (476 participants in total), and the intervention of interest was evaluated within a follow-up period of 0 to 6 weeks. Compared to the placebo group, patients in the topical aminosalicylates group showed a higher frequency of induction of clinical remission from 0 to 2 weeks (risk ratio [RR]: 3.84; 95% CI: 2.05-7.19), endoscopic remission from 0 to 2 weeks (RR: 7.54; 95% CI: 2.08-27.36), clinical and endoscopic remission from 2 to 4 weeks (RR: 10.27: 95% CI: 0.62-169.16), and clinical and endoscopic remission from 4 weeks to 6 weeks (RR: 10.21; 95% CI: 1.52-68.49). In addition, this intervention did not increase the frequency of adverse events (RR: 0.29; 95% CI: 0.04-2.14) or serious adverse events during treatment (RR: 0.26; 95% CI: 0.03-2.29), nor did it reduce the frequency of in-hospital care during treatment due to clinical deterioration (RR: 0.26; 95% CI: 0.03-2.29). The quality of evidence was moderate due to limitations in terms of precision of results and risk of bias28.
Aminosalicylates suppositories vs. liquid enema
A systematic review identified by the group in charge of the development of the NICE guideline (AMSTAR score 2, low quality) evaluated the safety and efficacy of using aminosalicylate suppositories, compared to liquid enema, for inducing remission in patients with mild to moderate active ulcerative proctitis extending up to 20 cm from the anal verge. The outcomes evaluated were the frequency of clinical and endoscopic remission from 0 to 2 weeks and from 2 to 4 weeks. One controlled clinical trial was retrieved (39 participants), and the intervention of interest was assessed within a follow-up period of 0 to 4 weeks. Compared to liquid enema, aminosalicylate suppositories therapy was not associated with a higher or lower frequency of clinical remission from 0 to 2 weeks (RR: 1.18; 95% CI: 0.58-2.42) or from 2 to 4 weeks (RR: 0.99; 95% CI: 0.72-1.36). Also, the use of enemas did not increase the frequency of endoscopic remission from 0 to 2 weeks (RR: 1.58; 95% CI: 0.7-3.59) or from 2 to 4 weeks (RR: 1.13; 95% CI: 0.75-1.72) when compared with the use of suppositories. The quality of the evidence was very low due to limitations regarding precision of results and risk of bias28.
Using topical aminosalicylates once per day vs. using them twice daily
A systematic review identified by the group in charge of the development of the NICE guideline (AMSTAR score 2, low quality) evaluated the safety and efficacy of using topical aminosalicylates once per day versus using them twice per day in the treatment of patients with mild to moderate UC extending 20 cm from the anal verge to the splenic flexure. The outcomes assessed were the clinical remission rate (defined as a DAI <3) and the frequency of adverse events during the follow-up period. Twelve controlled clinical trials were retrieved (2143 patients in total), and the intervention of interest was evaluated within a follow-up period ranging from 2 to 8 weeks. The administration of topical aminosalicylates twice per day did not increase the proportion of patients experiencing clinical remission from 2 to 4 weeks (RR: 0.94; 95% CI: 0.62-1.41) or from 4 to 6 weeks (RR: 1.08; 95% CI: 0.85-1.36), but neither was it associated with a higher or lower frequency of adverse events (RR: 0.96; 95% CI: 0.67-1.38). The quality of the evidence was very low due to limitations regarding precision of results and risk of bias28.
Topical aminosalucylates vs. oral aminosalicylates
A systematic review (AMSTAR score 2, moderate quality), compared the safety and efficacy of topical and oral administration of aminosalicylates to treat patients with proctitis and mild to moderate UC extending in the rectum up to 15 cm from the anal verge and in the sigmoid colon up to 50 cm from the anal verge. The outcomes assessed in this review were the frequency of remission, relapse and adverse events; twelve controlled clinical trials were retrieved for a total of 322 patients and the intervention of interest was evaluated during a follow-up period ranging from 3 weeks to 24 months. When compared to patients in the oral administration of aminosalicylates group, those in the topical administration arm did not show a higher or lower frequency of clinical remission from 4 to 8 weeks (RR: 0.82; 95% CI: 0.52-1.28), but did have a lower frequency of relapse from 6 to 24 months (RR: 0.64; 95% CI: 0.43-0.95). There were no statistically significant differences between groups regarding the frequency of adverse events (RR: 0.61; 95% CI: 0.24-1.52). The quality of the evidence was very low due to some limitations in terms of risk of bias, applicability, precision, and consistency of the results29.
Oral aminosalicylates vs. oral plus topical aminosalicylates
A moderate quality systematic review (AMSTAR score 2) compared the safety and efficacy of oral aminosalicylates therapy versus oral and topical aminosalicylates combination therapy in the treatment of patients with mild to moderate UC, ranging from proctitis to pancolitis. The following outcomes were assessed: the clinical remission (defined as the resolution of rectorrhagia, and having an endoscopic activity index [EAI] <4) and relapse rates. The review retrieved 12 controlled clinical trials (322 patients in total), and the intervention of interest was evaluated during a follow-up period ranging from 3 weeks to 24 months. Compared to oral aminosalicylates monotherapy, combination therapy with oral plus topical aminosalicylates was associated with an increased clinical remission rate (RR: 0.65; 95% CI: 0.47-0.91); however, it was not associated with a lower frequency of relapse (RR: 0.48; 95% CI: 0.17-1.38) or of adverse events (RR: 0.77; 95% CI: 0.55-1.19). The quality of the evidence was very low due to some limitations regarding results precision, consistency, and risk of bias30.
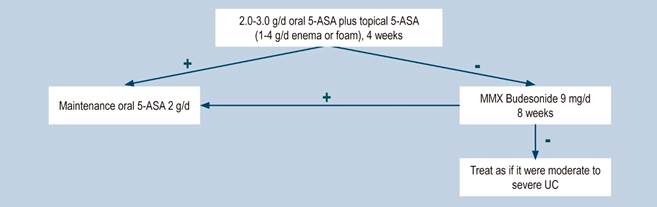
Efficacy and safety of oral 5-aminosalicylic acid therapy for induction of remission in ulcerative colitis
A moderate-quality systematic review (AMSTAR score 2) evaluated the efficacy and safety of using 5-ASA to induce remission in adult patients diagnosed with mild to moderate UC, as defined by the Truelove and Witts criteria (1955). In this review, oral 5-ASA therapy was compared with the following interventions: placebo, sulfasalazine or 5-ASA comparators (other formulations of 5-ASA), including different dosing schedules (once daily dose versus two or three doses per day) and commercial drugs Asacol, Claversal, Salofak and Pentasa. Efficacy outcomes assessed included the proportion of patients who failed to achieve clinical or overall remission according to the criteria established by the authors of the studies included in the review, the frequency of clinical improvement, endoscopic remission or endoscopic improvement, and the proportion of patients who failed to adhere to treatment; on the other hand, the following safety outcomes were evaluated: the occurrence of at least one adverse event, the frequency of withdrawal due to adverse events, and the proportion of patients who were lost to follow-up.
The systematic review retrieved 53 studies, and according to its results, 5-ASA therapy was superior to placebo, as lower frequencies of failure to induce clinical or overall remission (11 studies, 2387 patients; RR: 0.86%; 95% CI: 0.82-0.89), failure to induce clinical improvement (3 studies, 231 patients; RR: 0.79; 95% CI: 0.64-0.97), failure to induce endoscopic remission (4 studies, 1154 patients; RR: 0.77; 95% CI: 0.77, 0.67-0.89), failure to induce endoscopic improvement (4 studies, 416 patients; RR: 0.71; 95% CI: 0.59-0.86) and of withdrawal due to adverse events (13 studies, 2372 patients; RR: 0.72; 95% CI: 0.54-0.97) were observed when 5-ASA therapy was used; however, there were no differences between groups regarding the frequency of adverse events.
On the other hand, when compared with sulfasalazine therapy, differences in favor of 5-ASA therapy regarding the frequency of withdrawal due to adverse events (RR: 0.40; 95% CI: 0.24-0.68; 10 studies, 640 patients) and the frequency of adverse events (RR: 0.48, 95% CI: 0.36-0.63; 12 studies, 909 patients) were reported, but there were no differences between both interventions in terms of reducing the frequency of failure to induce remission or clinical improvement, induction of endoscopic remission or improvement, and induction of overall remission.
Finally, there were no statistically significant differences between the single-dose administration of mesalazine using MMX (longer extended release), Salofalk (pH-dependent release) and Pentasa (controlled release) and the administration of two or three doses per week in terms of reducing the frequency of failure to induce remission or clinical or endoscopic improvement, nor in the frequency of adverse events or withdrawal due to adverse events. Similarly, there were no differences between the different presentations of the drug regarding the induction of clinical response or clinical or endoscopic remission, nor in the adverse events occurrence rate31.
The quality of the evidence was moderate due to limitations regarding the precision of results.
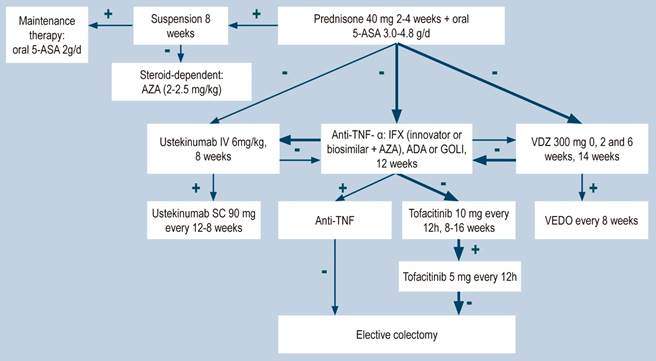
Clinical evidence: oral corticosteroids vs. placebo (taken from the clinical practice guideline for the management of ulcerative colitis developed in 2015).
A systematic review (AMSTAR score 2, moderate quality), assessed the efficacy of using glucocorticoids (hydrocortisone, cortisone, prednisolone, methylprednisolone, prednisone, betamethasone, beclomethasone, or fluticasone), whether through oral or parenteral administration, for inducing remission in patients with active UC with different degrees of severity. The outcomes evaluated in the review were the frequency of failure to achieve clinical and endoscopic remission, defined as having ≤2 nonbloody stools per day, absence of fever or tachycardia, having normal (or improved) hemoglobin and erythrocyte sedimentation rate values, and experiencing weight gain. The review retrieved five controlled clinical trials (445 patients in total), and the intervention of interest was assessed within a follow-up range of 3 to 8 weeks. When compared to placebo, glucocorticoid administration decreased the proportion of patients who failed to achieve clinical remission (RR: 0.65; 95% CI: 0.45-0.93)32. The quality of evidence was very low due to limitations related to inconsistency of results and the precision of results.
Efficacy and safety of using budesonide for the induction of remission in ulcerative colitis
A moderate quality systematic review (AMSTAR score 2) evaluated the efficacy and safety of using budesonide to induce remission in patients with UC. The review included studies conducted in patients diagnosed with UC and in which disease activity had been measured using any index and the definition established by the authors of each study, and compared the use of standard formulation of budesonide or extended-release (MMX) budesonide with the use of placebo. The following efficacy and safety outcomes were assessed within a period ranging from 2 to 9 weeks: clinical remission (as defined by the authors of each study included in the review), clinical, endoscopic or histological improvement, endoscopic mucosal healing, changes in the disease activity index scores used by in each primary study, quality of life, need for intravenous administration of corticosteroids, need for surgery, and frequency of adverse events.
In total, six clinical trials were retrieved. When compared to placebo, extended-release budesonide 9 mg was more likely to increase the probability of clinical and endoscopic remission (RR: 2.25; 95% CI: 1.50-3.39, 3 studies, 900 patients), resolution of symptoms (RR: 1.86, 95% CI: 1.25-2.77, 2 studies, 442 patients), endoscopic improvement (RR: 1.29, 95% CI: 1.01-1.66) and endoscopic remission (RR: 1.56, 95% CI: 1.13-2.16); there were no significant differences between groups regarding the frequency of adverse events or the probability of clinical improvement. On the other hand, when compared to placebo, differences in favor of budesonide 6 mg dose were found in terms of symptoms resolution (RR: 1.56; 95% CI: 1.04-2.35, 2 studies, 440 patients), but no differences were observed in endoscopic remission or improvement, the frequency of adverse events or serious adverse events, and the frequency of withdrawal due to adverse events.
Furthermore, additional comparison analyses were performed in the systematic review. When standard budesonide and prednisolone were compared, there were no differences in terms of clinical improvement or endoscopic remission, histologic remission, frequency of adverse events or frequency of withdrawal due to adverse events. In the case of the standard budesonide versus mesalazine comparison, a higher remission rate was found in the group of patients who were administered mesalazine (RR: 0.72; 95% CI: 0.57-0.91), but there were no differences regarding endoscopic remission or improvement, histologic remission, frequency of adverse events or withdrawal due to adverse events. Finally, in the extended-release budesonide versus Entocort EC comparison, there were no differences between both groups in terms of clinical or endoscopic remission or improvement, histologic remission, resolution of symptoms, or the frequency of serious adverse events33. The quality of the evidence was very low due to limitations in terms of the consistency and precision of results.
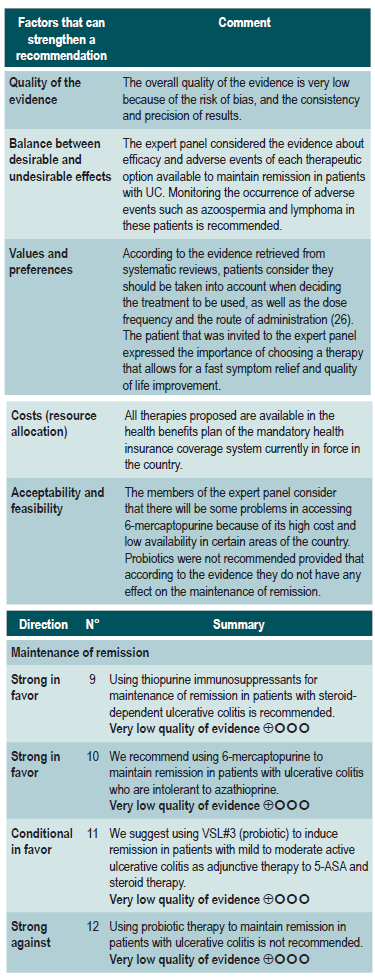
Clinical evidence: azathioprine vs placebo (taken from the clinical practice guideline for the management of ulcerative colitis developed in 2015)
A systematic review identified by the group in charge of the development of the NICE guideline (AMSTAR 9/11) evaluated the safety and efficacy of azathioprine to induce remission in patients with mild to moderate UC. The outcomes assessed were the frequency of clinical remission, which was defined using the Truelove and Witts severity index, and the proportion of patients achieving endoscopic remission. The review retrieved a controlled clinical trial for a total of 80 patients and the intervention of interest was evaluated during a follow-up period of 2 to 4 weeks. When compared to placebo, azathioprine therapy did not increase the frequency of clinical (RR: 1.15; 95% CI: 0.87-1.51) or endoscopic (RR: 1.67; 95% CI: 0.83-3.36) remission, both assessed from 2 to 4 weeks28. The quality of evidence was very low because there were some limitations related to the precision and consistency of results, and the risk of bias.
Therapies for maintenance of remission
Efficacy and safety of using azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis
A moderate quality systematic review (AMSTAR score 2) assessed the efficacy and safety of using oral azathioprine or 6-mercaptopurine for maintenance of remission in UC. The review included patients with UC in remission, which was defined as the presence of mild symptoms or their absence after complete discontinuation of corticosteroid therapy and endoscopic findings reporting grade 1 mucosal inflammation or absence of it. Regarding the interventions, studies performing the following comparisons were included: azathioprine versus placebo, 6-mercaptopurine versus 5-ASA, azathioprine versus sulfasalazine, 6-mercaptopurine versus methotrexate, and azathioprine versus cyclosporine. Failure to maintain clinical or endoscopic remission at 12 months (defined as the presence of relapse or withdrawal), and the frequency of adverse events and withdrawal due to adverse events were considered the efficacy and safety outcomes, respectively.
A total of seven clinical trials were included in the review. With regard to the studies that evaluated azathioprine, it was found that, when compared to sulfasalazine, there were no differences in the proportion of patients who failed to maintain remission or in the frequency of adverse events (1 study, 25 patients). Similarly, when compared to cyclosporine, there were no differences in terms of maintenance of remission, withdrawal due to adverse events or the occurrence of any adverse event (1 study, 16 patients). On the other hand, azathioprine was superior to placebo in maintaining remission (RR of failure to maintain remission: 0.68, 95% CI: 0.54-0.86; 4 studies, 232 patients), but no differences were found in the frequency of adverse events or of withdrawal due to adverse events. With regard to comparisons including 6-mercaptopurine, it was found that, when compared to 5-ASA, it had a lower frequency of failure to maintain remission (RR: 0.53; 95% CI: 0.31-0.9; 1 study, 22 patients), but no differences were observed regarding the frequency of adverse events or of withdrawal due to adverse events. Similarly, when compared with methotrexate, 6-mercaptopurine therapy showed a better maintenance of remission (RR of failure to maintain remission: 0.55, 95% CI: 0.31-0.95; 1 study, 26 patients), but there were no differences in the frequency of withdrawal due to adverse events34. The quality of the evidence was very low due to limitations found in the consistency and precision of the results.
Efficacy and safety of using probiotics in patients with ulcerative colitis
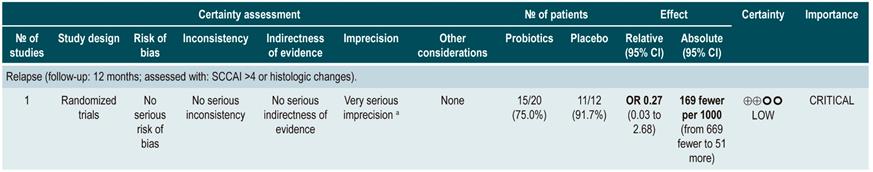
A critically low quality systematic review (AMSTAR score 2) evaluated the efficacy and safety of using probiotics, fructans, inulin-type prebiotics, and synbiotics for inducing or maintaining remission of disease activity in patients with UC. Adults and children with active or inactive UC were included in the review (no further specifications were provided) and the following interventions were considered: use of probiotics, prebiotics (defined as a substrate that is selectively used by host microorganisms to obtain a health benefit) and synbiotics, which were defined as compounds containing both probiotics and prebiotics. Disease remission was considered as the efficacy outcome, without further specifications. Comparators were not explicitly reported by the authors of the review.
The review retrieved 18 studies conducted in a total of 1491 patients, of which 16 evaluated the efficacy of using probiotics; 1, the efficacy of using prebiotics, and 1, the efficacy of using synbiotics. In the case of probiotic use versus an unspecified control intervention in patients with active UC, there were no differences regarding the frequency of remission when assessed using the scales proposed in the primary studies (RR: 1.46; 95% CI: 0.94-2.27), nor in the maintenance of remission in patients with inactive UC (RR: 1.38; 95% CI: 0.86-2.21). In addition, a subgroup analysis was performed to evaluate the frequency of remission according to the microorganism included in the product, finding a higher frequency of remission (95% CI: 1.99; 1.25-3.15) when the VSL#3 strain was used as adjunctive therapy to other treatments in mild to moderate active UC. No differences regarding remission were found when probiotics including bifidobacteria, other non-bifidobacteria and mutaflora were used. The review did not provide information on the efficacy of prebiotics or synbiotics, nor did it consider the assessment of adverse events35.
The quality of the evidence was very low due to limitations regarding risk of bias and the consistency and precision of results.
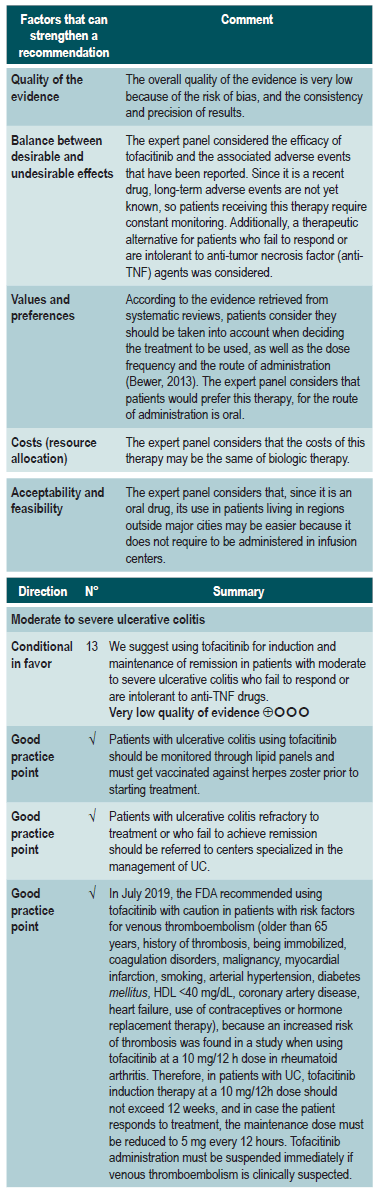
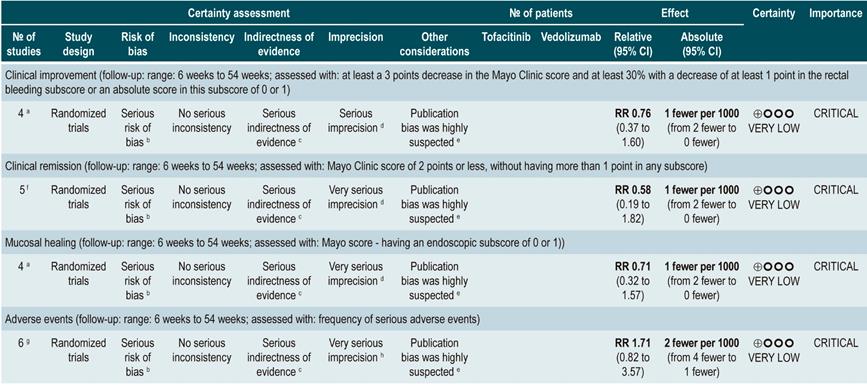
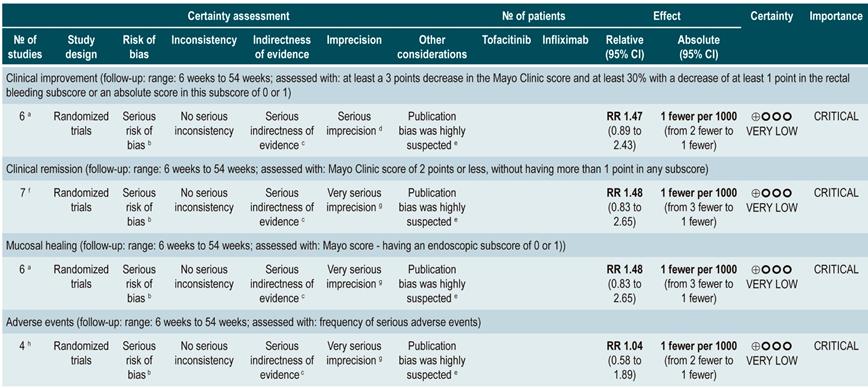
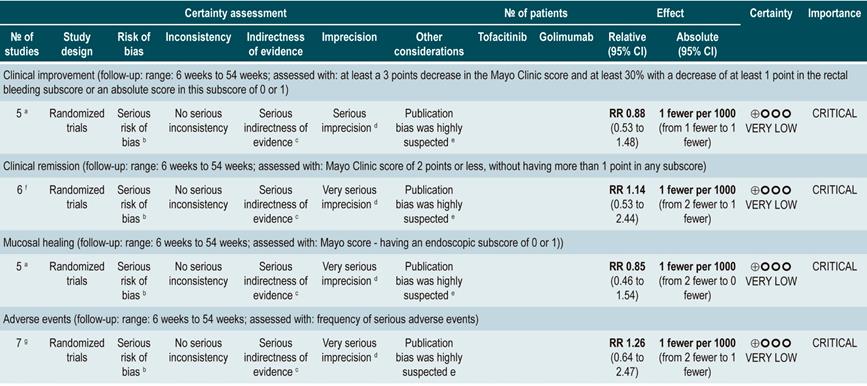
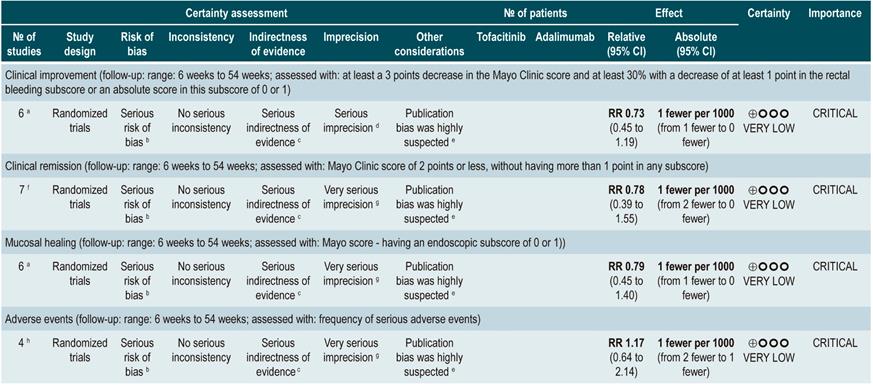
Efficacy and safety of using tofacitinib vs. biologic drugs to treat patients with moderate to severe ulcerative colitis. Results of a network meta-analysis
A critically low quality systematic review and network meta-analysis (AMSTAR score 2) evaluated the efficacy and safety of using tofacitinib in patients with moderate to severe UC in comparison with the use of placebo or biologic drugs. Patients diagnosed with moderate to severe UC, defined as having 6 to 12 points in the Mayo Clinic Score for ulcerative colitis disease activity and an endoscopic subscore of 2 or 3 points were included in the review; no further specifications were provided about the characteristics of the patients. The interventions considered in this systematic review were tofacitinib, adalimumab, golimumab, infliximab, and vedolizumab, and the efficacy outcomes assessed were clinical response, clinical remission and mucosal healing status at the end of induction and at the end of the maintenance phase; clinical response was defined as: (a) a 3 points decrease in the Mayo score and a decrease of at least 1 point in the rectal bleeding subscore or an absolute score in this subscore of 0 or 1; clinical remission was defined as obtaining 2 points or less in the Mayo score, without having more than 1 point in any subscore; mucosal healing was measured using the endoscopic subscore of the Mayo score, and healing was defined as having a score 0 or 1. On the other hand, the frequency of any adverse event and the frequency of serious adverse events were evaluated as safety outcomes. Outcomes were assessed within a follow-up period ranging from 6 to 54 weeks.
The review retrieved 19 clinical trials, and according to the results of the direct comparisons analysis, all interventions considered were superior to placebo in terms of clinical response and clinical remission. In the case of tofacitinib, the authors reported a 2.42-fold higher frequency of clinical response than placebo (95% CI: 1.61-3.63; 2 studies, 577 patients), a 2.47-fold higher frequency of clinical remission than placebo (95% CI: 1.41-4.34; 3 studies, 577 patients) and a higher frequency of mucosal healing (RR: 2.06; 95% CI: 1.25-3.39; 2 studies, 521 patients). Furthermore, in the analysis of indirect comparisons between interventions, there were no differences in the frequency of clinical response or clinical remission between tofacitinib and any of the biologic drugs considered in the review.
Regarding the frequency of adverse events, no statistically significant differences were found in the frequency of serious adverse events in the direct comparisons analysis between tofacitinib and placebo (RR: 0.69, 95% CI: 0.43-1.09; 4 studies, 1812 patients), nor in the frequency of any adverse event and of serious adverse events in the indirect comparisons analysis.
Finally, it was determined that infliximab had the highest probability of being the best therapy in relation to clinical response (60%) and mucosal healing (51.4%) outcomes, while tofacitinib had the following probabilities of being the best therapy in terms of clinical response, clinical remission and mucosal healing: 3.7% (third place), 3% (fourth place) and 5.2% (third place), respectively. In relation to adverse events, vedolizumab had the highest probability of being the best treatment regarding the frequency of adverse events (40.2%) and of serious adverse events (87.7%). In the case of tofacitinib, its probabilities of being the safest therapy in terms of adverse events and serious adverse events were 35.6% (second place) and 6.5% (second place), respectively36. The quality of the evidence was very low because of limitations regarding direct evidence, precision of results, and suspected publication bias.
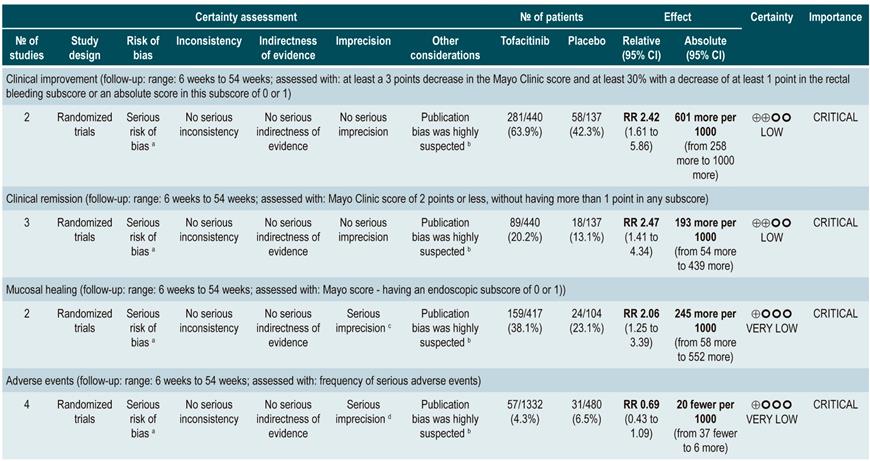
On the other hand, a critically low quality systematic review (AMSTAR 2 score) evaluated the efficacy and safety of tofacitinib, compared to placebo, for the induction of remission in patients with moderate to severe UC (defined according to the criteria established in the primary studies included in the review). Clinical remission or clinical response, mucosal healing, endoscopic or symptom remission and quality of life, as defined by the primary studies, were assessed as efficacy outcomes, while the frequency of adverse events, serious adverse events and serious infections were evaluated as safety outcomes.
Three studies, for a total of 1220 patients, were included in the systematic review. Statistically significant differences in favor of tofacitinib were found in terms of clinical remission (OR: 3.84; 95% CI: 2.29-6.44), clinical response (OR: 2.95; 95% CI: 2.21-3.95), endoscopic remission (OR: 5.65; 95% CI: 2.25-14.17), symptom remission (OR: 2.85; 95% CI: 1.46-5.54), mucosal healing (OR: 2.7; 95% CI: 1.81-4.03) and changes in quality of life scores in the Inflammatory Bowel Disease Questionnaire (IBDQ) (mean difference [MD]: 13.3; 95% CI: 9.7-16.91), and in the SF-36 scale physical domain (MD: 3.45; 95% CI: 2.44-4.45) and mental domain (MD: 3.94; 95% CI: 2.69-5.19); however, patients who were administered tofacitinib had a higher frequency of infections (OR: 1.51; 95% CI: 1.05-2.19). Finally, there were no differences between tofacitinib and placebo regarding the frequency of adverse events (OR: 0.93; 95% CI: 0.68-1.28), serious adverse events (OR: 0.63; 95% CI: 0.34-1.15), withdrawal due to adverse events (OR: 0.94; 95% CI: 0.34-2.6) or serious infections (OR: 3.17; 95% CI: 0.56-17.94)37.
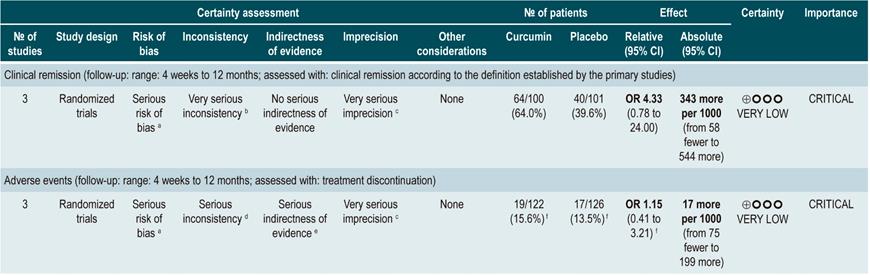
Efficacy and safety of using curcumin in patients with mild to moderate active ulcerative colitis
A critically low quality systematic review (AMSTAR score 2) evaluated the efficacy and safety of curcumin therapy in active UC. Adult patients with clinically and endoscopically detected UC with a mild to moderate disease activity (as defined by the indexes used in the primary studies) were included in the review, and the intervention of interest (oral administration of curcumin as an adjuvant therapy of UC) was compared with the use of placebo or no adjuvant treatment. The following outcomes were assessed: efficacy outcomes: proportion of patients achieving remission (measured with the Colitis Activity Index [CAI], the Simple Clinical Colitis Activity Index [SCCAI] and the Ulcerative Colitis Disease Activity Index [UCDAI], maintenance of remission, changes in disease activity scores, endoscopic remission (mucosal healing) and clinical response; safety outcomes: frequency of adverse events. Outcomes were evaluated during a follow-up period ranging from 4 weeks to 12 months.
In total, four clinical trials (241 participants combined) were included in the review. No significant differences between the comparisons were found in terms of clinical remission (OR: 4.33; 95% CI: 0.78-24), the frequency of patients with changes in in the score of the disease activity index (curcumin, range: 20% to 61%; placebo, range: 12.5% to 36%) and mucosal healing (curcumin, range 22% to 34%; placebo, range 0% to 30%). Regarding safety outcomes, no differences were found in relation to the frequency of withdrawal (OR calculated based on the data reported by the review: 1.15; 95% CI: 0.41-3.21) or of adverse events38.
The quality of the evidence was very low due to limitations regarding risk of bias, consistency, precision, and publication bias.
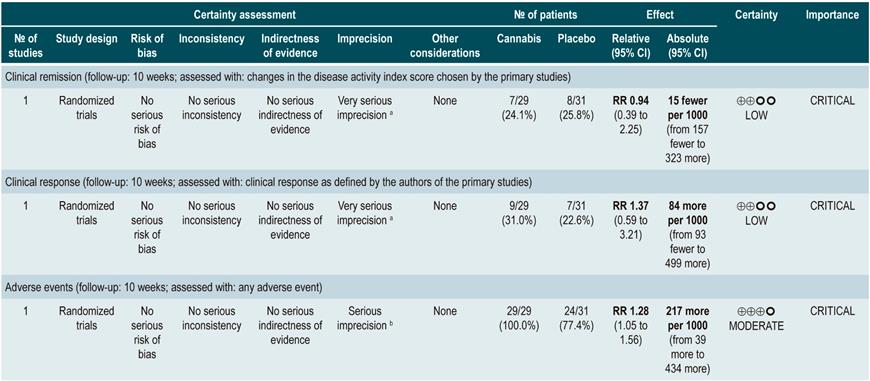
Efficacy and safety of cannabis in patients with ulcerative colitis
A moderate-quality systematic review (AMSTAR score 2) evaluated the efficacy and safety of using cannabis for the treatment of patients with UC. Patients included in the review were over 18 years of age and had a diagnosis of active or quiescent UC, which was defined using the Mayo Score or the Disease Activity Index (DAI). The intervention of interest was the use of cannabis or derivative cannabinoids in any presentation and administration route and it was compared with the use of placebo or any active therapy for the treatment of UC. Clinical remission (as defined by the primary studies included in the review), maintenance of remission, clinical response, endoscopic remission, histological response, quality of life and symptom improvement were assessed as efficacy outcomes, while the frequency of adverse events, the frequency of serious adverse events and the frequency of withdrawal due to adverse events were evaluated as safety outcomes.
The review included two clinical trials (92 patients) and the follow-up period was 10 weeks. According to the results of this systematic review, patients in the cannabinoids group had a higher frequency of adverse events (RR: 1.28; 95% CI: 1.05-1.56; 1 study, 60 patients), but no significant differences were found in terms of clinical remission (RR: 0.94; 95% CI: 0.39-2.25: 1 study, 60 patients), clinical response (RR: 1.37; 95% CI: 0.59-3.21; 1 study, 60 participants), symptom control (MD in the pain scale: 0.32; 95% CI: -0.51-1.15; MD rectal bleeding: -0.09; 95% CI: -0.47-0.29), stool frequency (MD: 0.00; 95% CI: -0.35-0.35), frequency of serious adverse events (RR: 0.12; 95% CI: 0.01-2.11) or frequency of withdrawal due to adverse events (RR: 2.14; 95% CI: 0.83-5.51)39.
The quality of the evidence was low due to limitations regarding the precision of the results.
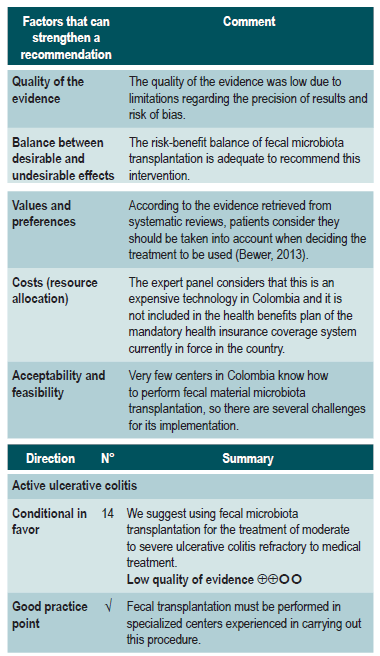
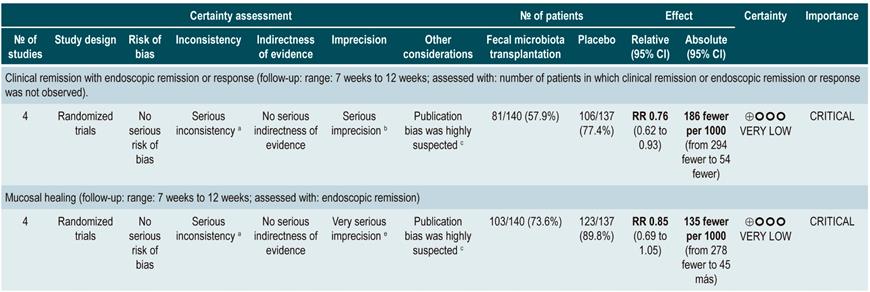
Efficacy and safety of fecal microbiota transplantation in patients with active ulcerative colitis
A critically low quality systematic review (AMSTAR score 2) assessed the efficacy and safety of fecal microbiota transplantation to treat adult patients with both clinically and endoscopically active UC measured with the Mayo score for ulcerative colitis disease activity and the Simple Clinical Colitis Activity Index (SCCAI). The review retrieved four clinical trials (277 patients in total) and the intervention of interest was any modality of fecal microbiota transplantation, while comparators were the use of placebo, defined as the excipient of fecal transplantation (without microbiota), or autologous fecal microbiota transplantation. The following outcomes were assessed in a follow-up period ranging from 7 to 12 weeks: efficacy outcomes: clinical remission together with endoscopic remission or response, clinical remission alone, and endoscopic remission alone; safety outcomes: frequency of serious adverse events. According to this systematic review, patients in the fecal microbiota transplantation group had better clinical remission alone (RR: 0.76; 95% CI: 0.62-0.93) and clinical remission with response or endoscopic remission (RR: 0.8; 95% CI: 0.71-0.89) rates when compared to those in the placebo group. However, no differences in endoscopic remission alone (RR: 0.85; 95% CI: 0.96-1.05) or in the frequency of serious adverse events (RR: 1.4; 95% CI: 0.55-3.58) were found40.
The quality of the evidence was low due to limitations regarding publication bias and the precision of results.
WHAT IS THE EFFICACY AND SAFETY OF BIOLOGIC THERAPY TO TREAT PATIENTS WITH MODERATE TO SEVERE ULCERATIVE COLITIS?
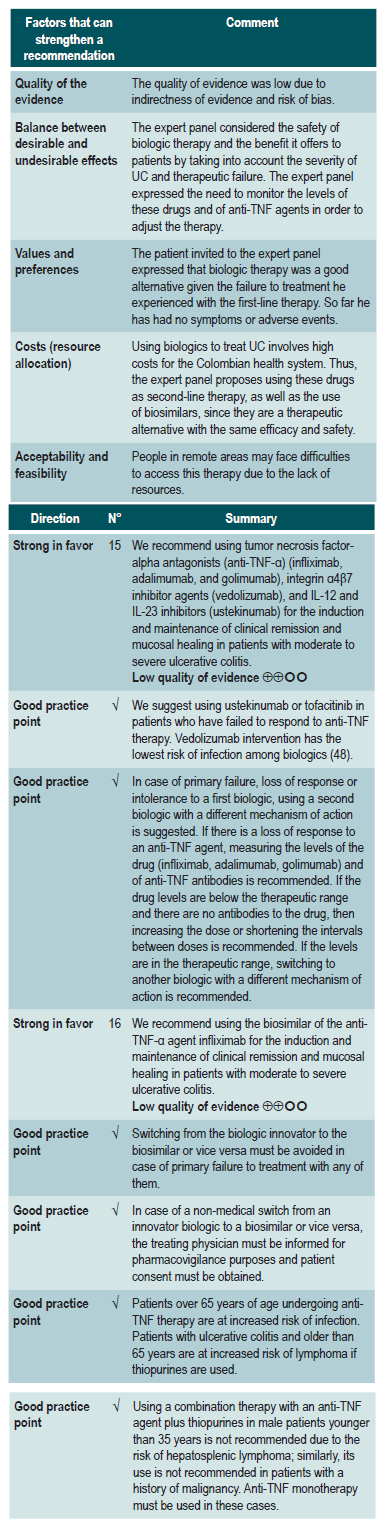
Clinical evidence: efficacy/effectiveness of anti-TNF-α agents and α4β7 integrin inhibitor agents in the treatment of moderate to severe ulcerative colitis
A moderate quality systematic review (AMSTAR score 2) evaluated the efficacy of using biologic therapies (adalimumab, infliximab, golimumab, and vedolizumab) to treat adults with moderate to severe active UC by assessing the following clinical outcomes both at induction and maintenance: clinical response, clinical remission and mucosal healing.
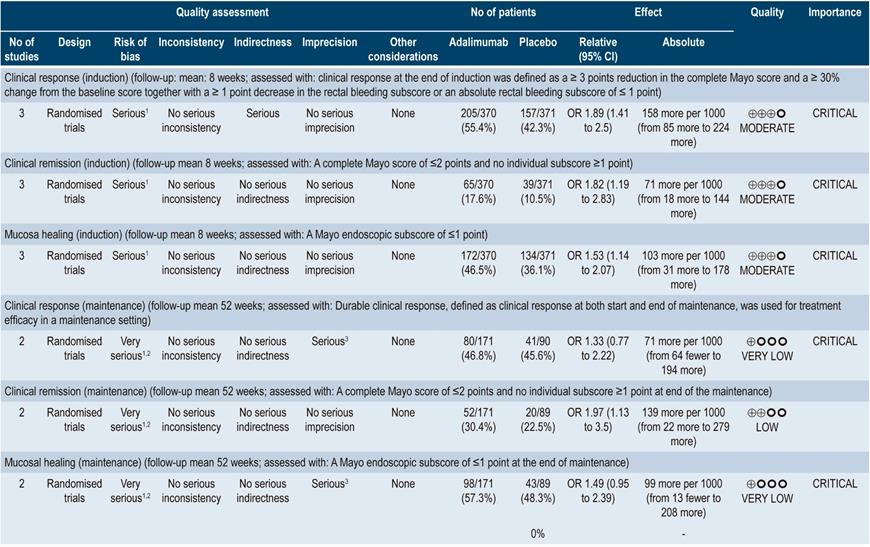
In three studies involving 741 patients, adalimumab at induction was superior to placebo in terms of clinical response (OR: 1.89; 95% CI: 1.41-2.5), clinical remission (OR: 1.82; 95% CI: 1.19-2.83) and mucosal healing (OR: 1.53; 95% CI: 1.14-2.07). The quality of the evidence was moderate due to some limitations associated with the presence of risk of bias. With respect to maintenance therapy (2 studies; 260 patients), no significant differences were found in terms of clinical response (OR: 1.33; 95% CI: 0.77-2.22) or mucosal healing (OR: 1.49; 95% CI: 0.95-2.39). In the case of clinical remission, the OR was 1.97 with a 95% CI of 1.13-3.5. The quality of evidence ranged from low to very low due to the presence of risk of bias and imprecision of results.
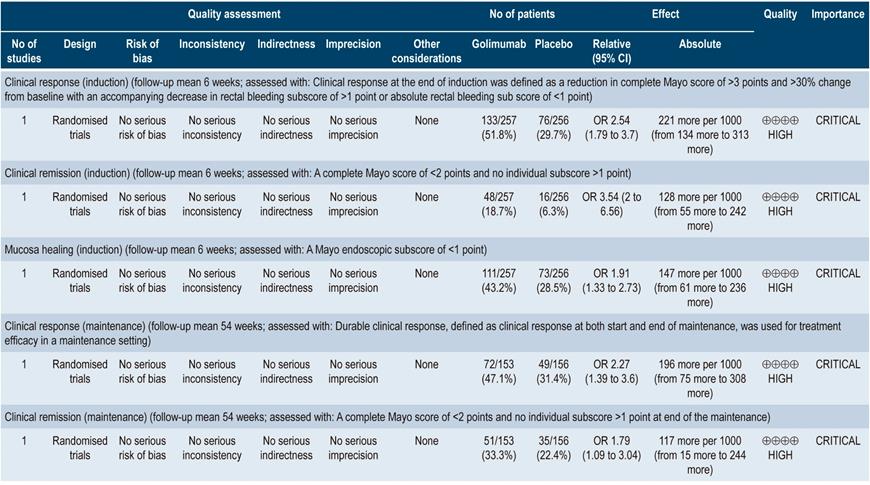
In the case of golimumab induction therapy, the review retrieved one study comparing this intervention with placebo in 309 patients. Said study reported differences in favor of golimumab in relation to clinical response (OR: 2.54; 95% CI: 1.79-3.70), clinical remission (OR: 3.54; 95% CI: 2.00-6.56) and mucosal healing (OR: 1.91; 95% CI: 1.33-2.73). In the case of maintenance therapy, golimumab was superior to placebo in the clinical response (OR: 2.27; 95% CI: 1.39-3.60) and clinical remission (OR: 1.79; 95% CI: 1.09-3.04) outcomes. The quality of the evidence was high.
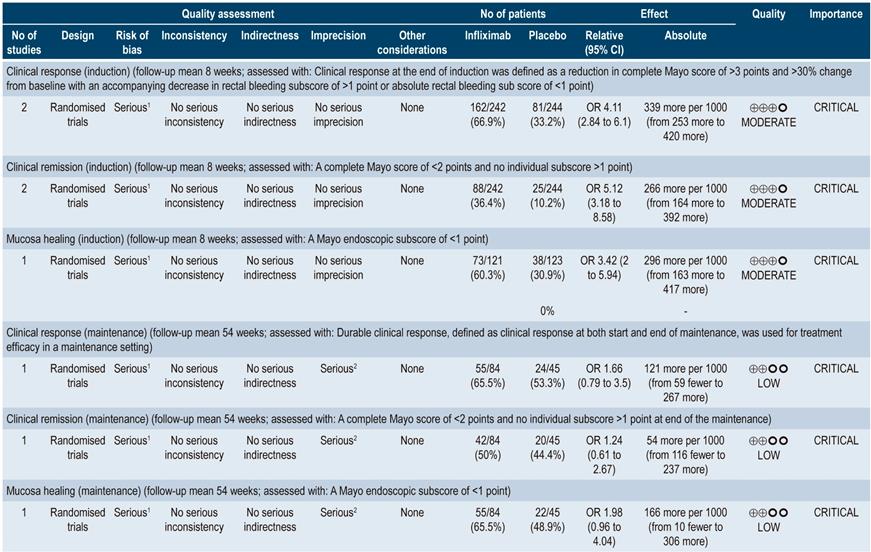
When compared with placebo, two studies (486 patients in total) reported differences in favor of infliximab at induction in terms of clinical response (OR: 4.11; 95% CI: 2.84-6.1), clinical remission (OR: 5.12; 95% CI: 3.18-8.58) and mucosal healing (OR: 3.42; 95% CI: 2.00-5.94). The quality of the evidence was moderate due to some limitations associated with the presence of risk of bias. In the case of maintenance therapy, based on one study conducted in 129 patients, the review reports there were no significant differences between infliximab and placebo regarding clinical response (OR: 1.66; 95% CI: 0.79-3.50), clinical remission (OR: 1.24; 95% CI: 0.61-2.67) and mucosal healing (OR: 1.98; 95% CI: 0.96-4.04). The quality of the evidence was low due to the presence of risk of bias, indirectness of evidence, and imprecision.
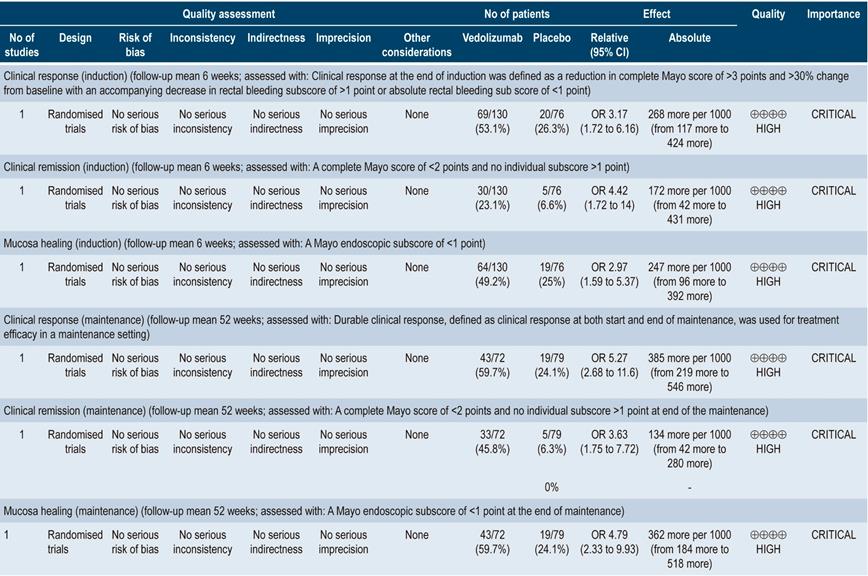
With regard to vedolizumab, the review retrieved one study conducted in 206 patients who were administered this α4β7 integrin inhibitor as induction therapy. Vedolizumab was superior to placebo in clinical response (OR: 3.17; 95% CI: 1.72-6.16), clinical remission (OR: 4.42; 95% CI: 1.72-14.00) and mucosal healing (OR: 2.97; 95% CI: 1.59-5.37). The quality of the evidence was high. Regarding its use as maintenance therapy, the review retrieved a study comparing vedolizumab with placebo in 151 patients, where it was superior to placebo in terms of clinical response (OR: 5.27; 95% CI: 2.68-11.6), clinical remission (OR: 3.63; 95% CI: 1.75-7.72) and mucosal healing (OR: 4.79; 95% CI: 2.33-9.93)41. The quality of the evidence was high.
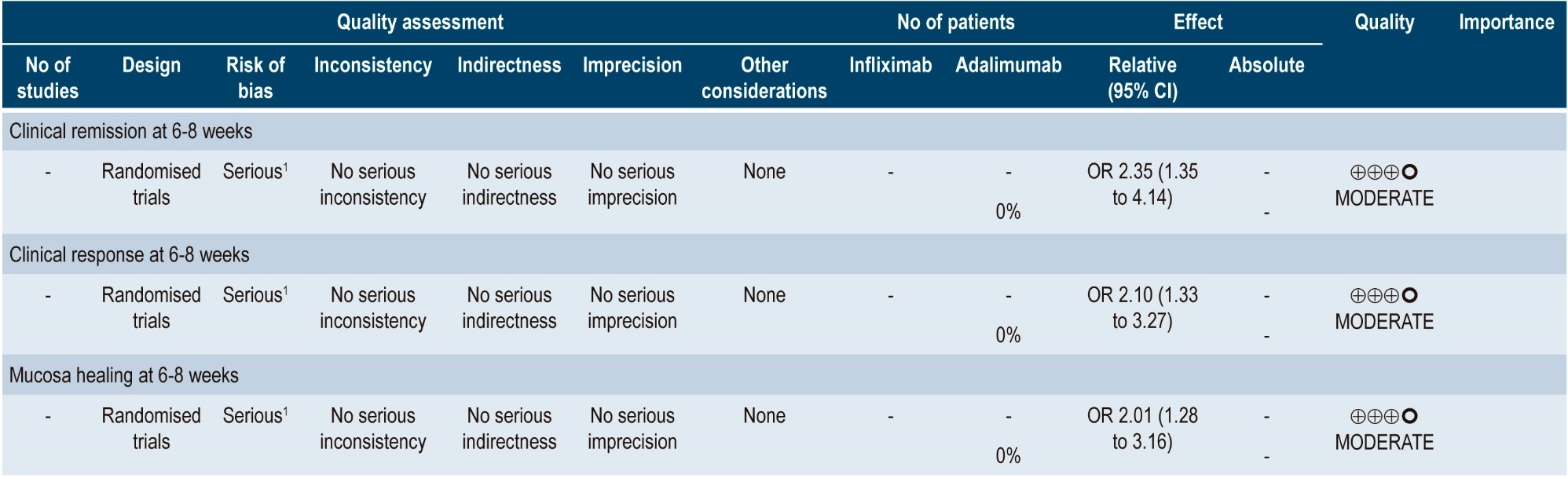
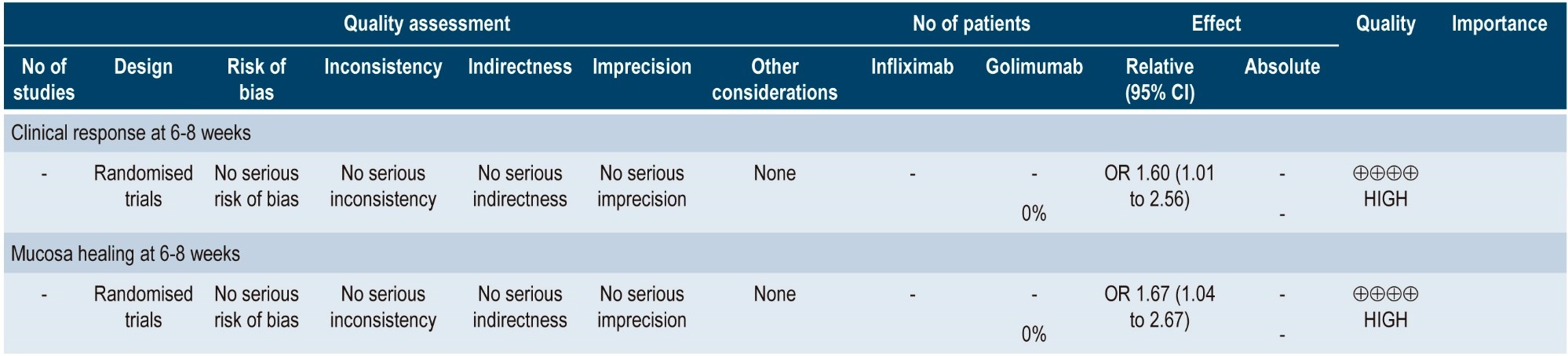
A more recent systematic review (AMSTAR score 8/11) performed indirect comparisons between different biologic drugs in relation to the treatment of moderate to severe UC, namely, adalimumab, golimumab, infliximab and vedolizumab. Only infliximab 5 mg/kg was superior to adalimumab in inducing clinical remission (6-8 weeks) (OR: 2.35; 95% CI: 1.35-4.14) and clinical response (6-8 weeks) (OR: 2.10; 95% CI: 1.33-3.27). Infliximab was also superior to golimumab in inducing clinical response (6-8 weeks) (OR: 1.60; 95% CI: 1.01-2.56). In the case of mucosal healing, infliximab was superior to adalimumab and golimumab (OR: 2.01; 95% CI: 1.28-3.16; and OR: 1.67; 95% CI: 1.04-2.67, respectively). There were no differences between the different comparisons in maintaining clinical remission (48-54 weeks)42. The quality of the evidence was low because of the presence of risk of bias and indirectness of evidence.
Finally, a study comparing the use of vedolizumab versus adalimumab in 769 adults with moderate to severe UC found that patients in the vedolizumab group had a higher rate of clinical remission (31.3% vs. 22.5%; 95% CI: 2.5-15.0; p=0.006) and endoscopic response (39.7% vs. 27.7%; 95% CI: 5.3-18.5; p<0.001) at week 52; however, there were no differences in terms of steroid-free clinical remission43.
Clinical evidence: efficacy/effectiveness and safety of CT-P13, a biosimilar of the anti-TNF-α agent infliximab, in the treatment of ulcerative colitis
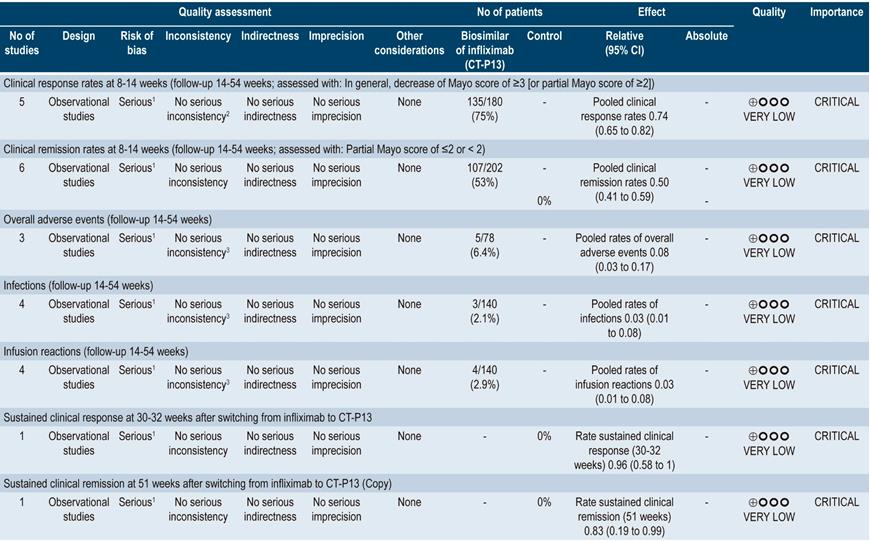
A moderate quality systematic review (AMSTAR score) (44) assessing the efficacy and safety of using biosimilars of anti-TNF-α agents in patients with inflammatory bowel disease was found. The outcomes evaluated were short-term clinical response (8-14 weeks), clinical remission (at 8-14 weeks), overall adverse events, infections, infusion reactions, sustained clinical response (30-32 weeks) and sustained clinical remission (at 51 weeks). No controlled clinical trials or studies assessing biosimilars other than CT-P13 were included in the review.
Regarding UC and the clinical response outcome, the review identified five observational studies conducted in 180 patients in total, reporting a pooled clinical response rate of 0.74 (95% CI 0.65-0.82) and a pooled clinical remission rate of 0.50 (95% CI 0.41-0.59). In addition, the pooled rates of sustained clinical response and sustained clinical remission were 0.96 (95% CI 0.58-1) and 0.83 (95% CI 0.19-0.99), respectively. In the case of safety outcomes, three observational studies involving 78 patients were identified and a pooled overall adverse events rate of 0.08 (95% CI: 0.03-0.17) was found. Similarly, a pooled rate of infections of 0.03 (95% CI: 0.01-0.08; 4 studies, 140 patients) and a pooled rate of infusion reactions of 0.03 (95% CI: 0.01-0.08; 4 studies, 140 patients) were found44. The quality of evidence was very low due to the presence of risk of bias.
Clinical evidence: efficacy/effectiveness of using ustekinumab to treat moderate to severe ulcerative colitis
A recent study conducted to assess the efficacy of ustekinumab, an antagonist of the p40 subunit of IL-12 and IL-23, as induction and maintenance therapy in patients with moderate to severe UC found a clinical remission rate at week 8 of 15.6% and 15.5% among patients who received an intravenous infusion of ustekinumab at a dose of 130 mg or 6 mg/kg, respectively, compared to a 5.3% rate in the placebo group (p<0.001, for both comparisons). In addition, the group of patients who experienced clinical response was randomized and treatment was continued by using 90 mg subcutaneous injections every 12 weeks, every 8 weeks or placebo, achieving clinical remission rates of 38.4%, 43.8% and 24.0%, respectively (p=0.002 and p<0.001); similarly, endoscopic response was achieved in 43.6%, 51.1% and 28.6%, respectively (p=0.002 and p<0.001)45.
Clinical evidence: safety of using anti-TNF-α agents and α4β7 integrin inhibitor agents to treat moderate to severe ulcerative colitis
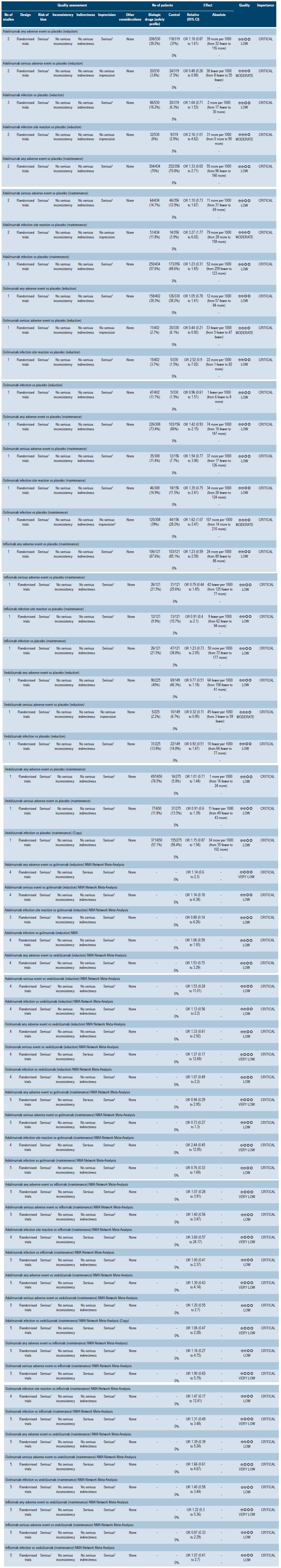
A moderate quality systematic review (AMSTAR score 2) that evaluated the safety profile of biologic drugs to treat patients with moderately to severely active ulcerative colitis was found. The following clinical outcomes were assessed both at the induction (6-8 weeks) and maintenance phase (52-54 weeks): any adverse event, serious adverse events, infections and injection site reactions.
In two clinical trials conducted in 849 patients in total, there were no differences between placebo and adalimumab as induction therapy in terms of any adverse event (OR: 1.18; 95% CI: 0.87-1.61) and infections (OR: 1.04; 95% CI: 0.71-1.53); however, the probability of injection site reactions was higher in the adalimumab group (OR: 2.16; 95% CI: 1.01-4.62), and in the serious adverse events outcome, an OR of 0.48 (95% CI: 0.26-0.89) was obtained. In the case of maintenance therapy, based on data retrieved from two studies conducted in 790 patients, no significant differences were found between adalimumab and placebo regarding any adverse event (OR: 1.33; 95% CI: 0.65-2.71), serious adverse events (OR: 1.10; 95% CI: 0.73-1.67) and infections (OR: 1.23; 95% CI: 0.31-1.65), although a higher probability of injection site reactions was observed compared to placebo (OR: 3.27; 95% CI: 1.77-6.02). The quality of the evidence ranged from moderate to low due to some limitations regarding the presence of risk of bias and imprecision.
With regard to golimumab as induction therapy, the review identified a clinical trial comparing the use of this intervention with placebo in 732 patients, where no significant differences between the two groups were found in the any adverse event (OR: 1.05; 95% CI: 0.78-1.41), injection site reactions (OR: 2.52; 95% CI: 0.9-7.02) and infections (OR: 0.96; 95% CI: 0.61-1.51) outcomes; in the case of the serious adverse events outcome it had an OR of 0.44 (95% CI: 0.21-0.92). On the other hand, a clinical trial comparing the use of golimumab as maintenance therapy versus placebo in 464 patients was retrieved. There were no significant differences between both interventions in terms of any adverse event (OR: 1.42; 95% CI: 0.93-2.15), injection site reactions (OR: 1.35; 95% CI: 0.75-2.41), infections (OR: 1.62; 95% CI: 1.07-2.47) and serious adverse events (OR: 1.54; 95% CI: 0.77-3.06). The quality of the evidence ranged from moderate to low due to some limitations in terms of presence of risk of bias, indirectness of evidence, and imprecision.
Regarding infliximab as maintenance therapy, one clinical trial comparing this intervention with placebo (242 patients) was found. There were no significant differences in terms of any adverse event (OR: 1.23; 95% CI: 0.59-2.59), serious adverse events (OR: 0.79; 95% CI: 0.44-1.45), injection site reactions (OR: 0.91; 95% CI: 0.4-2.1) and infections (OR: 1.23; 95% CI: 0.73-2.05). The quality of the evidence was low because of some limitations regarding the presence of risk of bias and imprecision.
In the case of the α4β7 integrin inhibitor agent vedolizumab, one study conducted in 374 patients receiving vedolizumab as induction therapy was found. According to said study, there were no significant differences between placebo and vedolizumab in terms of any adverse event (OR: 0.77; 95% CI: 0.51-1.18) and infections (OR: 0.92; 95% CI: 0.51-1.67), but the OR for the vedolizumab intervention in the serious adverse events outcome was 0.32 (95% CI: 0.11-0.95). The quality of the evidence varied from moderate to low due to some limitations related to the presence of risk of bias and imprecision. Regarding its use as maintenance therapy, one clinical trial conducted in 925 patients was identified, where no significant differences were found between vedolizumab and placebo in terms of any adverse event (OR: 1.01; 95% CI: 0.71-1.44), serious adverse events (OR: 0.91; 95% CI: 0.6-1.69) and infections (OR: 1.15; 95% CI: 0.87-1.54). The quality of the evidence was low due to the presence of risk of bias and imprecision.
This systematic review also performed indirect comparisons between several biologic agents in the treatment of patients with moderate to severe UC. There were no significant differences between adalimumab and golimumab in the any adverse event outcome in both the induction and the maintenance phases (OR: 1.14, 95% CI: 0.6-2.3; and OR: 0.94, 95% CI: 0.94, 95% CI: 0.29-2.95, respectively), nor in the serious adverse events (OR: 1.14; 95% CI: 0.18-6.38 at induction; OR: 0.73; 95% CI: 0.27-1.3 at maintenance), injection site reactions (OR: 0.88; 95% CI: 0.14-6.26 at induction; OR: 2.44; 95% CI: 0.45-12.95 at maintenance) and infections (OR: 1.06; 95% CI: 0.59-1.93; at induction, OR: 0.76; 95% CI: 0.33-1.69 at maintenance) outcomes. Similarly, no significant differences were found between adalimumab and vedolizumab in terms of any adverse event (OR: 1.53; 95% CI: 0.75-3.29 at induction; OR: 1.30; 95% CI: 0.43-4.14 at maintenance), serious adverse events (OR: 1.53; 95% CI: 0.24-11.01 at induction; OR: 1.20; 95% CI: 0.55-2.7 at maintenance) and infections (OR: 1.13; 95% CI: 0.56-2.2 at induction; OR: 1.06; 95% CI: 0.47-12.28 at maintenance).
On the other hand, when adalimumab and infliximab were compared as maintenance therapy, no significant differences were found regarding the any adverse event (OR: 1.07; 95% CI: 0.28-3.91), serious adverse events (OR: 1.40; 95% CI: 0.56-3.47), injection site reactions (OR: 3.60; 95% CI: 0.57-24.17), and infections (OR: 1.00; 95% CI: 0.41-2.37) outcomes.
Likewise, there were no significant differences between golimumab and vedolizuma in terms of the any adverse event (OR: 1.33; 95% CI: 0.61-2.92 at induction; OR: 1.39; 95% CI: 0.39-5.24 at maintenance), serious adverse events (OR: 1.37; 95% CI: 0.17-12.69 at induction; OR: 1.39; 95% CI: 0.39-5.24 at maintenance), and infections (OR: 1.07; 95% CI: 0.49-2.2 at induction; OR: 1.40; 95% CI: 0.58-3.44 at maintenance) outcomes. In the case of the golimumab versus infliximab comparison, no significant differences were found in the maintenance phase in the any adverse event (OR: 1.16; 95% CI: 0.27-4.75), serious adverse events (OR: 1.90; 95% CI: 0.65-5.79), and infections (OR: 1.47; 95% CI: 0.17-12.41) outcomes.
Finally, no significant differences were found between infliximab and vedolizumab at maintenance in the any adverse event (OR: 1.22; 95% CI: 0.3-5.36), serious adverse events (OR: 0.87; 95% CI: 0.33-2.29) and infections (OR: 1.07; 95% CI: 0.41-2.7) outcomes46.
All indirect comparisons had a low quality of evidence due to the presence of risk of bias, indirectness of evidence, and imprecision.
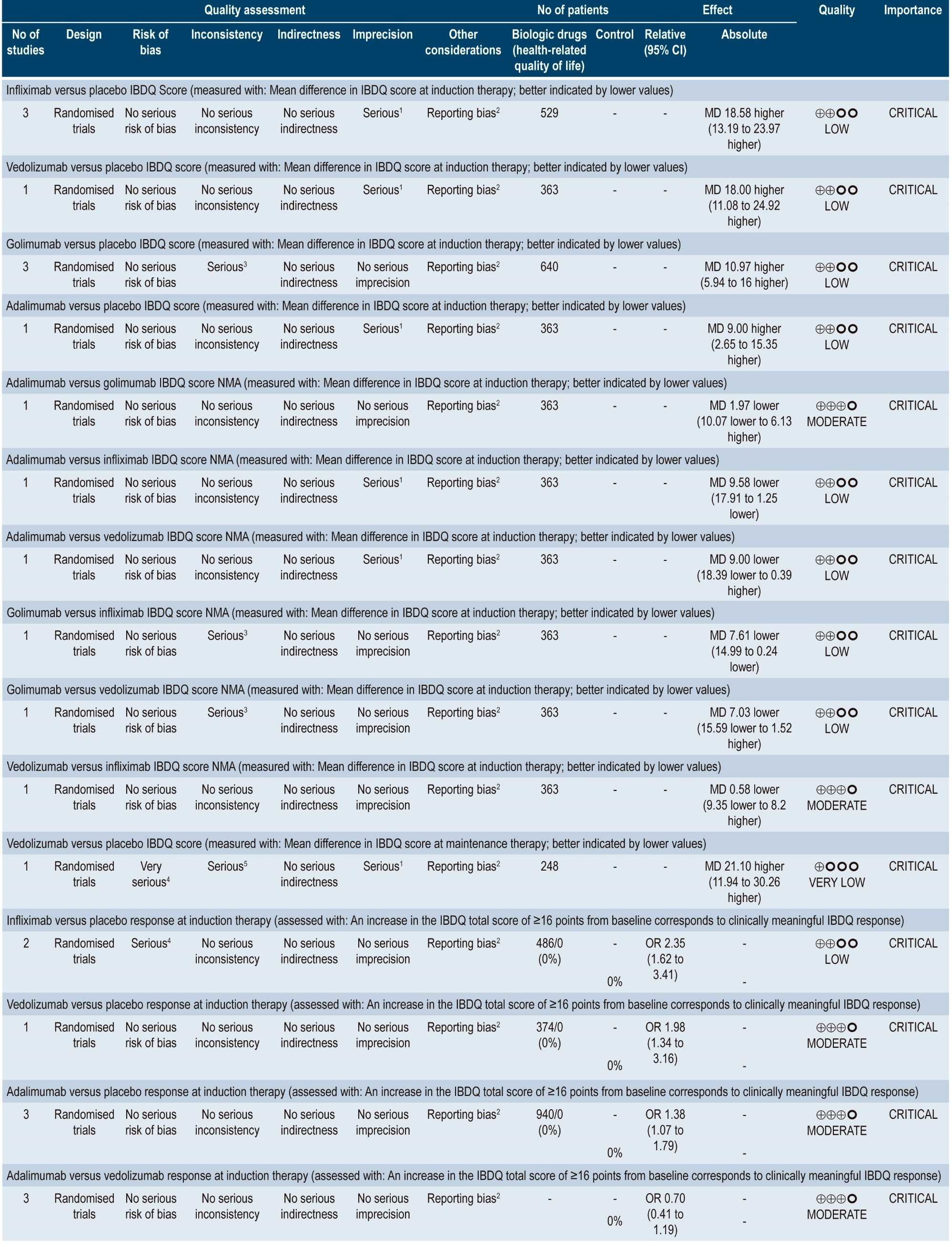
Clinical evidence: quality of life in patients with moderate to severe ulcerative colitis treated with anti-TNF-α agents and α4β7 integrin inhibitor agents
A moderate quality systematic review (AMSTAR score) comparing the impact of different interventions to treat moderate to severe UC on health-related quality of life was found47. Outcomes assessed in the review included changes in quality of life scores and the proportion of patients who experienced improvement in their quality of life.
In this systematic review, indirect comparisons between several biologic agents used to treat patients with moderate to severe UC were made, finding that, when compared to the placebo group, a greater improvement in the mean Inflammatory Bowel Disease Questionnaire (IBDQ) score was observed in the infliximab (MD: 18.58; 95% CI: 13.19-23.97) and vedolizumab (MD: 18.00; 95% CI: 11.08-24.92) groups, followed by the golimumab (MD: 10.97; 95% CI: 5.94-16.00) and adalimumab (MD: 9.00; 95% CI: 2.65-15.35) groups. In addition, when all interventions were compared among each other, infliximab was superior to adalimumab (MD: 9.58; 95% CI: 1.25-17.91) and golimumab (MD: 7.61; 95% CI: 0.24-14.99).
Similarly, all interventions were associated with a higher proportion of patients who showed a clinically significant increase in their IBDQ score (at least 16 points compared to the baseline score) in comparison with placebo (infliximab: OR: 2.35; 95% CI: 1.62-3.41; vedolizumab: OR: 1.98; 95% CI: 1.34-3.16; adalimumab: OR: 1.38; 95% CI: 1.07-1.79). However, no significant differences were found between adalimumab and vedolizumab (OR: 0.70; 95% CI: 0.41-1.19)48. The quality of the evidence ranged from moderate to very low due to imprecision, indirectness of evidence, and reporting bias problems.
WHAT IS THE EFFICACY OF COLONOSCOPIC SCREENING AND SURVEILLANCE FOR THE DETECTION OF COLORECTAL CANCER IN PATIENTS WITH ULCERATIVE COLITIS?
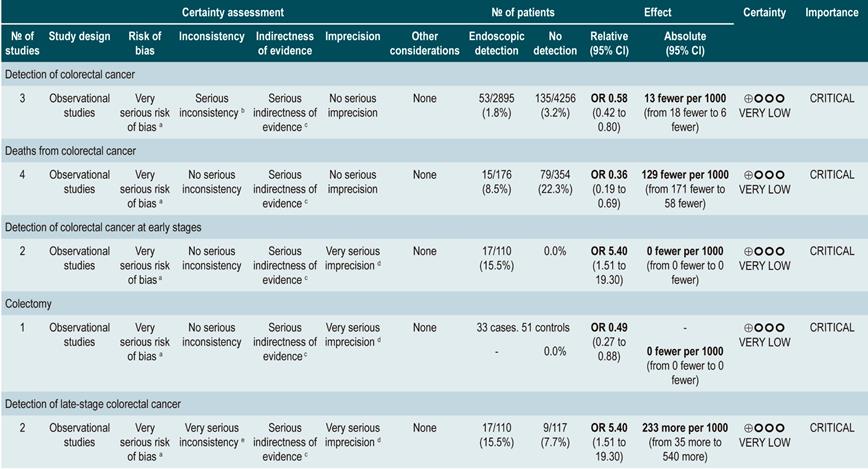
A high quality systematic review (AMSTAR score II) evaluated the efficacy of the different strategies for detecting colorectal cancer (CRC) through colonoscopy in patients with inflammatory bowel disease (including patients with UC) in order to reach a CRC diagnosis and carry out colonoscopic surveillance and, this way, reduce CRC-associated mortality. Five observational studies conducted in a total of 7199 patients with inflammatory bowel disease (IBD) were identified.
Three studies found a high rate of cancer detection in patients who underwent surveillance colonoscopy compared to those who were not monitored. CRC was detected in 1.83% of patients who were not monitored compared to 3.17% of those who were monitored (OR: 0.58; 95% CI: 0.42-0.80). In terms of the mortality rate associated with CRC, 8% of the patients in the surveillance group died due to CRC compared to 22% in the non-surveillance group (OR: 0.36; 95% CI: 0.19-0.69). Two studies reported a higher rate of early stage CRC detection in the surveillance group (16%) compared to the non-surveillance group (8%) (OR: 5.40; 95% CI: (1.51-19.30), being this difference significant (p=0.009); besides, a higher rate of late-stage CRC was observed in the patients of the non-surveillance group compared to those in the surveillance group (OR: 0.46; 95% CI: 0.08-2.51), although the difference was not statistically significant49. The quality of the evidence is very low because of high risk of bias, heterogeneity, and inconsistency
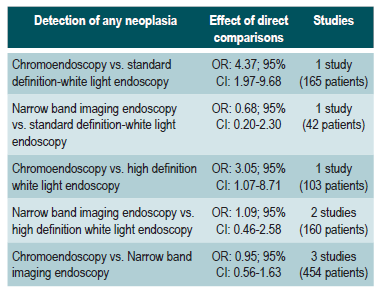
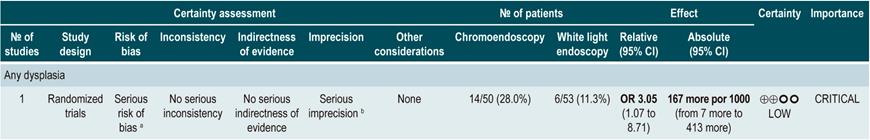
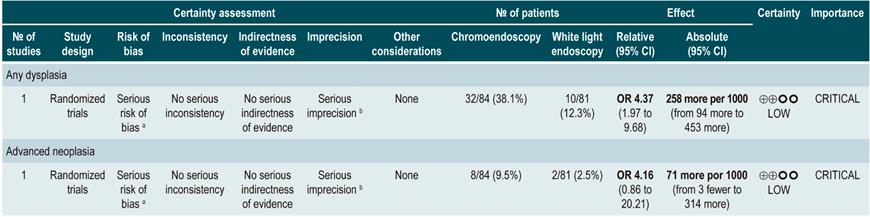
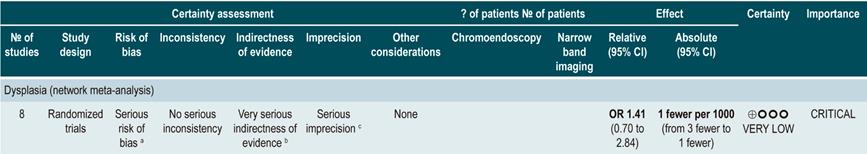
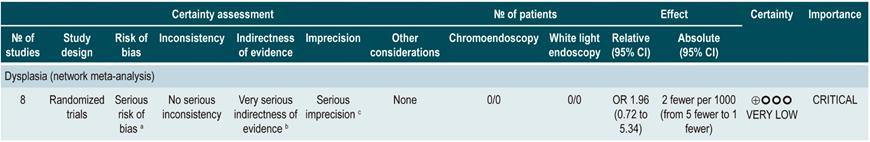
A low quality systematic review (AMSTAR score II) evaluated the comparative efficacy of different dysplasia detection techniques in UC patients. Eight randomized clinical trials (924 patients in total) assessing surveillance colonoscopy with standard definition-white light endoscopy (SD-WLE), high definition white light endoscopy (HD-WLE), narrow band imaging (NBI) and dye-based chromoendoscopy were included.
According to the results of this review, when direct comparisons were performed, dye-based chromoendoscopy was superior to SD-WLE and HD-WLE in detecting any dysplasia (p<0.05). No significant differences were found between the other different endoscopy techniques in terms of dysplasia detection (p>0.05). The estimators for each comparison are presented below.
Regarding the detection of advanced neoplasms, there was no superiority among the interventions when direct comparisons were made (p>0.05). Likewise, when indirect comparisons were made, none of the techniques were superior to the others (p>0.05). There were no significant differences between SD-WLE (OR: 1.96; 95% CI: 0.72-5.34), NBI (OR: 1.41; 95% CI: 0.7-2.84) and HD-WLE (OR: 2.37; 95% CI: 0.81-6.94) in detecting any dysplasia50. The quality of the evidence is low due to risk of bias and imprecision.
SUMMARY OF THE RECOMMENDATIONS
What is the most useful scale to determine the disease activity of ulcerative colitis in patients diagnosed with it?
What is the most effective and safe treatment for the induction and maintenance of remission in ulcerative colitis according to its extent and severity in patients older than 16 years?
What is the efficacy and safety of using other therapeutic alternatives to treat patients with moderate to severe ulcerative colitis?











 text in
text in