Rationale and theoretical framework
Inflammatory bowel disease (IBD) is a term used to describe two rare chronic inflammatory conditions of the gastrointestinal tract that mainly affect the colon and the small intestine: Crohn’s disease (CD) and ulcerative colitis (UC). IBD is characterized by multiple relapses; besides, an increased frequency of occurrence has been detected worldwide in recent years1,2. Its etiology is unknown, but it results from a complex interaction between the host’s genotype, intestinal microbiota and environmental factors, which trigger an alteration in the intestinal immune system response3. Despite CD has traditionally been considered an autoimmune disease, it does not meet the criteria to be considered as such4 and, therefore, some authors think it is actually an autoinflammatory disease5,6.
Historically, studies reporting the highest prevalence rates of IBD have been conducted in Scandinavian countries, the United Kingdom, and North America. IBD affects approximately 5 million people worldwide, including 1.4 million in the United States and about 3 million in Europe7. In this regard, a systematic review of epidemiological studies about IBD found a CD prevalence of 0.6-322, 16.7-318.5, and 0.88-67.9 cases per 100,000 people in Europe, North America, and Asia, respectively. Also, an increase in its incidence over time has been described in 75% of CD studies8. In addition, a recent systematic review that included 147 studies reported high prevalence rates of CD in Europe (where the highest prevalence was found in Germany with 322 cases per 100,000 people) and North America (being the highest prevalence found in Canada with 319 cases per 100,000 people), which have remained stable9. However, population-based studies conducted since 1990 have shown an increase in the incidence and prevalence of CD in developing countries from Asia and South America such as Brazil, Mexico, and Colombia9-13.
In Colombia, CD has been found to be less frequent than UC: in 1991, in one of the first studies published in the country about this topic, and that included 108 patients diagnosed with IBD (98 with UC and 10 with CD) between 1968 and 1990 in Bogota (Colombia), a UC/CD ratio of 9.8:1 was described14. Then, in 2010, a study conducted at the Hospital Pablo Tobón Uribe in Medellín (Colombia) found that out of 202 patients diagnosed with IBD between 2001-2009, only 15.8% were CD cases, while 80.7% had UC (classifying the type of IBD was not possible in 3.5% of the study population), that is, a UC/CD ratio of 5.1:115. In addition, in a recent work conducted at the same hospital, a UC/CD ratio of 3.0:1 was found, since out of 649 patients with IBD, 478 had UC (73.7%), 159 had CD (24.5%). and in 12 (1.8%) it was not possible to determine the form of IBD; besides, in said study, CD was predominant in men 13. Other case series carried out in Colombia have also described a higher frequency of UC cases compared to CD cases16,17. These data show that in Colombia, more and more patients are diagnosed with CD in the context of IBD, which is a similar situation to what has been reported in developed countries18.
Because of its low prevalence, CD, unlike UC, meets the criteria to be considered an orphan disease. In Colombia, an orphan disease is defined as a severe, chronic debilitating and life-threatening disease with a prevalence (the number of individuals affected by a disease within a particular period of time) of less than 1 case per 5000 people (Law 1392 of 2010/Law 1438 of 2011)19.
CD usually occurs between the second and fourth decade of life, with a small additional peak in people aged 50-60 years20. Its diagnosis is based on the simultaneous evaluation of, on the one hand, clinical signs and symptoms and, on the other, alterations in endoscopic or radiological and biochemical and histopathological studies. Isolated alterations in the histopathology report are not enough to reach a CD diagnosis. In fact, the European Crohn’s and Colitis Organisation, in their recently published guidelines on CD, has confirmed that there is not a “gold standard” for the diagnosis of CD, and that genetic or serological tests should not be used for diagnosing it21,22. Clinically, CD is a chronic inflammatory disorder of the gastrointestinal tract that mainly affects the colon and the small intestine; however, it can affect any part of the gastrointestinal tract from the mouth to the perianal area. It can also affect organs outside the gastrointestinal tract, and its clinical course is variable with alternating periods of activity and remission of symptoms. CD produces a segmental, asymmetric and transmural inflammation, and, from a clinical perspective, is a heterogeneous, insidious and progressive disorder. Depending on the severity, location and behavior of CD, the most common symptoms and clinical signs are abdominal pain, diarrhea, gastrointestinal bleeding, and weight loss1. Smoking, living in urban areas, exposure to antibiotics, and oral contraceptive use have been described as risk factors for CD23.
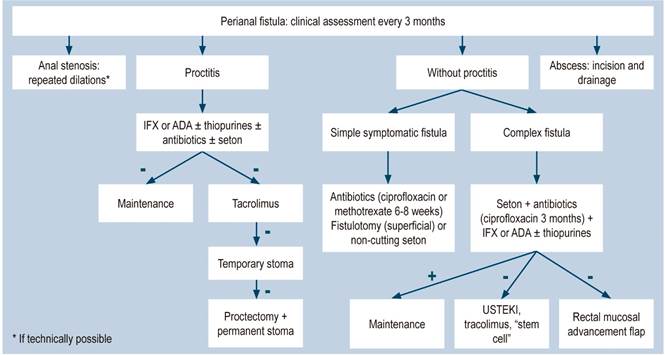
When performing a physical examination in these patients, signs of systemic toxicity, dehydration, malnutrition, anemia and malabsorption, as well as abdominal pain or palpable abdominal masses must be looked for. Physical examination of the perianal area is mandatory since perianal involvement occurs in up to one third of patients1. The most frequent laboratory findings are anemia, thrombocytosis, hypoalbuminemia, and high C-reactive protein (CRP) levels; however, the latter does not correlate well with endoscopic findings and in one third of patients, CPR levels never increase21,24. Fecal biomarkers such as calprotectin have been correlated with inflammatory activity by neutrophils in the intestine and are being used as a screening test with high sensitivity and specificity for the diagnosis of IBD25. In this regard, it has been reported that patients with irritable bowel syndrome (IBS) symptoms and with a fecal calprotectin concentration <40 μg/g, have a 1% chance of having IBD26. On the other hand, between 60% and 70% of patients with CD may have elevated antimicrobial antibody levels, being anti-Saccharomyces cerevisiae antibodies (ASCA) the most prevalent; somehow, the sensitivity and specificity of these antibodies are too low to reach a CD diagnosis27.
Endoscopic findings reported by means of ileocolonoscopy are key to the diagnosis of CD. Typical findings include the presence of multiple inflammatory aphthous ulcers with segmental involvement, associated with longitudinal and serpentiginous ulcers. Additionally, the presence of perianal involvement, fistulas, ileitis, and strictures supports the diagnosis of CD22,28. In case of negative results in the ileocolonoscopy, but clinical suspicion of CD, small bowel capsule endoscopy is indicated in the absence of obstructive symptoms or known stenosis21. Histologically, inflammatory involvement is chronic, focal, discontinuous, and transmural. Epithelioid granuloma is the histological marker of CD, but it is only found in 15% of mucosal biopsies and 70% of surgical specimens1,29. Imaging studies such as magnetic resonance (MR) enterography and Computed tomography (CT) enterography are useful to reach a CD diagnosis, as well as to determine its extent and rule out complications such as strictures and fistulas; furthermore, they can be useful for follow-up purposes, since they can be used to measure the activity of the disease and the patient’s response to treatment30. It should be noted that MR enterography is preferred due to the lower radiation exposure it involves compared to CT enterography. Finally, the use of pelvic MRI is recommended for the study of perianal and fistulizing CD30.
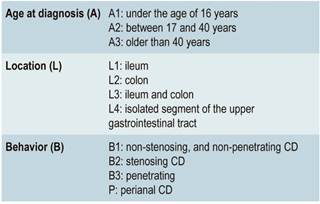
Once the diagnosis has been reached, patients with CD should be phenotyped according to the Montreal classification31 in order to determine its location and clinical behavior. The location of the disease tends to be stable, but its behavior usually changes over time32.
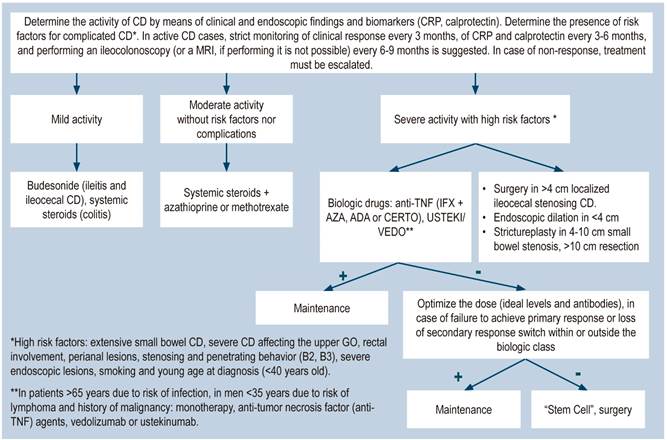
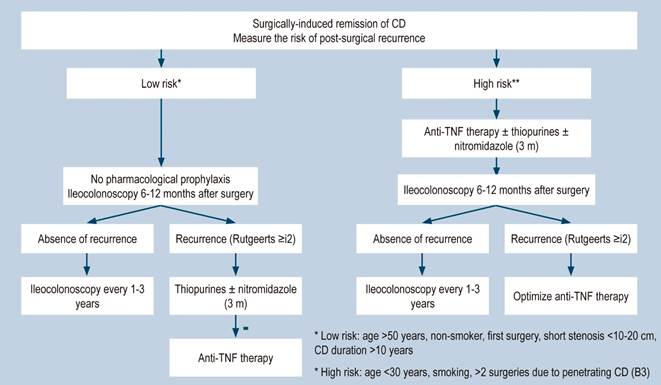
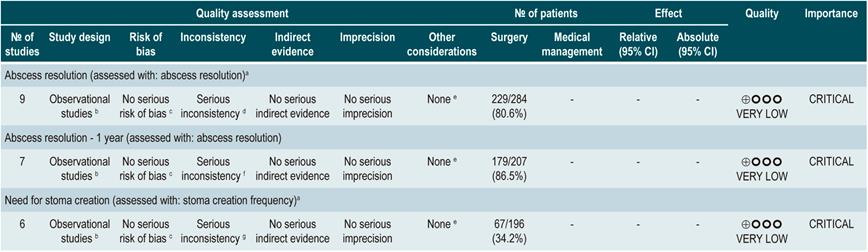
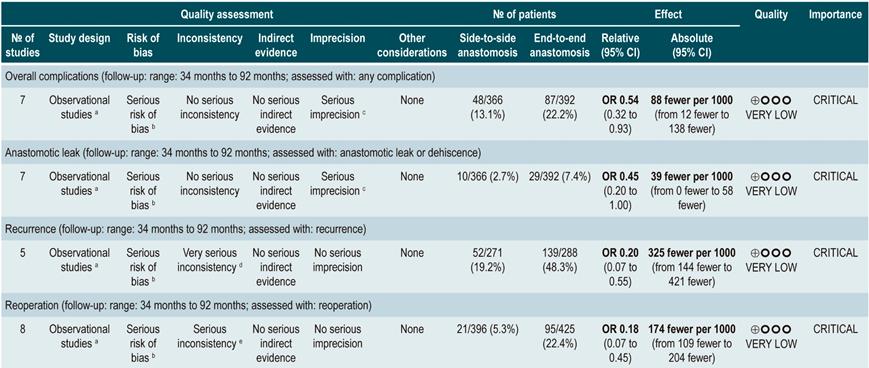
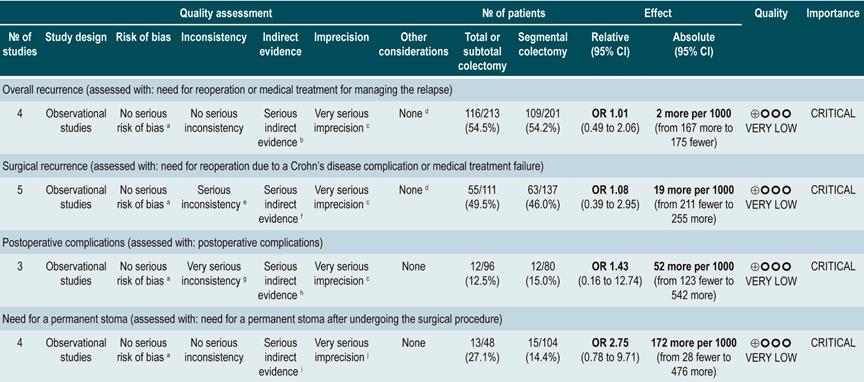
A systematic review that included population-based studies found that the risk of surgery in patients with CD was 16.3%, 33.3%, and 46.6% at 1, 5, and 10 years of follow-up, respectively33. Unfortunately, CD cannot be cured with surgery: 50% of patients report clinical recurrence, 80%, endoscopic recurrence, and 30% require additional surgical management34. In addition, patients with CD are classified based on the risk of developing complications35. On the other hand, conditions that are associated with a poor prognosis in these patients include isolated location in the ileum or location in the ileum and colon, severe involvement of the small bowel or extensive involvement of the upper gastrointestinal tract, perianal lesions, severe rectal involvement, deep ulcers on colonoscopy, history of intestinal surgical resections, stenosing and penetrating behavior of the disease, the need for steroid use at the time of diagnosis, young age at diagnosis (<30 years) and being a smoker35,36.
The current objective of CD treatment is to induce and maintain the clinical and endoscopic remission of its symptoms in order to prevent its progression37,38. In the past, the goal was to control CD symptoms; however, they do not correlate with the inflammatory activity of the involved areas. Therefore, the lack of this correlation has shifted the treatment goals to achieve a “deep remission”, which includes both, clinical remission, and endoscopic healing1,39. When “deep remission” is achieved, there are fewer relapses, fewer surgeries, and less intestinal damage (38). Considering that deep remission is the current goal of treatment for CD, therapeutic targets (“Treat to Target”) have been identified40, and recent studies suggest that this strategy, besides being cost-effective, should have an impact on CD progression and improve outcomes in these patients41.
Drugs used in the treatment of CD aim to attenuate or reduce the chronic abnormal inflammatory activity by acting on the immune pathways of the disease1. It should be noted that none of the available treatment cures CD. Drugs used to treat CD include 5-aminosalicylates (5-ASA); systemic (prednisone, prednisolone) or topical (budesonide) steroids; immunomodulators (azathioprine, 6-mercaptopurine, methotrexate); biological therapy (tumor necrosis factor-alpha [TNF-α] inhibitors): infliximab, adalimumab, and certolizumab pegol; anti-integrins: natalizumab, vedolizumab, anti-L12/23 p40 subunit, ustekinumab; probiotics, and antibiotics (ciprofloxacin, metronidazole). Surgical treatment is also available. Furthermore, the expiration of the patent of infliximab has allowed the introduction of its biosimilar37,42-44.
Given the complexity of CD, management by a multidisciplinary team is necessary, which, in addition to the gastroenterologist, includes the fundamental participation of a colon and rectal surgeon expert in the disease, as well as a nurse, a radiologist, a pathologist, a nutritionist and a psychologist, among others45.
The standard of care includes the education of patients and their families, the absolute prohibition of smoking and the vaccination of patients46-49.
Once the CD diagnosis has been established, ruling out the presence of diseases that may recur or be exacerbated when starting immunosuppressive treatment (steroids, immunomodulators or biological therapy) is essential50. Therefore, performing some tests is required, including tuberculin test; chest x-rays; hepatitis B virus panel: hepatitis B surface antigen, HBsAb (hepatitis B surface antibody), anti-HBc or HBcAb (hepatitis B core antibody); HCV antibody (anti-HCV test); human immunodeficiency virus (HIV), varicella zoster, and Epstein-Barr virus tests51. On the other hand, a concern when using biological therapy is the risk of infection, serious infection, and opportunistic infection. A serious infection occurs when the patient requires hospitalization or intravenous administration of antibiotics, and an opportunistic infection is caused by the weakening of the immune system, since it does not occur in immunocompetent individuals. Some examples of this type of infection include those caused by Clostridium difficile, mycobacterium, candida, cytomegalovirus, and varicella zoster50. Per se, CD activity also increases the risk of infections: when the disease is moderate-severe, the risk of serious disease increases twofold. For every 100 points of activity, the risk of serious infection and opportunistic infections increases by 39% and 31%, respectively51.
CD treatment involves an induction pharmacological regimen and then a maintenance pharmacological regimen. The choice of medication depends on the severity of the disease, the use of the medication in any previous treatment, and the presence of risk factors for developing complications35,37,52,53.
Currently, there are several pharmacological therapies and surgical interventions to treat CD; however, and despite there are multiple randomized studies, some clinical conditions related to CD are still managed based on clinical judgment and experts’ opinion, which has been reflected in the existing conceptual differences regarding the treatment of these patients. Taking this into account, and the fact that CD is a chronic disease that mostly affects young people, as well as the resulting social and economic implications, the development by multidisciplinary groups of a clinical practice guideline (CPG) for the treatment of this disease based on the best and more recent available evidence is necessary to unify the criteria for the successful management of CD in Colombia. The wide variety of clinical scenarios and the diverse individual and social circumstances of these patients difficult the provision of medical care to them, which is one of the justifications for developing this GPC with the aim of reducing the unjustified variability of criteria for treating patients with CD. Although the concepts presented here are based on the best published scientific evidence, this CPG provides recommendations that shall be used according to the clinical judgment of the treating physician.
Objectives
This CPG was developed taking into account the following objectives:
To make evidence-informed recommendations for the treatment of patients with CD.
To contribute to the timely and safe treatment of patients with CD, considering the minimization of the need for hospitalization and sequelae.
To support decision makers in the development of policies for the proper management of CD.
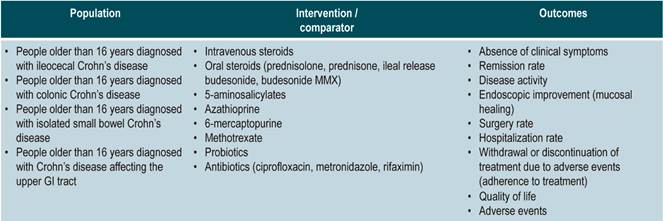
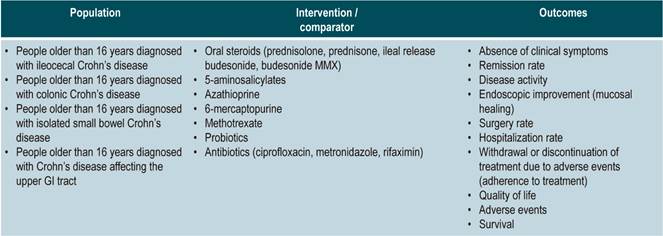
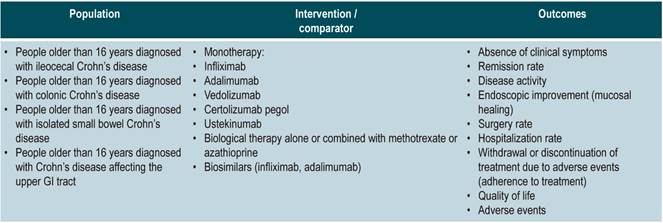
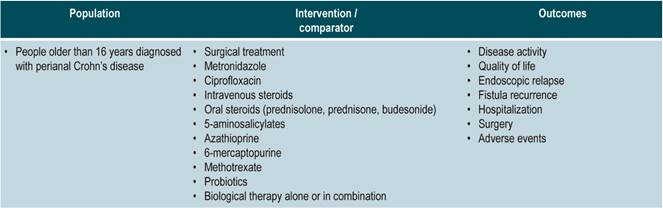
Population
Population groups to be considered
Patients older than 16 years diagnosed with CD, regardless of the time of progression and the clinical stage of the disease, nor the health care insurance plan they are enrolled in.
Users of the clinical practice guideline
This CPG is intended for health care workers such as gastroenterologists, colorectal surgeons (coloproctologists), gastrointestinal surgeons, internal medicine specialists, family medicine specialists, general practitioners, as well as for patients and other health care professionals interested in the management of CD.
Funding of the clinical practice guideline
The development of this GPC was funded by the Colombian Association of Gastroenterology.
Editorial independence statement
The funding organization provided support to the group in charge of the development of the guidelines (GDG) during its development, thus guaranteeing that its contents are transferable and applicable in the Colombian context. The scientific research work, as well as the recommendations included in this document were carried out independently by the GDC. The funding organization did not have any influence on the contents of the CPG.
Scope
This CPG is directly intended for health care professionals who provide medical care to patients with CD, but it is also indirectly intended for health care decision makers in the context of health care provision, and health insurance companies, health care payers, and health care policy makers. This CPG is intended to establish guidelines for the treatment of CD, and its scope is limited to the target population.
Health care provision setting
This CPG aims to help medical care providers treating patients older than 16 years with CD in any level of care. It should be noted that the management of very specific conditions by health care professionals involved in the care of patients with CD requires specific recommendations, which are beyond the scope of this guideline.
This CPG provides recommendations for all levels of care in which patients with CD are treated. It also provides health care professionals with enough information to provide guidelines for the proper management of CD.
Main clinical aspects
Clinical aspects addressed by the CPG:
The CPG will address the medical and surgical treatment of CD and poor prognostic factors in patients older than 16 years.
Aspects related to the diagnosis of the disease or the rehabilitation of these patients will not be addressed since, due to their length and complexity, these should be addressed in separate CPGs to be developed de novo (NICE, 2010).
Guideline audit support
Review criteria and assessment indicators have been included in the development of the CPG.
Updating the clinical practice guideline
Recommendations made in this guideline must be updated within the next three (3) years or sooner if new evidence modifying said recommendations becomes available. This process should be carried out by creating an expert panel responsible for making the necessary changes.
Glossary
Active disease: CD is clinically classified as mild, moderate, and severe. The severity of the disease is determined using the Crohn’s Disease Activity Index (CDAI). In most clinical trials, CD is classified depending on the score as follows: mild: 150-220; moderate: 220-450, and severe: >450.
Steroid-dependent Crohn’s disease:
patients in which reducing the steroid dose below the equivalent of prednisone 10 mg/day (budesonide 3 mg/day) within the first 3 months after being administered the steroids is not possible without experiencing disease recurrence; or
patients who relapse within the first 3 months after steroids are suspended.
total length of steroid therapy should not exceed 3 months.
Extensive disease: intestinal involvement >100 cm, regardless of CD location. The sum of inflammation areas alternating with non-involvement areas is included.
Localized disease: intestinal involvement of less than 30 cm.
Steroid refractory disease: patients with CD activity despite the administration of up to 1 mg/kg/day prednisone for 4 weeks.
Relapse: exacerbation of symptoms in a patient with CD who was in clinical remission, either if it occurs spontaneously or after medical treatment; a 70-point increase in the CDAI. Confirming the relapse with laboratory, endoscopic or imaging studies in the clinical practice is suggested.
Early relapse: exacerbation of symptoms in less than 3 months in a patient with CD in clinical remission and undergoing medical treatment.
Recurrence: recurrence of endoscopic lesions after undergoing surgical resection.
Clinical recurrence: recurrence of symptoms after performing the complete macroscopic resection of the disease, and after confirming the recurrence of endoscopic lesions. Confirming the presence of lesions is important, since there are conditions with symptoms that can mimic those of CD (bile salts malabsorption, motility disorders, bacterial overgrowth, among others).
Morphological recurrence: appearance of new CD lesions after performing the macroscopic resection of the disease, usually in the neo-terminal ileal stricture or in the anastomosis; it is usually detected through endoscopy, imaging studies or surgery. Endoscopic recurrence is classified according to the RUTGEERTS score: 0: no evident lesions. 1: less than 5 anastomotic aphthous lesions. 2: more than 5 apthous lesions with normal mucosa between the lesions. 3: diffuse aphthous ileitis with diffusely inflamed mucosa. 4: ileitis with ulcers, nodules, and/or strictures.
Clinical remission: CDAI <150 points. Confirming clinical remission by means of objective parameters based on laboratory (fecal calprotectin, CRP), endoscopic or imaging studies in the clinical practice is suggested.
Response to treatment: a change in the CDAI score, a ≥100 points decrease in the CDAI score.
Methodology
This section has been adapted from the Pan American Health Organization (PAHO) template available in the Strengthening national evidence-informed guideline programs. A tool for adapting and implementing guidelines in the Americas document, published in 2018.
Composition of the group
The GDG was composed by experts in gastroenterology, colorectal surgery, gynecology, epidemiology, pharmaceutical chemistry, and public health. In addition, cooperation by the Colombian Cochrane STI (Sexually Transmitted Infections) Group was received by the GDG. The Cochrane STI Group performed the systematic search of the relevant literature, retrieved the full text of the studies and created the GRADE tables.
Conflicts of interest statement
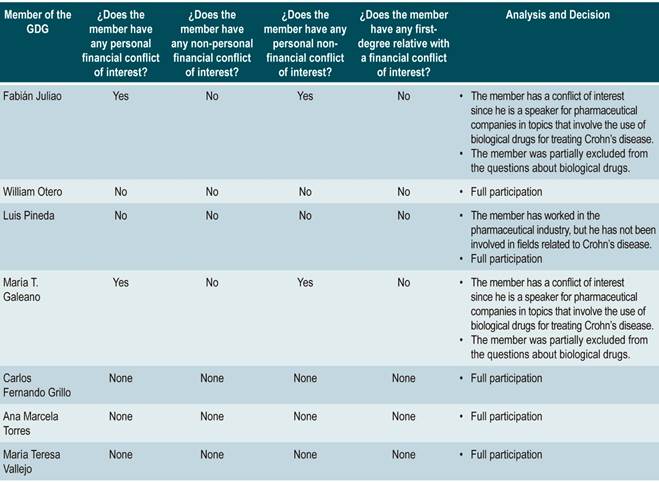
Those who took part and are responsible for the making of the recommendations presented in this CPG shall state in writing and in advance any conflicts of interest regarding said recommendations.
An analysis of the conflicts of interest was carried out and, based on the conflict or conflicts stated, partial or full participation was decided. Two experts were excluded from the formulation process of recommendations related to biological drugs for they are speakers for several pharmaceutical companies on the use of these drugs for treating CD. This analysis is available in Annex 1.
Definition of the scope and objectives of the clinical practice guideline
The scope and objectives of this CPG were defined by the Colombian Association of Gastroenterology with the purpose of supporting health professionals that provide medical care to patients with CD, so that they can provide high quality, equitable, efficient, and homogeneous medical care to these patients. After conducting a literature review on CD, the GDG wrote a document considering the heterogeneity in clinical practice, the availability of new evidence, the existence of new therapeutic alternatives, the inadequate use of resources, and the quality problems found in clinical practice resulting from the health care provision system. The topics that were addressed and those who were not addressed, as well as the target population and main clinical aspects of the CPG were also defined.
Decision on the development or adaptation of the clinical practice guideline
The GDG conducted a systematic search of the literature in order to identify all Colombian and international CPGs addressing the management of patients with CD and that had a similar scope and objective to those proposed for this CPG. The quality of the CPGs retrieved was evaluated using the AGREE II tool54 and each document was graded independently by two raters in order to determine the overall quality of each guideline. According to the guidelines proposed by international CPG developers, once the grading process was completed, discrepancy levels for each guideline were assessed to identify the domains that required to be reviewed. This discrepancy was evaluated using the rating system proposed by the AGREE group54.
Once the overall quality of each guideline was determined and the domains that needed to be reviewed were identified, informal consensus meetings were held to establish the possibility of adapting or developing de novo the CPG. Bearing this in mind, the GDG used the criteria included in the CPG adaptation or de novo development decision matrix as input55.
The following aspects are considered in the decision matrix:
The scope and objective of the retrieved CPGs must be related to the scope and objectives of the CPG to be developed.
The CPGs retrieved were developed using evidence-based methodologies, have evidence tables and are less than 5 years old.
The CPGs must have a have an adequate score in terms of methodological quality and editorial independence when evaluated using the AGREE II tool.
The CPGs must be recommended by both raters.
Based on the results of the decision matrix, the GDG considered that none of the eligible CPGs met all the necessary criteria to be adapted, so the de novo development of the CPG was started.
Formulation of the clinical questions of the clinical practice guideline
The GDG reviewed the relevant clinical aspects to be included in the CPG and, based on them, formulated basic questions which were then restructured according to the PICO (population, intervention, comparison, and outcome) framework. The resulting questions can be found in Annex 2.
Identification and grading of the clinical practice guideline outcomes
Literature search for the de novo development
The first step for the de novo development was conducting a search of systematic reviews in the following databases: MEDLINE (via Ovid) EMBASE (via embase.com) and Cochrane Library, which in turn includes the Health Technology Assessment (HTA) database, the Database of Abstracts of Reviews of Effects (DARE) and the NHS Economic Evaluation Database (NHS EED).
Search strategies were developed and performed by the search coordinator of the Cochrane STI Group; the GDG contributed to this process too. For this purpose, identification forms of words related to the clinical questions were used to select controlled language and free language terms, which in turn allowed creating the search syntaxes for each database (Annex 4). No publication date or language restrictions were applied in the search. Classic and relevant studies about CD proposed by experts were also included for full analysis. Clinical questions that were not addressed by systematic reviews were answered by including primary studies.
Grading of the evidence
Evidence was graded according to the type of evidence. The systematic reviews (SR) that were retrieved were assessed using the AMSTAR checklist56; besides, the contents, quality and clinical relevance of each SR were evaluated to identify those with the highest methodological quality, which were finally included in the GPC. When there were no high-quality systematic reviews, primary studies were assessed using the risk of bias tool recommended by Cochrane57.
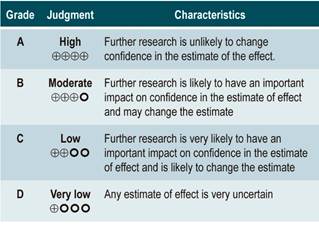
Evidence profiles were created using the www.guidelinedevelopment.org website to summarize the evidence found, and the levels of evidence were graded according to the GRADE classification, which grades the quality of the evidence in four levels58.
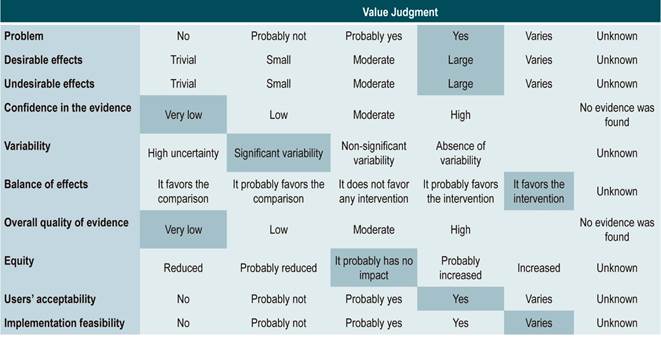
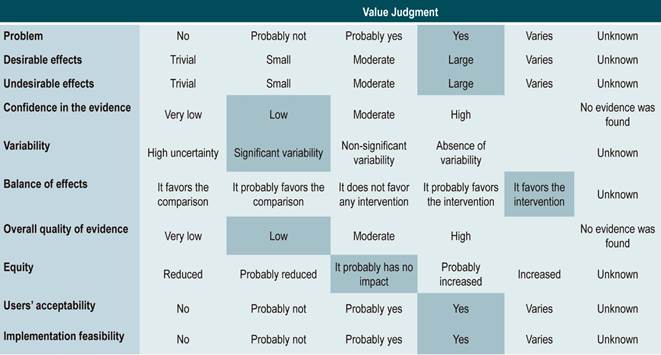
Grading the strength of the recommendations
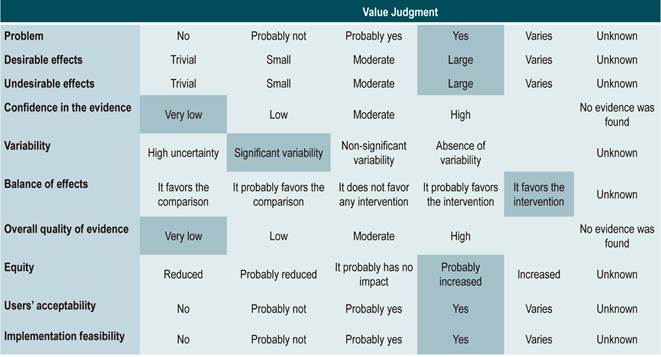
Recommendations were formulated in two steps. First, the GDG made the preliminary recommendations considering the risk-benefit balance, the preferences of patients, and the Colombian context. Then, the recommendations were discussed and adjusted in an expert panel with the representatives of users and patients. The strength of each recommendation was determined based on the level of evidence and other additional considerations that were fully reviewed by the GDG, the managing body of the CPG, and the expert panel taking into account the different scenarios of the Colombian context.
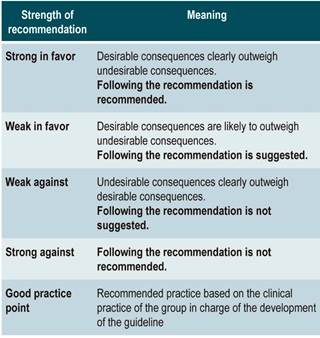
The GRADE methodology grades the strength of a recommendation as “Strong” or “Weak”. Once the risk-benefit balance, the quality of evidence, the values and preferences of patients, and the Colombian context were considered, the strength of each recommendation was determined using the following structure:
Finally, expert panel agreement with the recommendations that were suggested and the inclusion of the participants’ perspective in them was verified. All recommendations and their grades were voted on.
Good practices
Good practices are operational suggestions based on the experience of the GDG and the GRADE working groups where different stakeholders participated; although they are not evidence-based, they are part of the good diagnosis, treatment, or follow-up practices of patients. Good practices are intended to support the recommendations made in the CPG.
Inclusion of costs and preferences of patients
A cost-effectiveness assessment was not performed in the development of this CPG. The preferences of patients were identified through a systematic review, where the values and treatment preferences of patients and their perception of CD were assessed, which in turn helped strengthen the recommendations.
Cost-related considerations were not included in the development of this CPG given the variability of the Latin American context, so they are not included in the value judgment table of the recommendations. In addition, cost-influenced recommendations were not identified. Regarding preferences and values of patients, a search was performed, but it yielded only one study. However, given the disease, the management strategy and the target population, there are few recommendations that can be influenced by the preferences of parents or caregivers.
Formulation of questions by expert consensus
In the case of clinical questions in which no evidence was found or where evidence was controversial, the GDG made clinical practice recommendations based on their professional experience and good practices; these recommendations were submitted to formal consensus in the GRADE working groups.
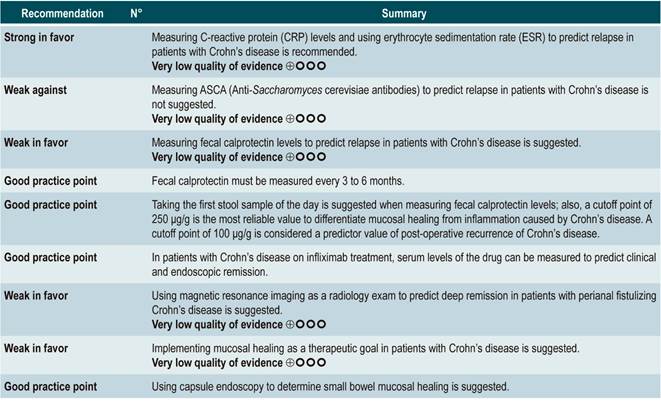
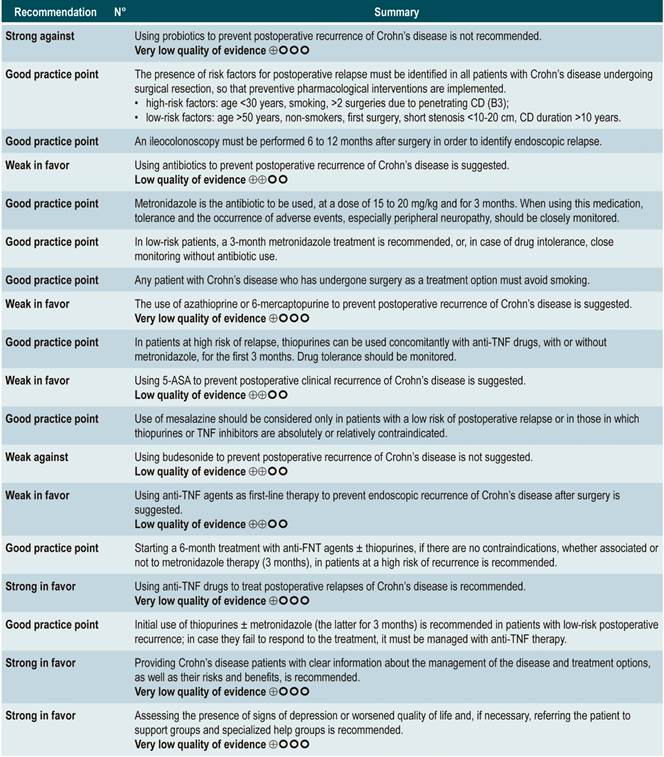
Question N° 1. What are the predictors of relapse of crohn’s disease in patients older than 16 years?
Summary of evidence
C-reactive protein, erythrocyte sedimentation rate, and ASCA
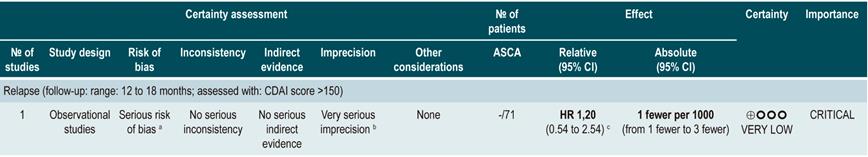
A systematic review (AMSTAR score 8/11) assessed the predictive ability of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and ASCA in patients with CD who were in remission. The outcome of interest was the occurrence t of relapse, defined as having a CDAI score >150; the prognostic value was expressed using hazard ratios (HR) or risk ratios (RR)59.
There was heterogeneity among studies included in the review regarding the reporting of outcomes and the cut-off points assessed; thus, summarizing the evidence using meta-analysis techniques was not possible.
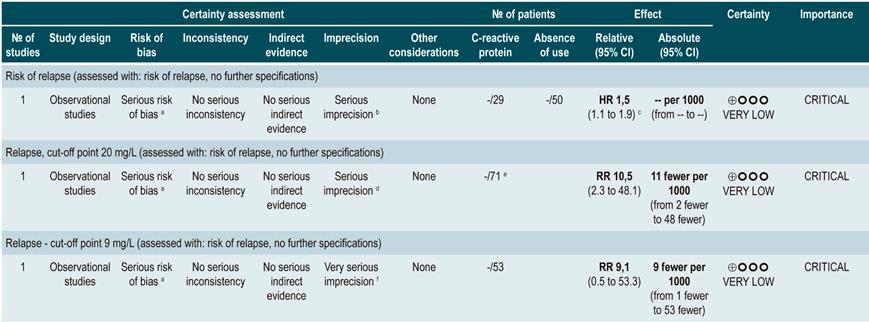
C-reactive protein: a cohort study performed a relapse prediction model based on the biological parameters of 101 patients with fistulizing, inflammatory or stenosing, intestinal or extraintestinal CD. Based on this model, it was established that, during the first year of follow-up, a CRP level greater than 10 mg/L substantially increases the probability of relapse (HR: 1.5; 95% confidence interval [CI]: 1.1-1.9) within the next 92 days. The model was adjusted by fistulizing CD, presence of colitis, and level of stress60.
A second cohort study assessed the prognostic value of CRP in 71 patients with ileal, ileocolonic or colonic CD, using a cutoff value of 20 mg/L. According to this study, a positive result in the CRP test markedly increases the risk of relapse (RR: 10.5; 95% CI: 2.3-48.1) within the next 6 weeks61. Finally, a third study analyzed the role of CRP together with other serological tests as predictive markers in 53 patients with colonic CD. In said study, when the model was adjusted by calprotectin levels, gender, presence of smoking, extent of the disease, and azathioprine consumption, no significant differences in the risk of recurrence were found when CRP levels exceeded 9 mg/L (HR: 9.1; 95% CI: 0.5-53.3)62.
In addition, in a meta-analysis that was already described above24, a CPR sensitivity of 49% (95% CI: 0.34-0.64) and specificity of 92% (95% CI: 0.72-0.96) for the detection of endoscopic disease activity in patients with inflammatory bowel disease was found.
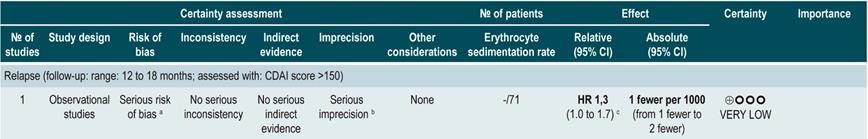
Erythrocyte sedimentation rate (ESR): a cohort study analyzed the predictive value of several biological tests in 101 patients with fistulizing, inflammatory or stenosing, intestinal or extraintestinal CD; in the bivariate analysis, no association between ESR values and subsequent risk of relapse was found (HR: 1.3; 95% CI: 1.0-1.7)60. On the other hand, another cohort study conducted in 71 participants determined the prognostic usefulness of ESR in patients with ileal, ileocolonic or colonic CD, reporting that when ESR exceeded 15 mm, the risk of relapse increased during the next 6 weeks (RR: 6.1; 95% CI: 1.9-18.9). Finally, according to the same study, high levels of CRP (>20 mg/L) and ESR (>15 mm) were significantly associated with recurrence of active Crohn’s disease within the next 6 weeks (RR: 9.9; 95% CI: 3.3-29.7)61.
Anti-Saccharomyces cerevisiae antibodies (ASCA): only one cohort study, conducted in 101 patients with fistulizing, inflammatory or stenosing, intestinal or extraintestinal CD, determined the role of this biomarker as a predictor of relapse in CD patients. The analysis of this study failed to show an association between positivity for this marker and the subsequent development of active CD (HR: 1.2; 95% CI: 0.54-2.5)60.
Quality of evidence: very low ⊕ΟΟΟ
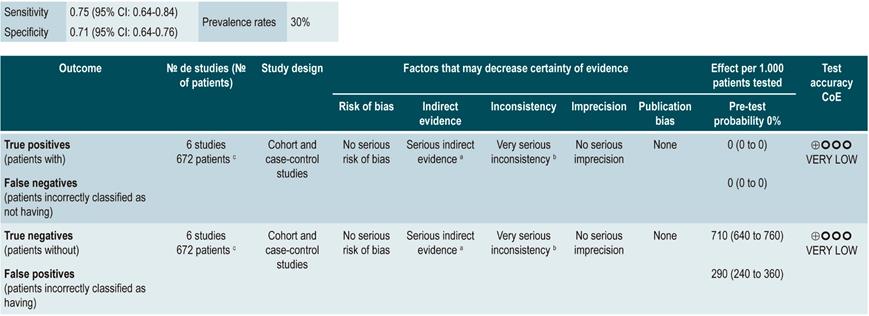
Fecal calprotectin
A systematic review and meta-analysis63 (AMSTAR score 8/11) evaluated the diagnostic accuracy of fecal calprotectin to predict relapse of CD. The presence of active Crohn’s disease, defined as having a CDAI score >150 or a Harvey-Bradshaw index score greater than 4 was determined as the standard of reference, and diagnostic accuracy was reported in terms of sensitivity, specificity, positive and negative likelihood ratios (LR+, LR-) and area under the curve values.
Based on the information provided by the six studies included in the systematic review, it was found that a positive value in fecal calprotectin levels (positivity range from 130 µg/g to 340 µg/g) acceptably predicts the development of recurrence of CD (sensitivity, 75%; 95% CI: 64%-84%; specificity, 71%; 95% CI: 64%-76%; LR+, 2.37; 95% CI: 1.56-3.61; LR-, 0.41; 95% CI: 0.27-0.61), with an area under the curve (AUC) of 0.79 (95% CI: 0.74-0.64). Besides, the test performance was quite similar in the case of patients with colonic CD (sensitivity, 76%; 95% CI, 59%-88%; specificity, 77%; 95% CI, 69%-83%; LR+, 3.26; 95% CI, 1.89-5.25; LR-, 0.34; 95% CI, 0.19-0.60), with an AUC of 0.81 (95% CI, 0.76-0.86)63.
In the case of post-operative endoscopic recurrence of CD, sensitivity and specificity were 0.90 and 0.36, respectively, for a fecal calprotectin cutoff point of 50 μg/g; 0.81 and 0.57, for a cutoff point of 100 μg/g; 0.70 and 0.69, for a cutoff point of 150 μg/g; and 0.55 and 0.71, for a cutoff point of 200 μg/g. In patients with a small bowel CD diagnosis confirmed by capsule endoscopy, the sensitivity and specificity values were 0.83 and 0.53, for a cutoff point of 50 μg/g, and 0.42 and 0.94, for a cutoff point of 200 μg/g, respectively64.
The recently published CALM study combined the use of fecal calprotectin and CRP levels, reporting a mucosal healing rate in 79% of patients with calprotectin and CRP levels <250 μg/g and <5 mg/L, respectively65.
Quality of evidence: very low ⊕ΟΟΟ
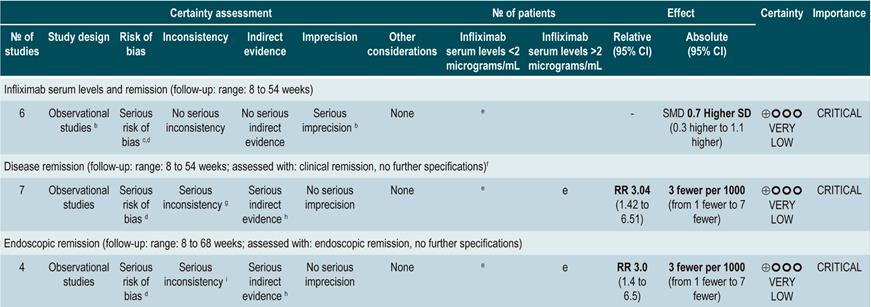
Serum levels of infliximab
A systematic review and meta-analysis66 (AMSTAR score 8/11) assessed the prognostic utility of serum levels of infliximab in patients with inflammatory bowel disease. The primary outcome was the frequency of clinical remission, while secondary outcomes included the proportion of patients with endoscopic remission and the need for colectomy. The review retrieved 22 observational studies, of which 11 were conducted exclusively in patients with CD, 4 in patients with ulcerative colitis, and 7 in individuals with indeterminate inflammatory bowel disease. Based on the findings of this systematic review, when infliximab levels exceeded 2 µg/mL at 14 weeks, patients with inflammatory bowel disease were more likely to achieve remission (RR: 2.91; 95% CI: 1.79-4.73) and intestinal mucosal healing (RR: 3.04; 95% CI: 1.42-6.51). Finally, the presence of undetectable serum levels of infliximab increased the probability of requiring colectomy compared to patients with detectable levels of this drug (RR: 5.4; 95% CI: 3.10-9.30)66.
Quality of evidence: very low ⊕ΟΟΟ
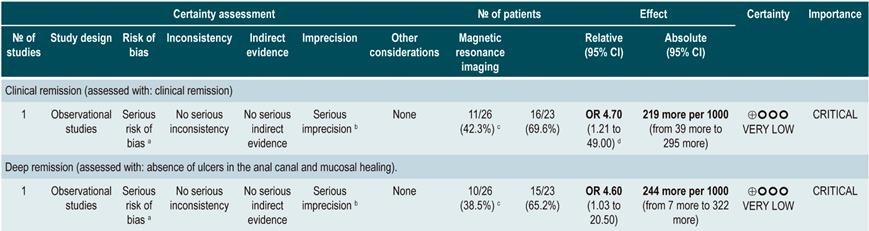
Using Magnetic resonance imaging to predict deep remission
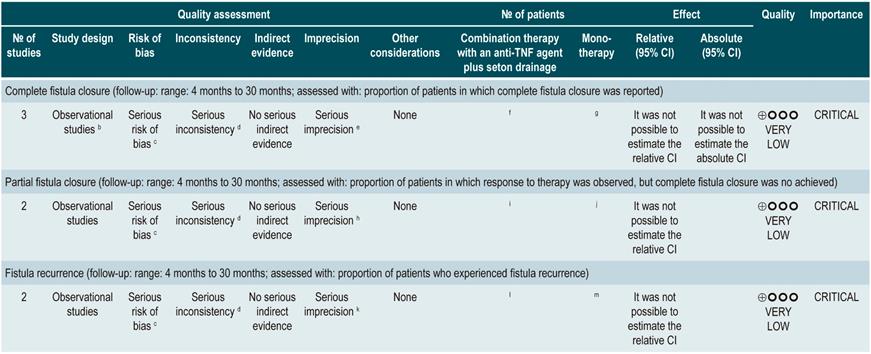
A prospective cohort study67 assessed the usefulness of MRI in predicting deep remission in patients with perianal fistulizing CD. The outcomes of interest were the presence of remission and deep remission (which was determined using the Van Assche index, which evaluates the complexity, extension, and location of the fistula), contrast medium uptake, rectal mucosa involvement, and the presence of abscesses; the assessment was performed independently by two radiologists with expertise in gastrointestinal tract imaging studies and who were not informed on the clinical signs and symptoms of the patients.
The cohort consisted of 49 patients who were undergoing anti-tumor necrosis factor (TNF)-α therapy and concomitant use of immunosuppressants to induce or maintain remission of the disease. According to this study, factors associated with the presence of remission were absence of rectal involvement on the MRI (OR: 4.7; 95% CI: 1.21-49.0) and absence of switch of anti-TNF-α (OR: 7.7; 95% CI: not reported; p<0.05). Regarding deep remission, absence of rectal involvement on the MRI was strongly associated with absence of ulcers in the anal canal (OR: 4.6; 95% CI: 1.03-20.5)67.
A meta-analysis that included 12 studies on the performance of magnetic resonance enterography using a new technique based on diffusion-weighted imaging (DWI), which, unlike the conventional technique, is faster and does not require the intravenous administration of a contrast medium, reported a sensitivity of 92.9% (95% CI: 85.8%-96.6%) and a specificity of 91% (95% CI: 79.7%-96.3%) for detecting inflammation; however, there was heterogeneity among the studies retrieved, and authors concluded that there may be an overestimation of the results68. In addition, a recent systematic review considers this new technique a valid alternative to the conventional technique that is less invasive, faster, and does not require fasting or bowel preparation69.
Quality of evidence: very low ⊕ΟΟΟ
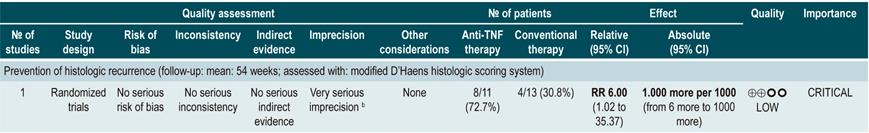
Use of capsule endoscopy to determine small bowel mucosal healing
In a systematic review and meta-analysis of 5 observational studies that included a total of 142 patients with CD who met the inclusion criteria, an association between a capsule endoscopy mucosal healing marker (Niv or Lewis score) and clinical remission at 12 weeks and 24 months of follow-up was found (odds ratio [OR]: 11.06; 95% CI: 3.74-32.73; p<0.001)70.
Mucosal healing
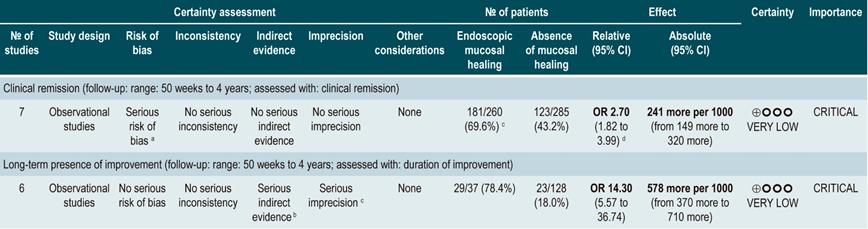
A systematic review38 (AMSTAR score 8/11) evaluated the prognostic value of intestinal mucosal healing in the occurrence of favorable clinical outcomes in CD patients. The following outcomes were considered: the frequency of long-term clinical remission (CDAI <150), the need for surgical treatment, and the rate of long-term mucosal healing.
The review retrieved 12 observational studies conducted in patients with stenosing or fistulizing, perianal, small bowel, ileocolonic, or colonic CD. According to this systematic review, the presence of healthy mucosa increases the likelihood of remaining in clinical remission for at least 50 weeks (OR: 2.7; 95% CI: 1.82-3.99; 304 patients), regardless of the type of treatment (biological therapy [OR: 2.89; 95% CI: 1.82-4.59] or non-biological therapy [OR: 2.48; 95% CI: 1.26-4.89]). Finally, the presence of healthy mucosa was also associated with a higher frequency of long-term endoscopic remission (OR: 14.3; 95% CI: 5.57-36.74), however it was not associated with a higher or lower frequency of patients requiring surgical intervention (OR: 0.22; 95% CI: 0.86-5.69)38.
Quality of evidence: very low ⊕ΟΟΟ
From evidence to the recommendation
The expert panel stated that CRP and ESR are low-cost tests and must be ordered simultaneously. The recommendation is strong because testing should be performed as part of a proper management of the disease that seeks to obtain the best outcomes for patients with CD.
The identification of serum levels of infliximab was not formulated as a recommendation due to its cost; only evidence about one biological drug was found, and this drug is not included in the health benefits plan of the mandatory health insurance coverage system in force in Colombia.
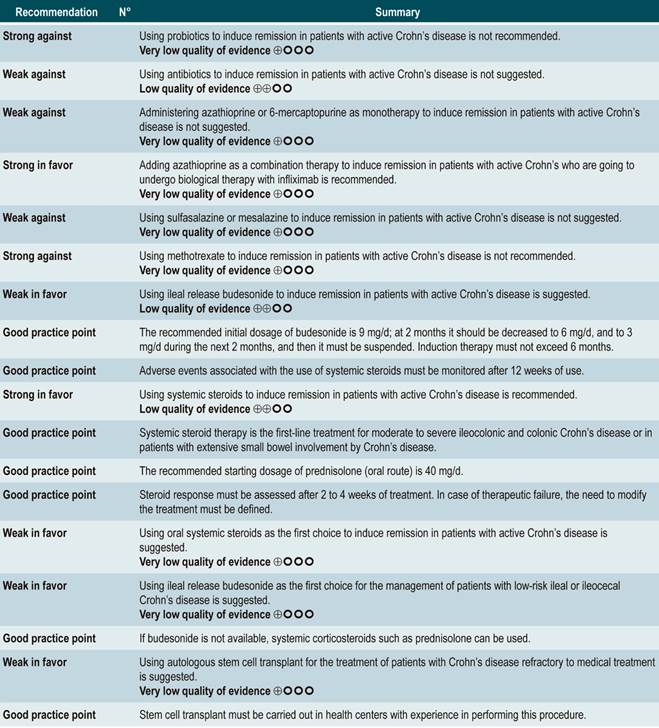
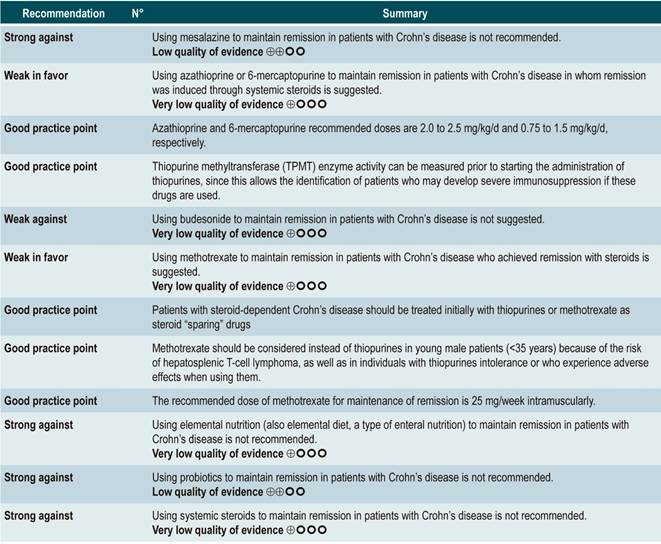
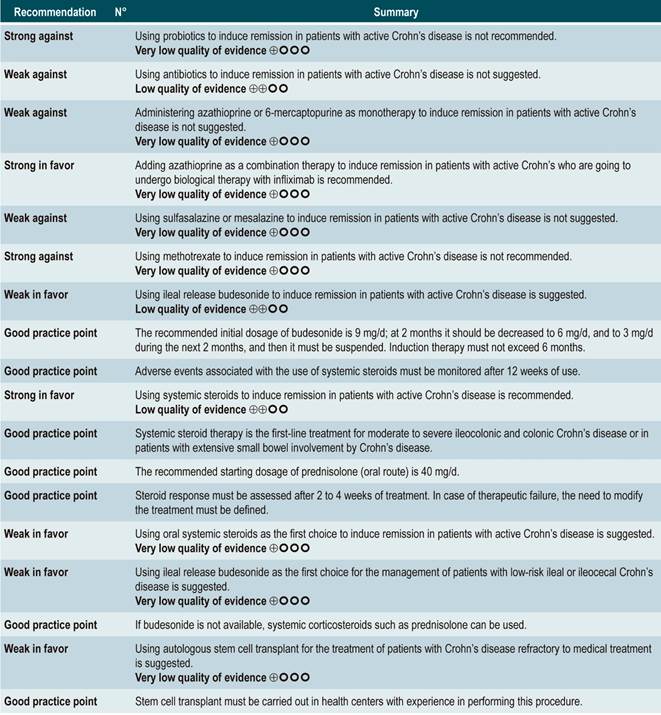
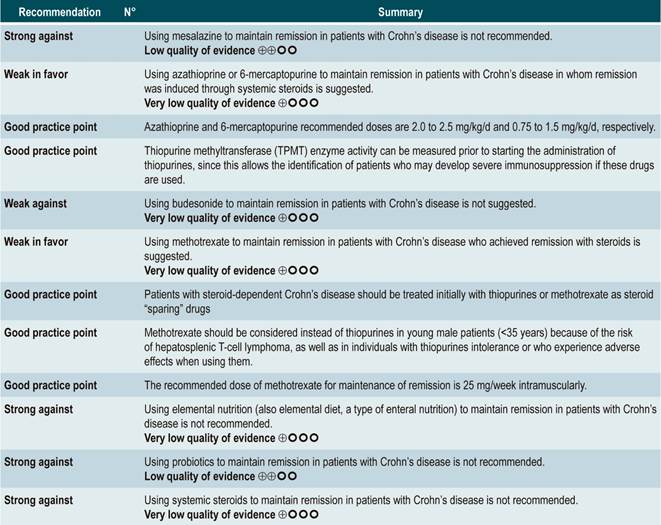
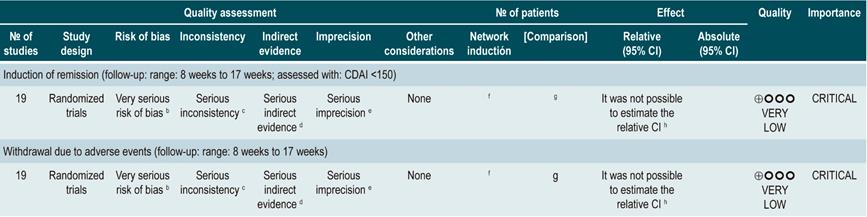
Question N° 2. What are the safest and most effective non-biological interventions to induce remission of crohn’s disease in patients older than 16 years?
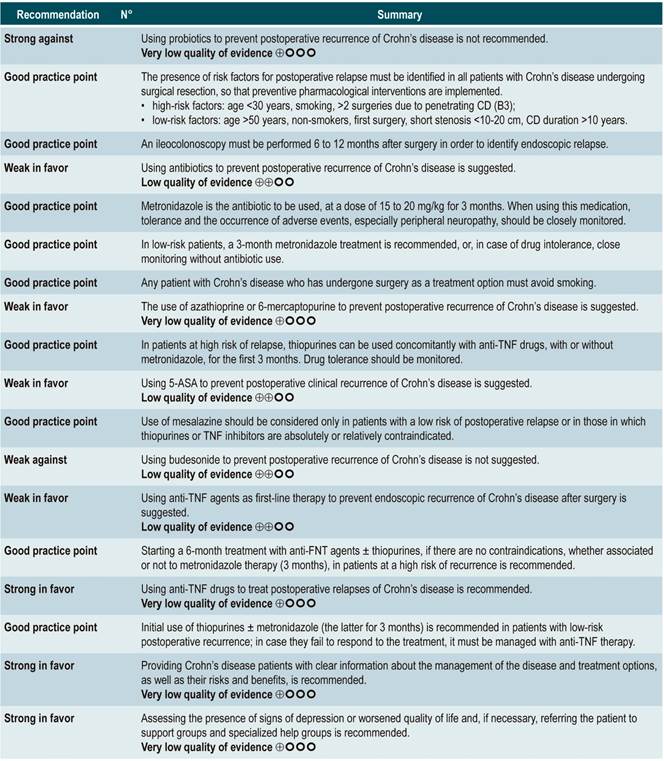
List of recommendations
Summary of evidence
Using probiotics to induce remission in patients with active Crohn’s disease
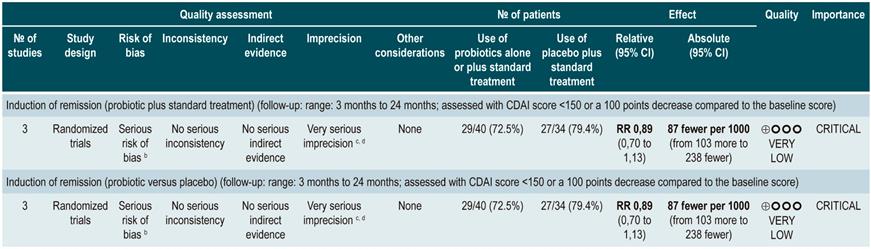
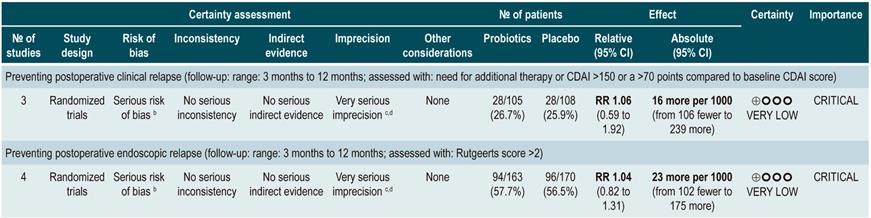
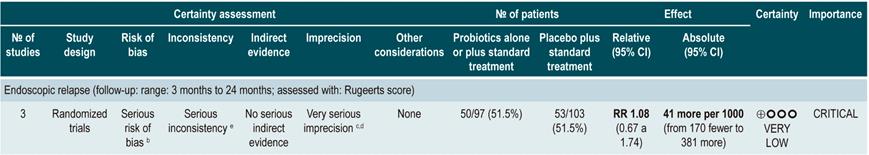
A systematic review and meta-analysis71 (AMSTAR score 8/11) evaluated the efficacy of using probiotics to induce remission in patients with CD. In said study, the assessed outcome was the frequency of patients who achieved clinical remission (defined as having a CDAI <150 or obtaining a score less than 100 points compared to the baseline score) during 3 to 24 months of follow-up. The review retrieved three controlled clinical trials with a total of 74 participants; based on the findings reported, probiotic administration failed to increase the proportion of patients achieving remission, either when used as monotherapy (RR: 0.89; 95% CI: 0.70-1.13) or as an adjuvant in conventional treatment (RR: 0.89; 95% CI: 0.70-1.13)71. Furthermore, in a recent Cochrane review that included 2 studies on the use of probiotics to induce remission in CD, it was concluded that available evidence about the efficacy or safety of using probiotics to induce remission in CD is very uncertain when compared to placebo72.
Quality of evidence: very low ⊕ΟΟΟ
Using antibiotics to induce remission in patients with active Crohn’s disease
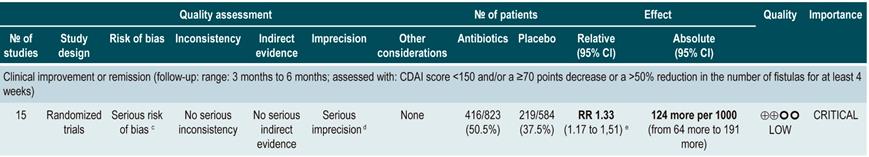
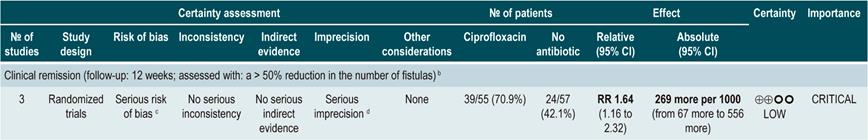
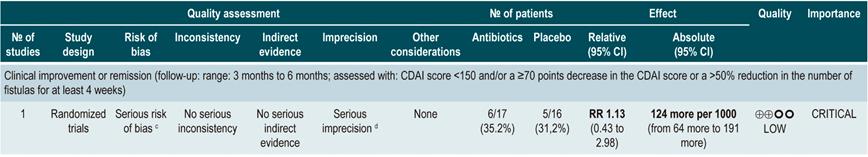
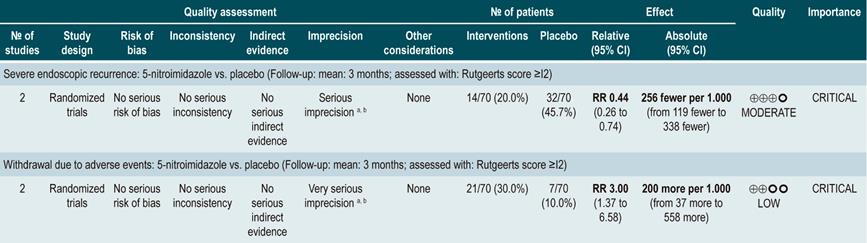
A systematic review73 (AMSTAR score 7/11) assessed the efficacy of using antibiotics to induce remission in patients with active CD. All studies included in the review allowed concomitant use of other interventions (immunomodulators) and the outcome of interest was the proportion of patients who achieved clinical improvement or remission (CDAI <150 and/or a ≥70 points decrease or a >50% reduction in the number of fistulas for at least 4 weeks) during follow-up.
The review retrieved three randomized clinical trials conducted in a total of 222 participants. When compared with placebo, the use of antibiotics during the first 3 to 6 months did not increase the proportion of participants experiencing clinical improvement or remission (RR: 1.15; 95% CI: 0.56-2.36). Also, when a subgroup analysis was performed according to the type of drug used, the administration of ciprofloxacin, rifaximin and 5-nitroimidazoles showed similar results73.
In addition, a recent Cochrane review of 13 studies suggests a modest benefit of using antibiotics in patients with active CD, and that their benefit for maintenance of remission is uncertain; therefore, according to this review, no solid conclusions regarding the efficacy of antibiotics in CD can be reached74.
Quality of evidence: low ⊕ΟΟΟ
Using azathioprine or 6-mercaptopurine to induce remission in patients with active CD
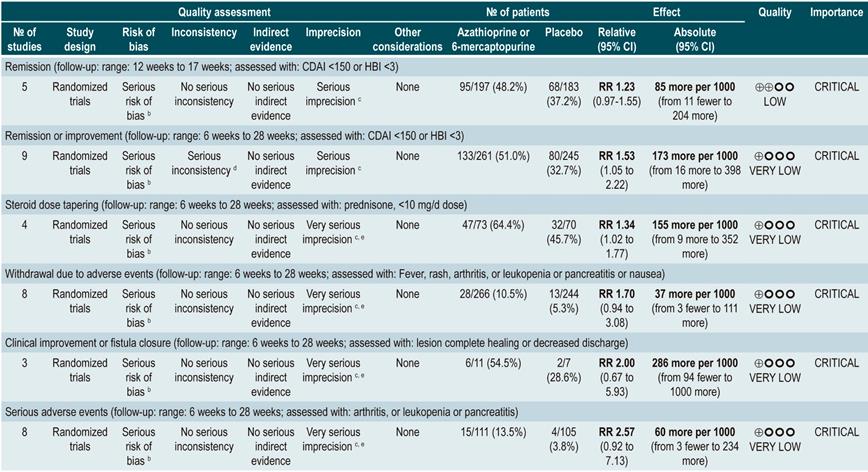
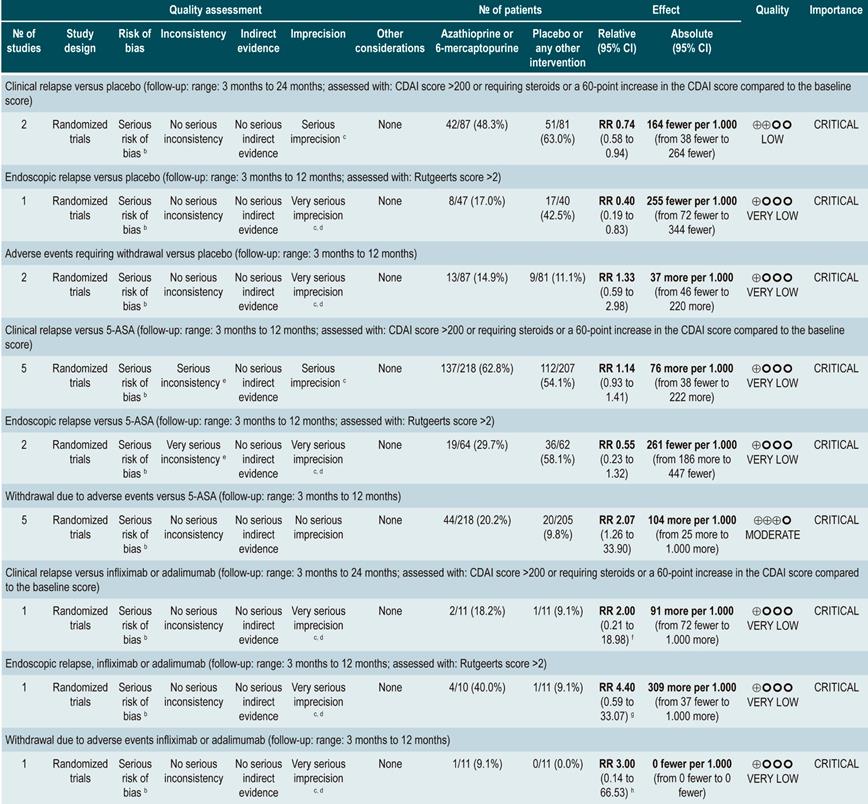
A systematic review and meta-analysis75 (AMSTAR score 10/11) evaluated the efficacy and safety of using azathioprine or 6-mercaptopurine for induction of remission in CD. The outcomes assessed in this study were the proportion of patients who achieved remission or clinical improvement (defined as a CDAI <150 points or a HBI <3), the proportion of patients in which steroid dose reduction was possible (assessed with prednisone at doses <10 mg/d) and who experienced improvement or fistula closure (complete lesion healing or decreased discharge) and, finally, the proportion of patients who experienced serious adverse events, and the frequency of withdrawal.
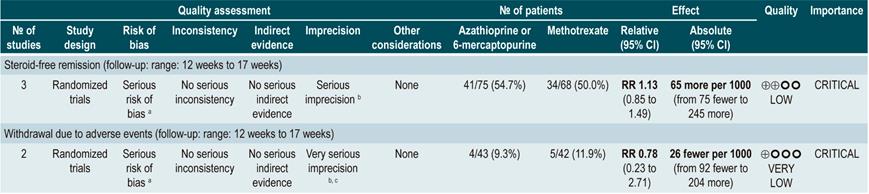
The review retrieved nine studies evaluating the effect of this intervention compared to placebo in 506 participants in total. According to this systematic review, in patients who were administered azathioprine or 6-mercaptopurine higher frequencies of clinical improvement (RR: 1.53; 95% CI: 1.05-2.22) and of lower steroid doses (RR: 1.34; 95% CI: 1.02-1.77) were observed, without this being reflected in a higher frequency of patients who achieved clinical remission criteria (RR: 1.23; 95% CI: 0.97-1.55) or who experienced clinical improvement or fistula closure (RR: 2.00; 95% CI: 0.67-5.93). However, the use of azathioprine or 6-mercaptopurine did not increase the risk of withdrawal (RR: 1.70; 95% CI: 0.94-3.08) or the frequency of adverse events (RR: 2.57; 95% CI: 0.92-7.13)75.
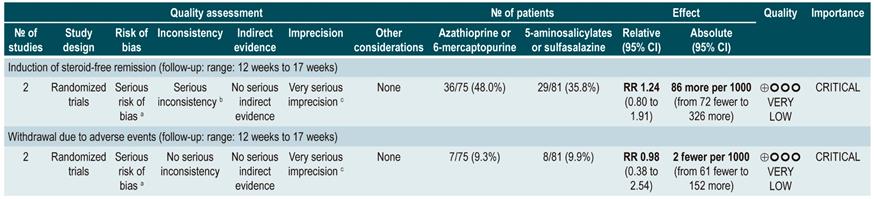
In a second analysis carried out in the same review, the efficacy and safety of azathioprine or 6-mercaptopurine administration versus any other pharmacological intervention to induce remission in patients with CD were compared. When compared with methotrexate, the use of azathioprine or 6-mercaptopurine did not increase the proportion of patients who had steroid-free remission (RR: 1.13; 95% CI: 0.85-1.49; 2 studies, 143 participants) or the frequency of withdrawal (RR: 0.78; 95% CI: 0.23-2.71; 2 studies, 85 participants). Likewise, when patients in the control group were administered 5-aminosalicylates or sulfasalazine, no statistically significant differences were found between groups (steroid-free remission [RR: 1.24; 95% CI: 0.80-1.91] and withdrawal [RR: 0.98; 95% CI: 0.38-2.54]; 2 studies, 156 patients)75.
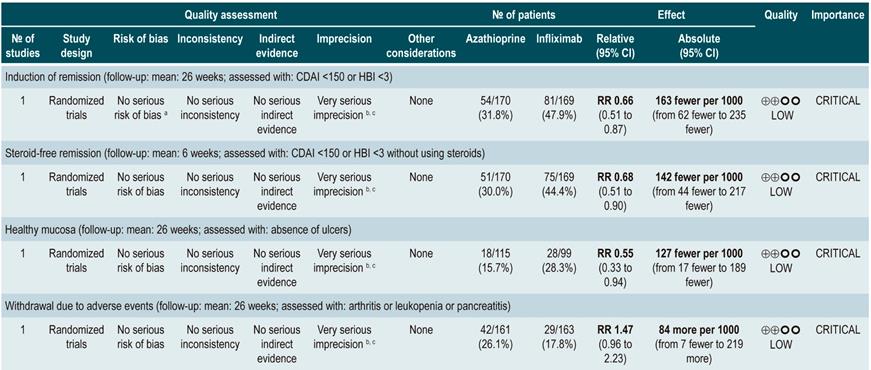
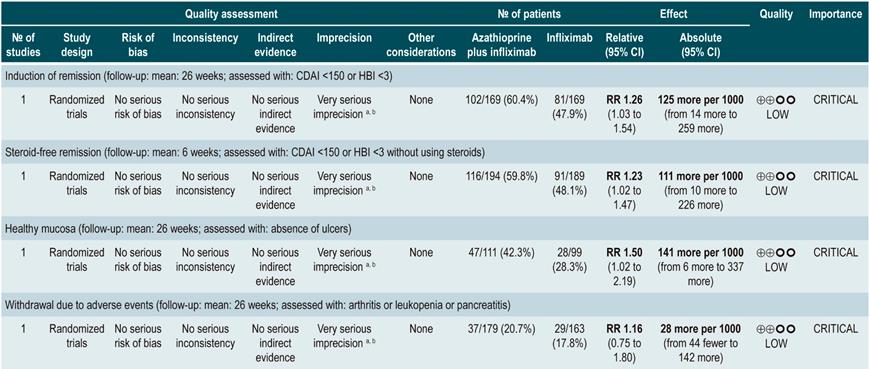
However, when azathioprine administration was compared with infliximab use (1 study, 339 participants), azathioprine was associated with a lower frequency of patients achieving clinical remission (RR: 0.66; 95% CI: 0.51-0.87), steroid-free remission (RR: 0.68; 95% CI: 0.51-0.90) or presenting healthy looking mucosa on endoscopic evaluation (RR: 0.55; 95% CI: 0.33-0.94); however, there were no significant differences regarding the frequency of adverse events between groups (RR: 1.47; 95% CI: 0.96-2.23). Finally, when combination therapy with azathioprine plus infliximab was compared with infliximab monotherapy, the use of combination therapy increased the proportion of patients who achieved clinical remission (RR: 1.26; 95% CI: 1.03-1.54), steroid-free remission (RR: 1.23; 95% CI: 1.02-1.47) or who had healthy-looking mucosa on endoscopic evaluation (RR: 1.50; 95% CI: 1.02-2.19), without increasing the frequency of adverse events (RR: 1.16; 95% CI: 0.75-1.80)75.
Quality of evidence: very low ⊕ΟΟΟ
Using 5-aminosalicylates to induce remission in patients with active Crohn’s disease
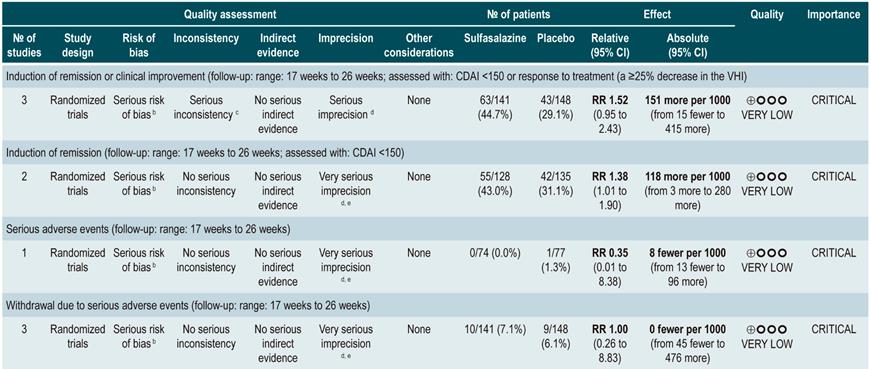
A systematic review76 (AMSTAR score 10/11) evaluated the safety and efficacy of using sulfasalazine to induce remission in patients with mild to moderate CD. This review reported findings on the following outcomes: the proportion of patients who achieved clinical improvement or remission (CDAI <150 or a >25% decrease in the VHI), the frequency of serious adverse events, and withdrawal due to undesirable effects.
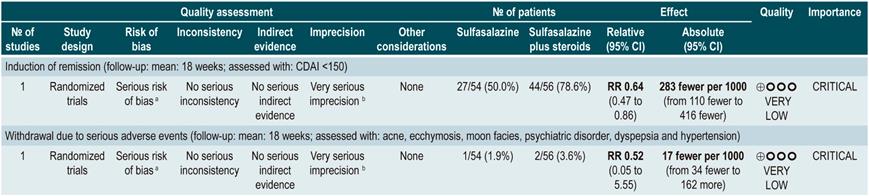
This review retrieved three controlled clinical trials comparing the use of this intervention versus placebo in 289 participants. Based on the results of the retrieved studies, it was found that sulfasalazine administration increased the frequency of remission (RR: 1.38; 95% CI: 1.01-1.90) but did not increase that of clinical improvement (RR: 1.52; 95% CI: 0.95-2.43), yet neither did it increase the incidence of serious adverse events (RR: 0.35; 95% CI: 0.01-8.38) or withdrawal (RR: 1.00; 95% CI: 0.26-8.83). In addition, in a second analysis, the efficacy and safety of sulfasalazine administration versus any other pharmacological intervention were compared. When compared with steroid therapy, the use of sulfasalazine was associated with a lower frequency of serious adverse events (RR: 0.43, 95% CI: 0.22-0.82; 2 studies, 159 participants) at the cost of a lower proportion of patients achieving remission (RR: 0.68, 95% CI: 0.51-0.91; 2 studies, 260 patients). No significant differences were found between groups regarding the rate of withdrawal (RR: 0.72; 95% CI: 0.33-1.59; 2 studies, 260 patients)76.
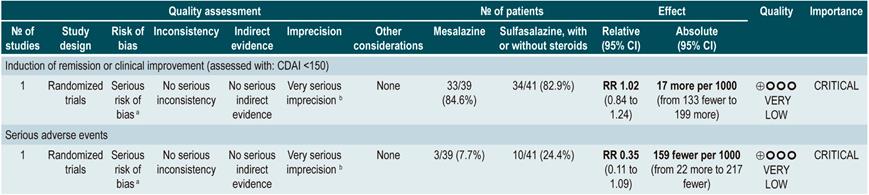
On the other hand, when 5-aminosalicylates monotherapy was compared with sulfasalazine-steroid combination therapy, sulfasalazine monotherapy was associated with a lower frequency of participants achieving clinical remission (RR: 0.64, 95% CI: 0.47-0.86; 1 study, 110 patients) and there was no statistically significant evidence regarding the rate of withdrawal (RR: 0.52, 95% CI: 0.05-5.55; 1 trial, 110 participants). Finally, a subgroup analysis based on the type of 5-aminosalicylates was performed in this review. When mesalazine was compared with sulfasalazine, no differences were found between groups in terms of efficacy (induction of remission [RR: 1.02; 95% CI: 0.84-1.24]) or safety ([serious adverse events: RR: 0.35; 95% CI: 0.11-1.09])76.
Quality of evidence: very low ⊕ΟΟΟ
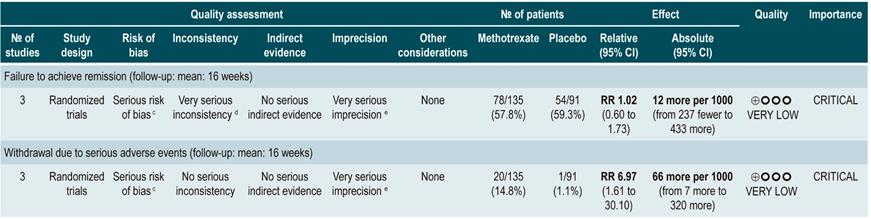
Using methotrexate to induce remission in patients with active Crohn’s disease
A systematic review77 (AMSTAR 8/11) assessing the safety and efficacy of using methotrexate for inducing remission in patients with CD was retrieved. The review included three studies conducted in a total of 226 participants. When compared to placebo, patients in the methotrexate arm did not experience a higher frequency of clinical remission (RR: 1.02; 95% CI: 0.60-1.73), but they did experience a higher frequency of withdrawal (RR: 6.97; 95% CI: 1.61-30.10)77.
Quality of evidence: very low ⊕ΟΟΟ
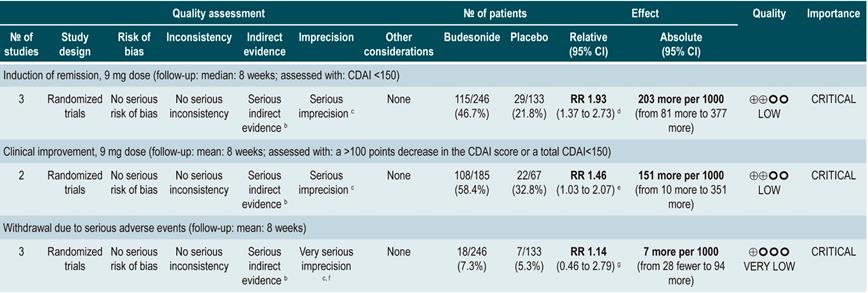
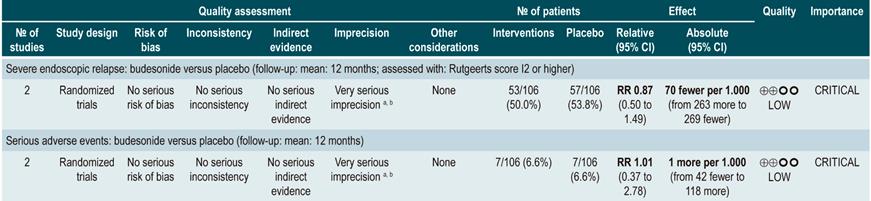
Using budesonide to induce remission in patients with active Crohn’s disease
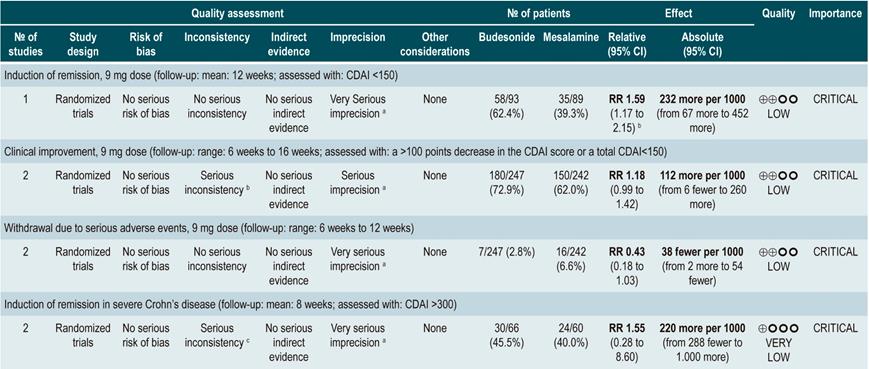
A systematic review and meta-analysis78 (AMSTAR score 10/11) evaluated the efficacy and safety of using budesonide to induce remission in patients with CD. Outcomes assessed in this study were the proportion of patients achieving remission (defined as a CDAI <150 points) and clinical improvement (a >100 points decrease in CDAI score or total CDAI<150 points), and the proportion of patients withdrawing due to serious adverse events. A comparison of the effect of this intervention with that of place was performed in three studies (379 patients in total). According to the findings of this systematic review, in the budesonide administration group higher proportions of remission (RR: 1.93, 95% CI: 1.37-2.73, at 9 mg dose; RR: 2.25, 95% CI: 1.35-3.76, at 15 mg dose) and clinical improvement (RR: 1.46, 95% CI: 1.03-2.07, at 9 mg dose; RR: 2.34, 95% CI: 0.83-6.63, at 15 mg dose) were observed, without increasing the proportion of patients who withdrew due to adverse events (RR: 1.14, 95% CI: 0.46-2.79, at 9 mg dose; RR: 1.55, 95% CI: 0.45-5.34, at 15 mg dose).
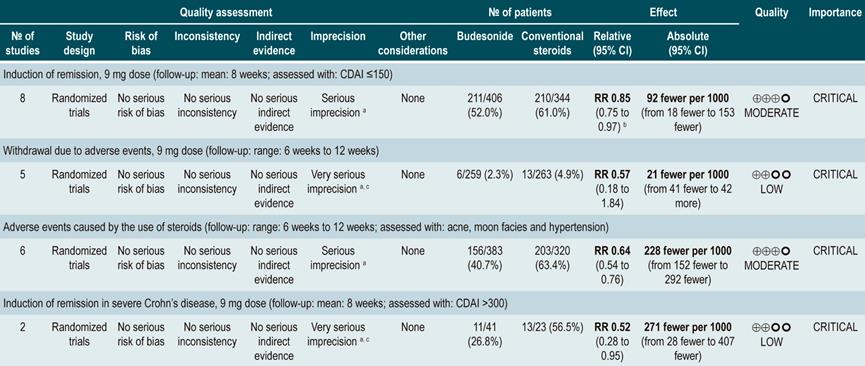
Also, in a second analysis, this review compared the efficacy and safety of budesonide administration for induction of remission in CD versus any other pharmacological intervention. When compared with mesalazine administration, budesonide use at a dose of 9 mg increased the number of patients who achieved remission at 12 weeks (RR: 1.59, 95% CI: 1.17-2.15; 1 study, 182 participants) and 16 weeks (RR: 1.79; 95% CI: 1.28-2.50; 1 study, 182 participants), but it did not increase the frequency of clinical improvement (RR: 1.18; 95% CI: 0.99-1.42; 2 studies, 489 patients) or withdrawal (RR: 0.43; 95% CI: 0.18-1.03; 2 studies, 489 patients). On the other hand, when participants in the control group were administered traditional steroids, patients in the budesonide 9 mg arm experienced a lower frequency of remission in the medium term (RR: 0.85, 95% CI: 0.75-0.97; for 8 weeks), but not in the long term (RR: 1.02, 95% CI: 0.81-1.30; for 12 weeks). Finally, patients who were administered budesonide reported a lower frequency of adverse events (RR: 0.64; 95% CI: 0.54-0.76), yet this was not reflected in a higher or lower frequency of withdrawal (RR: 0.57; 95% CI: 0.18-1.84)78.
Quality of evidence: low ⊕⊕ΟΟΟ
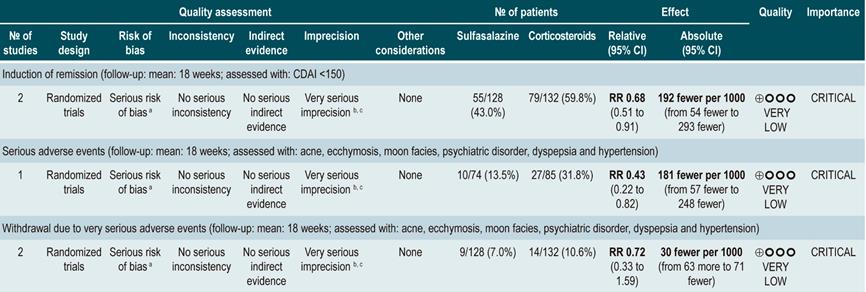
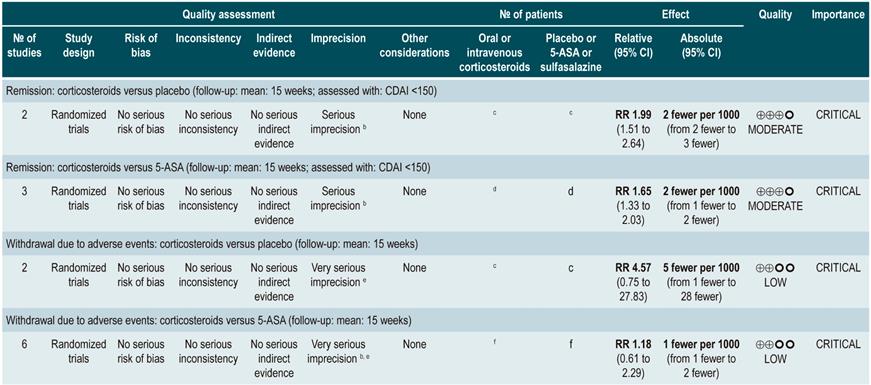
Oral or intravenous administration of corticosteroids to induce remission in patients with active CD
A systematic review79 (AMSTAR score 8/11) assessed the efficacy and safety of using corticosteroids (orally or intravenously) to induce remission in patients with CD. The outcomes reported in this study were the proportion of patients who achieved remission (defined as a CDAI <150 points) and the frequency of withdrawal due to serious adverse events. The effect of this intervention was compared with the effect of placebo in two studies (267 participants). According to the findings of the review, in the corticosteroid administration group, a higher frequency of remission was observed (RR: 1.99; 95% CI: 1.51-2.64), and the proportion of patients who withdrew did not increase (RR: 4.57; 95% CI: 0.75-27.83). On the other hand, a second analysis carried a comparison between the administration of steroids and 5-aminosalicylates use. Compared to the use of 5-aminosalicylates, corticosteroids were also associated with a higher frequency of remission (RR: 1.65, 95% CI: 1.33-2.03; 2 studies, 332 participants), without resulting in a higher frequency of withdrawal (RR: 1.18, 95% CI: 0.61-2.29; 6 trials, 478 patients)79.
Quality of evidence: low ⊕⊕ΟΟΟ
Safety and efficacy of using mesalazine, sulfasalazine, corticosteroids, and budesonide to induce remission in patients with active Crohn’s disease. Results of a network meta-analysis
A systematic review and network meta-analysis80 (AMSTAR score 8/11) evaluated the efficacy and safety of different pharmacological strategies for the induction of remission in CD. The review included studies conducted in patients with active Crohn’s disease (CDAI score between 150 and 400 points) located in the ileum, colon, cecum, or rectum. Studies that allowed the use of combination therapy or included individuals with postoperative recurrence of CD were excluded. The following interventions were assessed: administration of mesalazine, sulfasalazine, corticosteroids or budesonide, and the following outcomes were reported: induction of remission (CDAI score ≤150 points or as defined by the author) and withdrawal due to adverse events (assessed according to clinical judgment).
The review retrieved 24 randomized clinical trials. When compared with the placebo group, the administration of high doses of corticosteroids (OR: 3.86; 95% CI: 2.51-6.06), budesonide (OR: 3.18; 95% CI: 2.11-4.30; higher than 6 mg/d) or mesalazine (OR: 2.11; 95% CI: 1.39-3.31; higher than 2.4 g/d) had a significantly better performance than placebo in inducing remission. However, sulfasalazine therapy did not show a clear benefit when compared to placebo (OR: 1.56; 95% CI: 0.83-2.88). On the other hand, when determining which intervention could be the best therapeutic option, corticosteroids ranked first, followed by budesonide at doses >6 mg/d, mesalazine at doses >2.4 g/d and, finally, with a similar probability, low-dose budesonide, and sulfasalazine80.
Steroids and high-dose budesonide were significantly superior to mesalazine, sulfasalazine, or low-dose budesonide. Corticosteroids were superior to high-dose mesalazine (OR: 1.83; 95% CI: 1.16-2.88), but their efficacy was similar to that of high-dose budesonide therapy (OR: 1.21; 95% CI: 0.84-1.76). Finally, the frequency of withdrawal in all interventions was similar to that of placebo: low-dose mesalazine (OR: 1.74; 95% CI: 0.33-8.99) and high-dose mesalazine (OR: 1.07; 95% CI: 0.36-3.43), sulfasalazine (OR: 0.79; 95% CI: 0.01-14.36), low-dose budesonide (OR: 0.35; 95% CI: 0.03-2.45) and high-dose budesonide (OR: 0.94; 95% CI: 0.36-2.81) and, finally, corticosteroids (OR: 2.19; 95% CI: 0.59-8.70). Somehow, in the corticosteroids group the likelihood of withdrawal due to adverse events was 93% and 90% higher when compared with budesonide or high-dose mesalazine therapy, respectively80.
Quality of evidence: very low ⊕ΟΟΟ
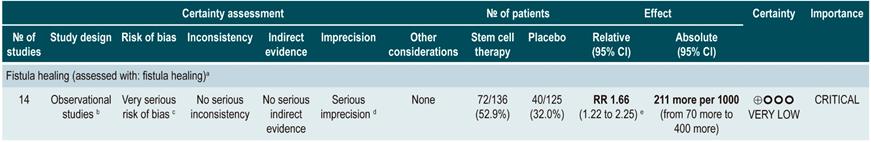
Stem cell therapy
A systematic review that included a meta-analysis of proportions81 (AMSTAR score 10/11) assessed the efficacy and safety of stem cell therapy in patients with active CD. The interventions considered in the review were the use of bone marrow mesenchymal stem cells, adipose tissue or hematopoietic stem cells, and the outcomes reported were their clinical efficacy, defined as clinical response or remission, and the frequency of adverse events, endoscopic remission, and clinical recurrence. A total of 20 prospective experimental studies (563 patients combined) were retrieved. The overall frequencies of clinical response, endoscopic remission, and recurrence were 56% (95% CI: 33%-76%), 15% (95% CI: 0%-50%), and 16% (95% CI: 4%-34%), respectively. The overall frequency of adverse events was 12% (95% CI: 0.06-0.23).
In addition, when a subgroup analysis was performed according to the route of administration, the frequencies of clinical response and clinical remission in patients who underwent systemic therapy were 66% (95% CI: 39%-86%) and clinical 46% (95% CI: 25%-69%), respectively81.
Quality of evidence: very low ⊕ΟΟΟ
From evidence to the recommendation
The expert panel highlighted that this group of recommendations updates the guidelines of international agencies, and the importance this entails. In general, methotrexate, sulfasalazine and azathioprine are not recommended drugs to induce remission. The periods of time in which steroids should be used are reported as a good practice in order to use them adequately, thus minimizing side effects.
Question N° 3. What are the safest and most effective non-biological interventions to maintain remission of crohn’s disease in patients older than 16 years with?
Summary of evidence: General considerations
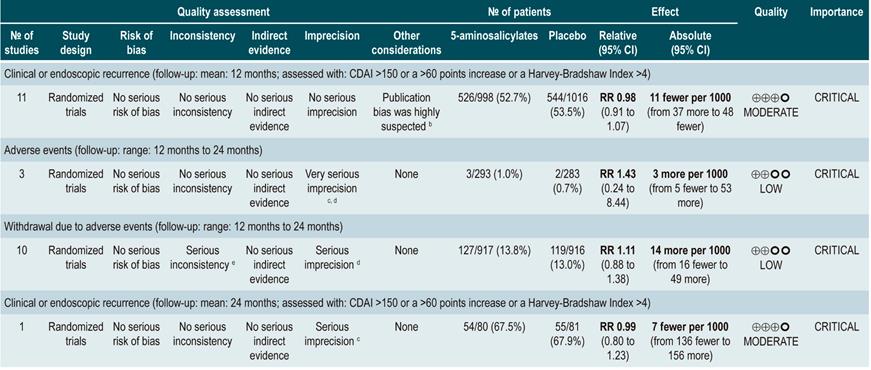
Using 5-aminosalicylates for maintenance of remission in Crohn’s disease
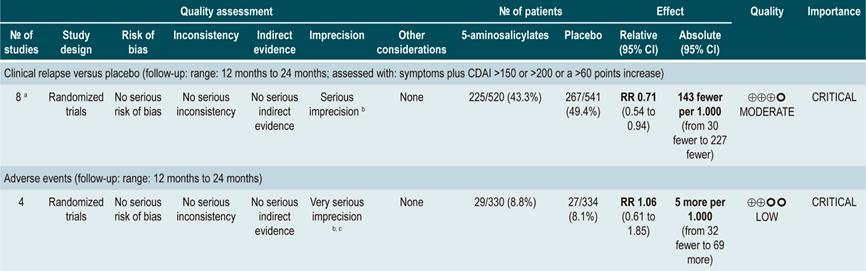
A systematic review82 (AMSTAR score 9/11) evaluated the safety and efficacy of using 5-aminosalicylates for maintenance of remission in CD. This review assessed the following outcomes: clinical or endoscopic recurrence at 12 or 24 months of follow-up (defined as having a CDAI >150 or a >60 points increase or a HBI >4) and the proportion of patients who experienced serious adverse events or who withdrew due to adverse events and serious adverse events.
The systematic review retrieved 11 randomized clinical trials conducted in a total of 2014 participants. When compared with placebo, the use of 5-aminosalicylates was not associated with lower clinical or endoscopic recurrence in the medium term (RR: 0.98, 95% CI: 0.91-1.07; 12 months) or the long term (RR: 0.99; 95% CI: 0.80-1.23; 24 months), nor with a higher frequency of serious adverse events (RR: 1.43; 95% CI: 0.24-8.44) or withdrawal due to adverse events (RR: 1.11; 95% CI: 0.88-1.38)82.
Quality of evidence: low ⊕⊕ΟΟ
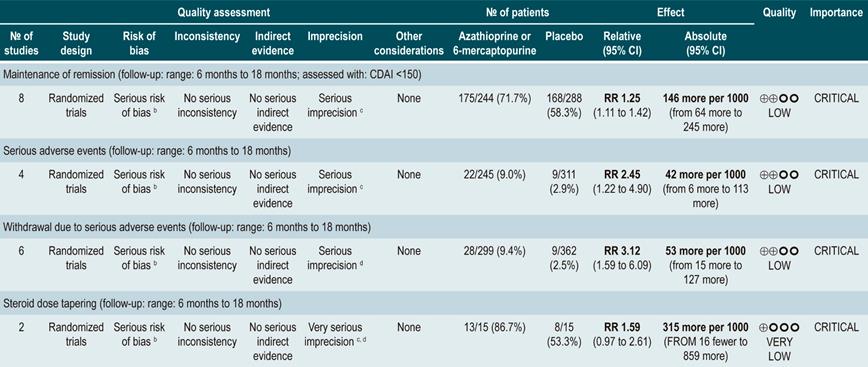
Using azathioprine or 6-mercaptopurine to maintain remission in patients with Crohn’s disease
A systematic review83 (AMSTAR score 9/11) assessed the safety and efficacy of using azathioprine or 6-mercaptopurine for maintenance of remission in CD. The following outcomes were reported: the proportion of patients who remained in remission (defines as having a CDAI <150), steroid sparing, and the frequency of serious adverse events or the frequency of withdrawals due to adverse events.
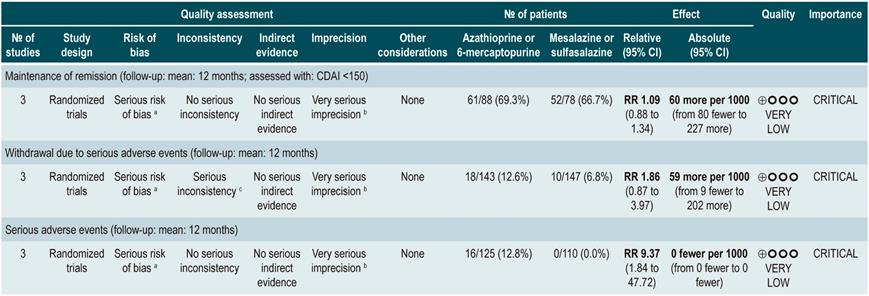
The review retrieved eight controlled clinical trials comparing this intervention versus placebo in 532 participants. Based on the evidence retrieved, the use of azathioprine or 6-mercaptopurine increased the proportion of patients who were in remission at study enpoint (RR: 1.25; 95% CI: 1.11-1.42) at the expense of a higher frequency of serious adverse events (RR: 2.45; 95% CI: 1.22-4. 90) or withdrawal due to adverse events (RR: 3.12; 95% CI: 1.59-6.09), without modifying the proportion of patients in which reducing the steroid dose was feasible (RR: 1.59; 95% CI: 0.97-2.61)83.
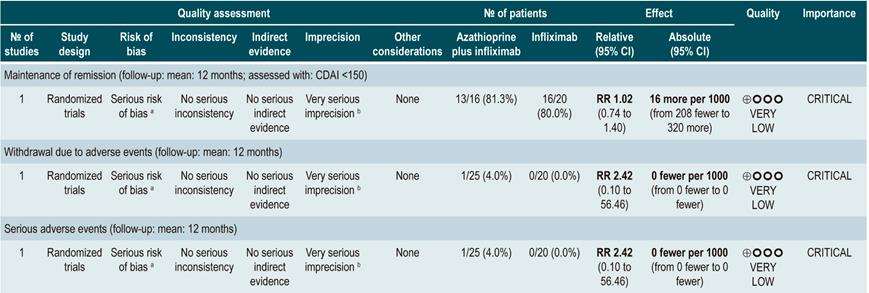
Furthermore, a second analysis was carried out to compare the administration of azathioprine or 6-mercaptopurine with mesalazine or sulfasalazine therapy. When compared with 5-aminosalicylates therapy, azathioprine or 6-mercaptopurine therapy was not associated with a higher or lower frequency of patients who remained in remission (RR: 1.09; 95% CI: 0.88-1.34) or who withdrew due to adverse events (RR: 1.86; 95% CI: 0.87-3.97), but it was associated with a higher incidence of serious adverse events (RR: 9.37; 95% CI: 1.84-47.72). Finally, in the last study retrieved in the review a comparison between combination therapy with azathioprine plus infliximab and monotherapy with infliximab was made. Combination therapy was not superior to infliximab in increasing the proportion of patients who remained in remission (RR: 1.02; 95% CI: 0.74-1.40). However, it did not increase the frequency of serious adverse events either (RR: 2.42; 95% CI: 0.10-56.46), nor of withdrawal (RR: 2.42; 95% CI: 0.10-56.46)83.
Quality of evidence: very low ⊕ΟΟΟ
Using budesonide for maintenance of remission in Crohn’s disease
A systematic review84 (AMSTAR score 10/11) evaluated the safety and efficacy of budesonide administration for maintenance of remission in CD. With this in mind, the following outcomes were assessed: proportion of patients who remained in remission (defined as having a CDAI <150), mean change in CDAI score compared to the baseline score, mean time to the first relapse episode (measured in days), and the frequency of withdrawal due to an adverse event.
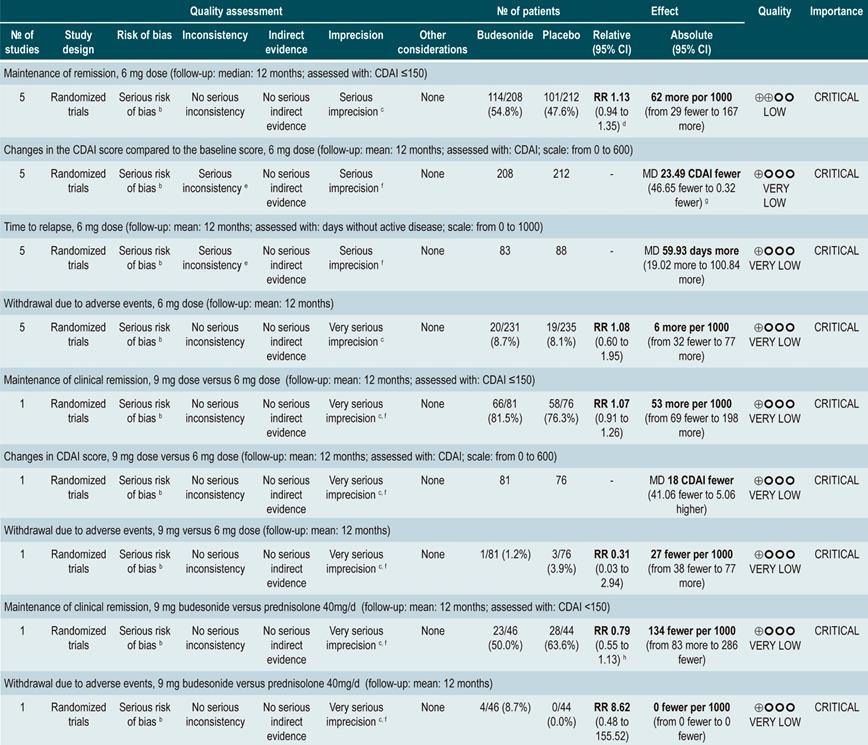
Five randomized clinical trials comparing the use of this intervention with the use of placebo in 420 patients combined were retrieved. Based on the evidence found, the use of budesonide at a dose of 6 mg/d did not increase the frequency of patients who remained in remission in the medium or the long term (RR: 1.15, 95% CI: 0.95-1.39; and RR: 1.13, 95% CI: 0.94-1.35; 6 and 12 months, respectively), although it did slightly decrease disease activity in the medium and the long term (MD CDAI score at 6 months: -24.30; 95% CI: -2.29 to -46.31; and CDAI score at 12 months: -23.49; 95% CI: -0.32 to -46.65) and slightly increased mean time to the first episode of relapse (MD: 59.93 days; 95% CI: 19.02-100.84 days), without significantly increasing the proportion of patients who withdrew due to adverse events (RR: 1.08; 95% CI: 0.60-1.95)84.
Also, when a subgroup analysis according to budesonide dose was performed, the increased dose of budesonide (9 mg versus 6 mg) did not increase the rate of remission (RR: 1.07; 95% CI: 0.91-1.26) or improve disease activity indices (MD CDAI score: -18; 95% CI: -41.06-5.06), but neither did it increase the frequency of withdrawal due to adverse events (RR: 0.31; 95% CI: 0.03-2.94). Finally, when budesonide therapy and the use of prednisolone 40 mg/d were compared, no significant differences were found between both groups regarding remission rate at 6 months (RR: 0.79; 95% CI: 0.56-1.12) or 12 months (RR: 0.79; 95% CI: 0.55-1.13) and the frequency of withdrawal due to adverse events (RR: 8.62; 95% CI: 0.48-155.52)84.
Quality of evidence: very low ⊕ΟΟΟ
Using methotrexate to maintain remission in patients with Crohn’s disease
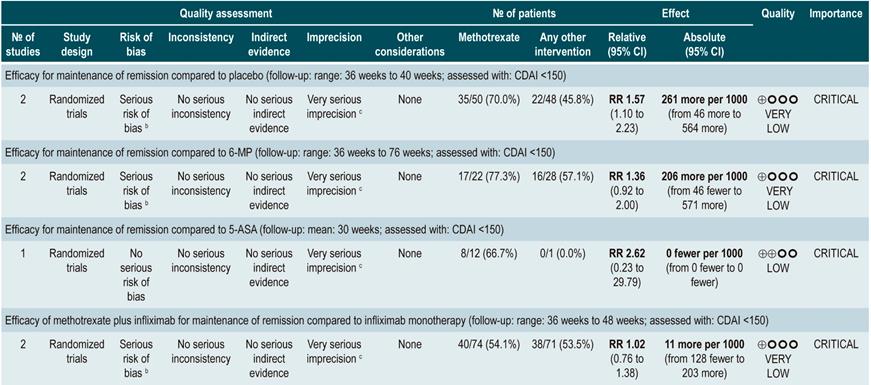
A systematic review and meta-analysis85 (AMSTAR score 8/11) evaluated the efficacy of methotrexate therapy for maintenance of remission in CD. The outcome assessed was the proportion of patients who remained in remission in a follow-up time ranging from 30 to 76 weeks (remission was defined as having a CDAI <150). Seven controlled clinical trials (306 participants in total) comparing methotrexate therapy with any other pharmacological intervention were retrieved. When placebo and methotrexate administration were compared, the latter increased the proportion of patients who remained in remission (RR: 1.57; 95% CI: 1.10-2.23); however, this finding was not consistent when methotrexate therapy was compared with 6-mercaptopurine (RR: 1.36; 95% CI: 0.92-2.00), 5-aminosalicylates (RR: 2.62; 95% CI: 0.23-29.79) or combination therapy with infliximab (RR: 1.02; 95% CI: 0.76-1.38)85.
Quality of evidence: very low ⊕ΟΟΟ
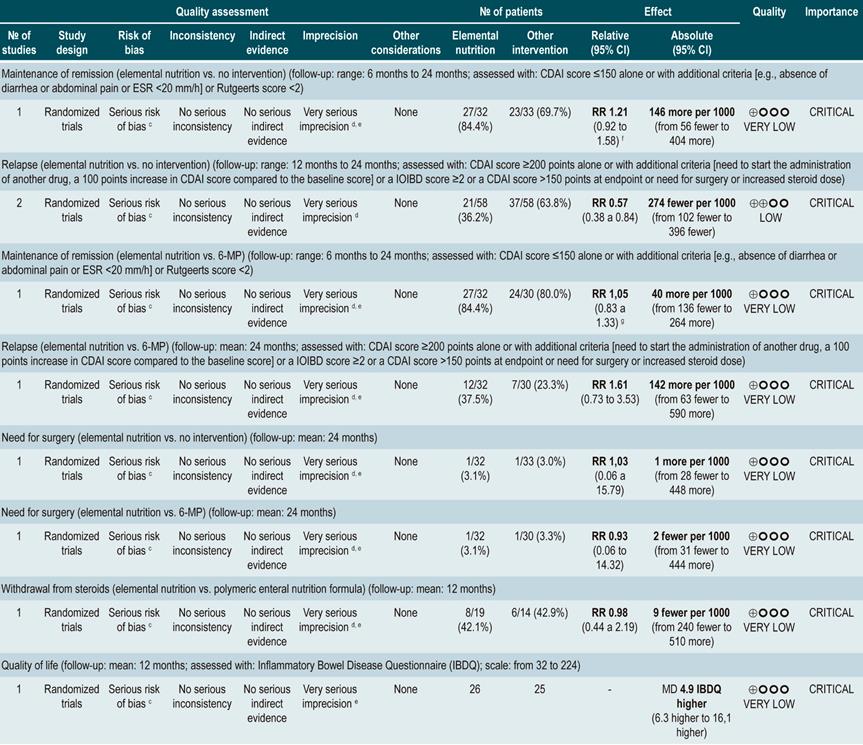
Using elemental nutrition (also elemental diet, a type o enteral nutrition) to maintain remission in patients with Crohn’s disease
A systematic review86 (AMSTAR score 7/11) evaluated the efficacy of elemental nutrition for the maintenance of remission in CD by assessing the following outcomes: maintenance of remission (CDAI score ≤150 alone or Rutgeerts score <2), relapse (CDAI score ≥200 or a 100 points increase, or an IOIBD score ≥2, or increasing the steroid dose), need for surgical intervention, withdrawal from steroids, and health-related quality of life (assessed with the Inflammatory Bowel Disease Questionnaire [IBDQ]).
Two controlled clinical trials conducted in a total of 116 participants were retrieved. When compared with the no intervention group (unrestricted diet), elemental nutrition did not significantly change the proportion of patients who remained in remission in the short (RR: 1.21; 95% CI: 0.92-1.58; at 6 months), medium (RR: 1.37; 95% CI: 0.86-2.17; at 12 months) or long term (RR: 2.06; 95% CI: 1.00-4.43; at 24 months) or the proportion of patients requiring surgical intervention (RR: 1.03; 95% CI: 0.06-15.79). However, it significantly reduced the risk the frequency of relapse episodes at 12 and 24 months of follow-up (pooled RR: 0.57; 95% CI: 0.38-0.84) and increased health-related quality of life scores during the first year of follow-up (MD IBDQ score=4.9; 95% CI: 6.3-16.1)86.
On the other hand, another study retrieved in this systematic review compared the efficacy of elemental nutrition versus 6-mercaptopurine therapy. No significant differences between both groups were found regarding maintenance of remission in the short (RR: 1.05; 95% CI: 0.83-1.33; at 6 months), medium (RR: 0.93; 95% CI: 0.64-1.35; at 12 months) or long term (RR: 0.77; 95% CI: 0.46-1.27), the frequency of relapse episodes (RR: 1.61; 95% CI: 0.73-3.53) or the need for surgical intervention (RR: 0.93; 95% CI: 0.06-14.32). Finally, there was also no difference in the frequency of patients who withdrew from taking steroids when elemental nutrition was compared with polymeric nutrition (RR: 0.98; 95% CI: 0.44-2.19)86.
A more recent Cochrane review comparing enteral nutrition with corticosteroids therapy in adult population reported a significant difference in favor of corticosteroids regarding remission rates in CD (45% vs. 73%) (RR: 0.65; 95% CI: 0.52-0.82); however, the quality of evidence was very low87.
Quality of evidence: very low ⊕ΟΟΟ
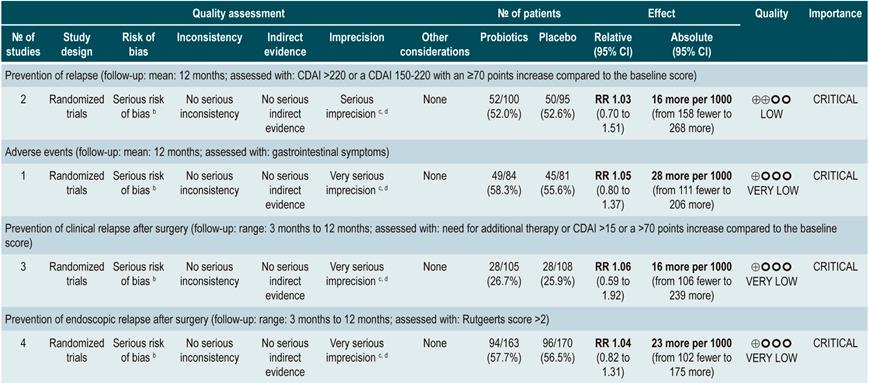
Using probiotics for maintenance of remission in Crohn’s disease
A systematic review88 (AMSTAR score 9/11) assessed the efficacy and safety of using probiotics for maintenance of remission in CD. The outcomes analyzed in the review were the frequency of relapse at 12-months (CDAI >220 or a CDAI 150-220 with an ≥70 points increase compared to the baseline score) and the incidence of adverse events resulting from the intervention. Four randomized clinical trials conducted in a total of 233 participants were included. Compared to placebo, the use of probiotics did not reduce the frequency of relapse episodes (RR: 1.03; 95% CI: 0.70-1.51), but neither did it increase the frequency of adverse events (RR: 1.05; 95% CI: 0.80-1.37)88.
Quality of evidence: low ⊕⊕ΟΟ
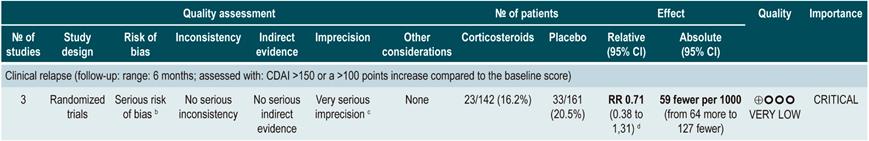
Using corticosteroids to maintain remission in patients with Crohn’s disease
A systematic review89 (AMSTAR score 8/11) evaluated the efficacy of corticosteroid therapy for the maintenance of remission in CD. Bearing this in mind, the review reported findings on the following outcome: frequency of relapse at 6, 12, and 24 months (a CDAI score >150 together by symptoms suggestive of CD). Three randomized clinical (303 participants combine) were included. Compared to the placebo group, corticosteroid therapy did not reduce the frequency of relapse episodes in the short (RR: 0.71; 95% CI: 0.38-1.31), medium (RR: 0.82; 95% CI: 0.47-1.44) or long term (RR: 0.72; 95% CI: 0.39-1.35)89.
Quality of evidence: very low ⊕ΟΟΟ
From evidence to the recommendation
Currently, thiopurine methyltransferase (TPMT) test is not available in Colombia; however, starting its use in the country would be an ideal scenario since patients treated with thiopurines may develop immunosuppression. The future inclusion of this test in the health benefits plan of the mandatory health insurance coverage system in force in Colombia is suggested.
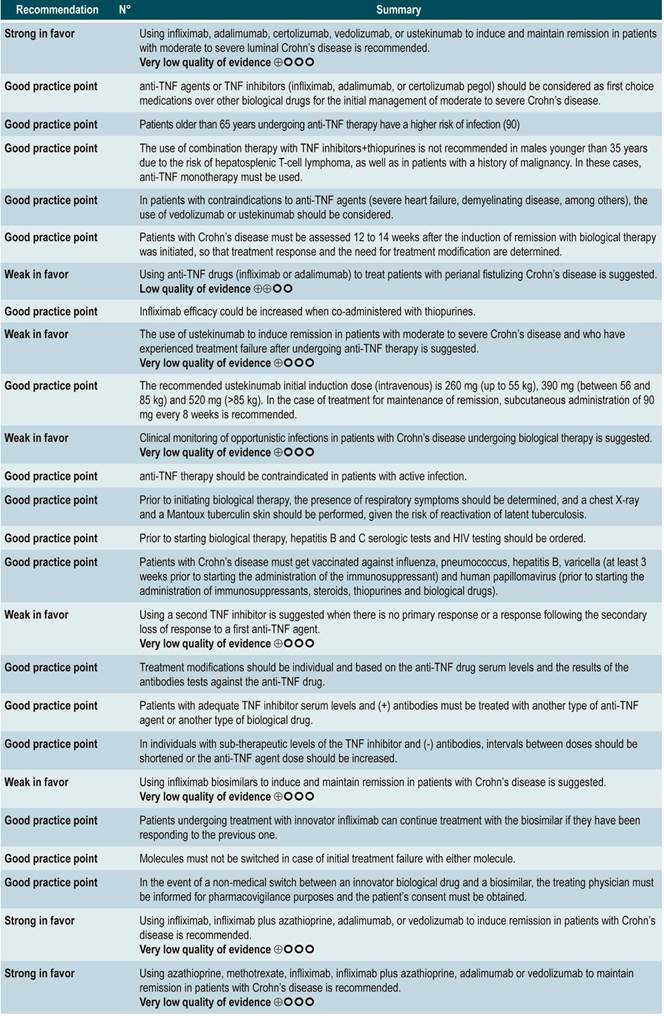
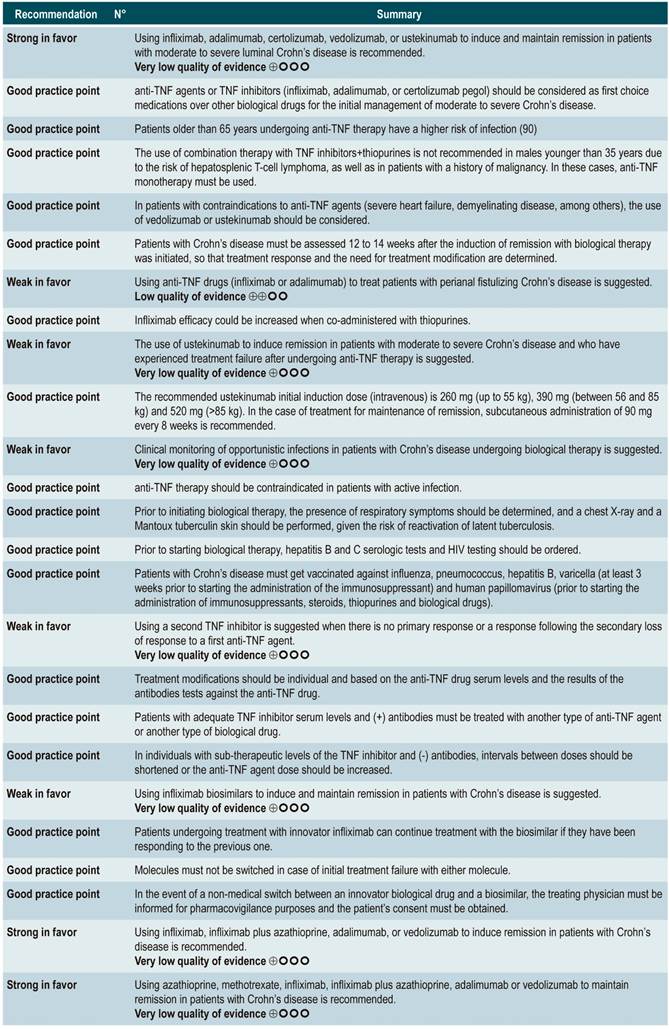
Question N°4. What is the safety and efficacy of using biological drugs to treat moderate to severe crohn’s disease in patients older than 16 years?
Summary of evidence: General considerations
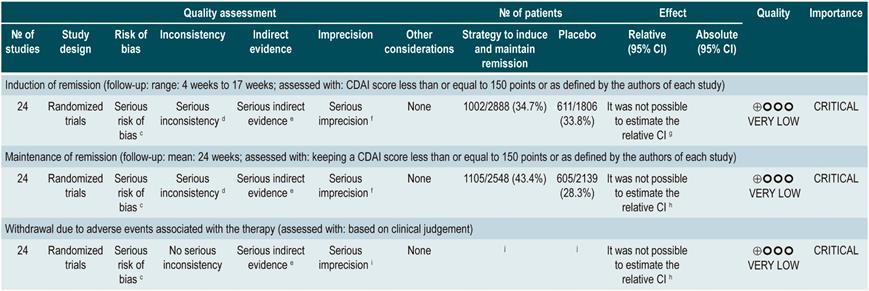
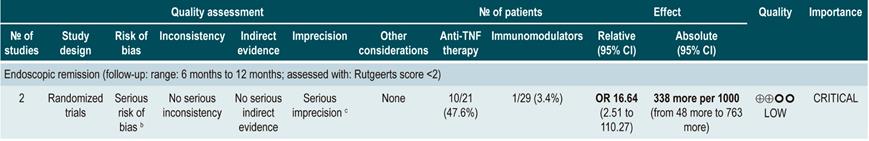
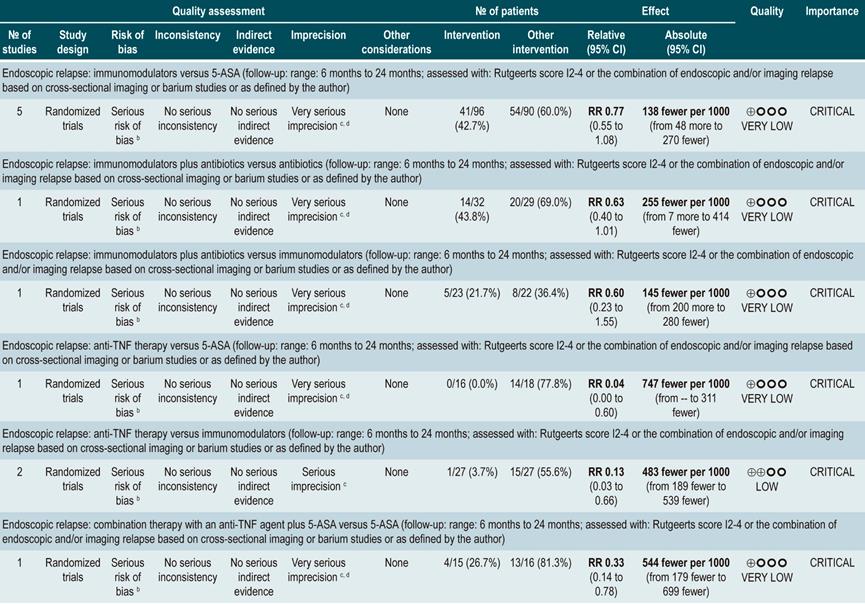
anti-TNF agents (infliximab, adalimumab or certolizumab pegol) or anti-integrins (natalizumab or vedolizumab) or IL-12/23 antagonists (ustekinumab) versus placebo for induction or maintenance of remission in moderate or severe luminal Crohn’s disease
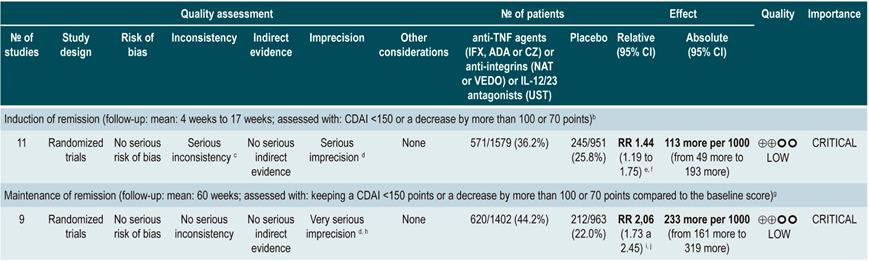
A systematic review and network meta-analysis91 (AMSTAR score 9/11) assessed the efficacy, compared to placebo, of using anti-TNF agents (infliximab, adalimumab or certolizumab pegol) or anti-integrins (natalizumab or vedolizumab) or interleukin 12/23 (IL-12/23) antagonists (ustekinumab) for induction or maintenance of remission in patients with moderate or severe luminal colonic, ileal or ileocolonic CD. All studies included in the review allowed concomitant use of immunomodulators, corticosteroids and/or 5-aminosalicylates. The following outcomes were assessed: induction of clinical remission (a CDAI <150 or a decrease by more than 100 or 70 points) and maintenance of remission (a CDAI <150 or a decrease by more than 100 or 70 points compared to the baseline score).
The review retrieved 11 randomized clinical trials (2530 patients in total). Compared to placebo, a higher proportion of patients in the biologic drugs group achieved clinical remission (RR: 1.44; 95% CI: 1.19-1.75) and remained in remission (RR: 2.06; 95% CI: 1.73-2.45). When subgroup analysis was performed and the outcome of interest was the induction of remission, compared to placebo, the use of anti-TNF agents significantly increased the proportion of patients in which this outcome was achieved (OR: 1.63; 95% CI: 1.24-2.14), however anti-integrins (OR: 1.20; 95% CI: 0.97-1.49) or IL-12/23 antagonist ustekinumab (OR: 0.79; 95% CI: 0.44-1.39) were not significantly superior to placebo. In addition, in the network meta-analysis, infliximab (RR: 6.11; 95% CI: 2.49-18.29) and adalimumab (RR: 2.98; 95% CI: 1.12-8.18) were significantly superior to placebo in terms of induction of remission, while certolizumab pegol (RR: 1.48; 95% CI: 0.76-2.93), natalizumab (RR: 1.36; 95% CI: 0.69-2.86), vedolizumab (RR: 1.40; 95% CI: 0.63-3.28) or ustekinumab (RR: 0.61; 95% CI: 0.15-2.49) were not significantly superior to placebo. Infliximab had an 86% probability of being the most effective therapy, followed by adalimumab, with a 16% probability91.
On the other hand, according to the subgroup analysis in which the maintenance of remission was the outcome of interest, when anti-TNF therapy (OR: 2.18; 95% CI: 1.65-2.88) or IL-12/23 antagonist ustekinumab (OR: 2.09; 95% CI: 1.49-2.92) were used, a higher proportion of patients remained in remission. These findings were not observed when anti-integrins were used (OR: 1.52; 95% CI: 0.96-2.42). Besides, in the network meta-analysis, adalimumab (RR: 5.16; 95% CI: 1.78-18.00) and infliximab (RR: 3.31; 95% CI: 0.98-14.01) were superior to placebo in terms of maintenance of remission, while certolizumab pegol (RR: 2.26; 95% CI: 0.38-13.57), natalizumab (RR: 4.26; 95% CI: 0.71-25.49), vedolizumab (RR: 2.20; 95% CI: 0.37-13.54) and ustekinumab (RR: 0.91; 95% CI: 0.31-12.31) were not. Adalimumab had a 48% probability of being the most effective therapy, followed by natalizumab and infliximab, with a 29% and 11% probability, respectively91.
Quality of evidence: very low ⊕ΟΟΟ
A recent systematic review compared the efficacy and safety of biologic drugs (infliximab, adalimumab, certolizumab, vedolizumab, and ustekinumab) as first-line (“biologic-naïve”) and second-line therapy (prior exposure to anti-TNF agents) in patients with moderate to severe CD. In total, 23 studies (randomized clinical trials; RCTs) were included: 8 using biologics as first-line agents, 6 using biologics as second-line agents, and 9 using them for maintenance of remission. Also, no head-to-head comparative studies were retrieved. In this review, the surface under the cumulative ranking curve (SUCRA), which represents the percentage of efficacy or safety achieved by an agent compared to an imaginary agent that is always the best choice and without uncertainty (i.e., SUCRA = 100%), for each biologic was determined. Infliximab (SUCRA, 0.93) and adalimumab (SUCRA, 0.75) had the highest SUCRA score for the induction of clinical remission in CD patients (moderate quality of evidence). On the other hand, in patients with prior exposure to anti-TNF agents (second-line therapy), adalimumab (SUCRA, 0.91) and ustekinumab (SUCRA, 0.71) achieved the highest rating for induction of remission (low quality of evidence). Adalimumab (SUCRA, 0.97) and infliximab (SUCRA, 0.68) had the highest scores for maintenance of remission, and Ustekinumab had the lowest risk of adverse events (SUCRA, 0.72) and infection (SUCRA, 0.71)92.
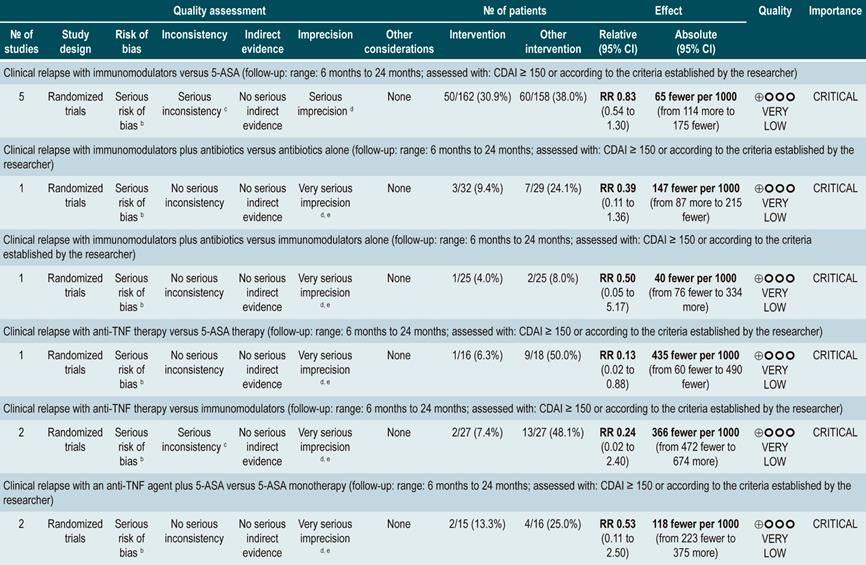
Efficacy of using a second anti-TNF agent in patients with inflammatory bowel disease and treatment failure or intolerance to a first biologic
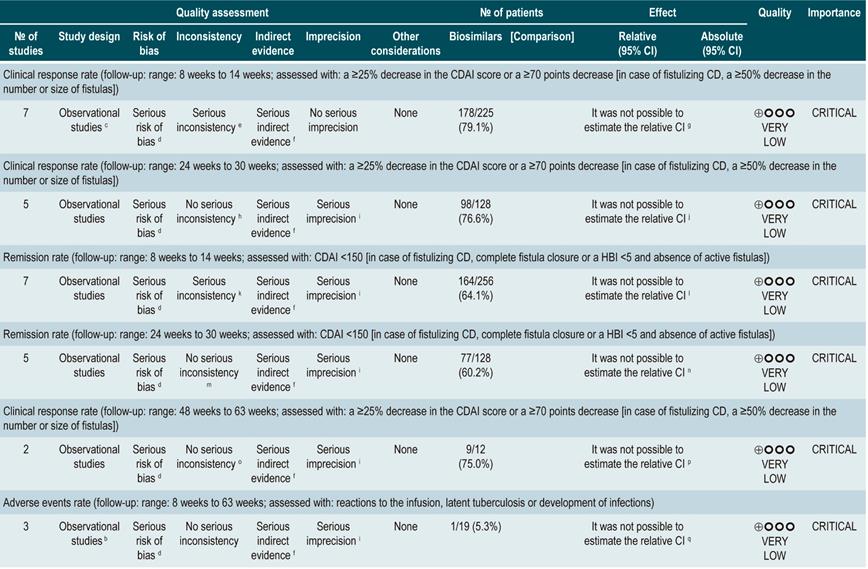
A systematic review93 (AMSTAR score 8/11) evaluated the efficacy of using a second anti-TNF agent in patients with inflammatory bowel disease and primary or secondary failure or intolerance to a first anti-TNF agent therapy. Patients included in the studies retrieved were characterized by having luminal or fistulizing Crohn’s disease with CDAI score ranging from 220 to 450 points or a HBI ≥7 or by having “moderate to severe Crohn’s disease” or “steroid-dependent” disease or having experienced “failure to treatment with immunomodulators”. Out of all the studies identified, 32 evaluated switching from infliximab to adalimumab therapy: 4, from infliximab to certolizumab, and 1, from adalimumab to infliximab. The outcomes assessed were the overall rates of remission or response after primary failure and switching from infliximab to adalimumab, and from infliximab to adalimumab or certolizumab pegol, and, finally, the overall rate of remission or secondary response in case of intolerance to infliximab. According to this meta-analysis, the percentage of remission in the short, medium and long term was 18%, 30% and 28%, respectively; also, the short-, medium‐ and long‐term response rates after primary failure to infliximab and switching to adalimubab were, 35%, 67% and 42%, respectively93.
In the case of primary failure to infliximab and switching to adalimumab or certolizumab, the remission rates in the short, medium and long term were 41%, 38% and 60%, and the response rates in the medium and long term were 66% and 42%, respectively. Finally, remission rates in the short, medium and long term were 50%, 60% and 83%, respectively, and response rates in the medium and long term were 70% and 77%, when infliximab therapy was switched to adalimumab therapy due to intolerance. The frequency of adverse events was not reported in this meta-analysis93.
Quality of evidence: very low ⊕ΟΟΟ
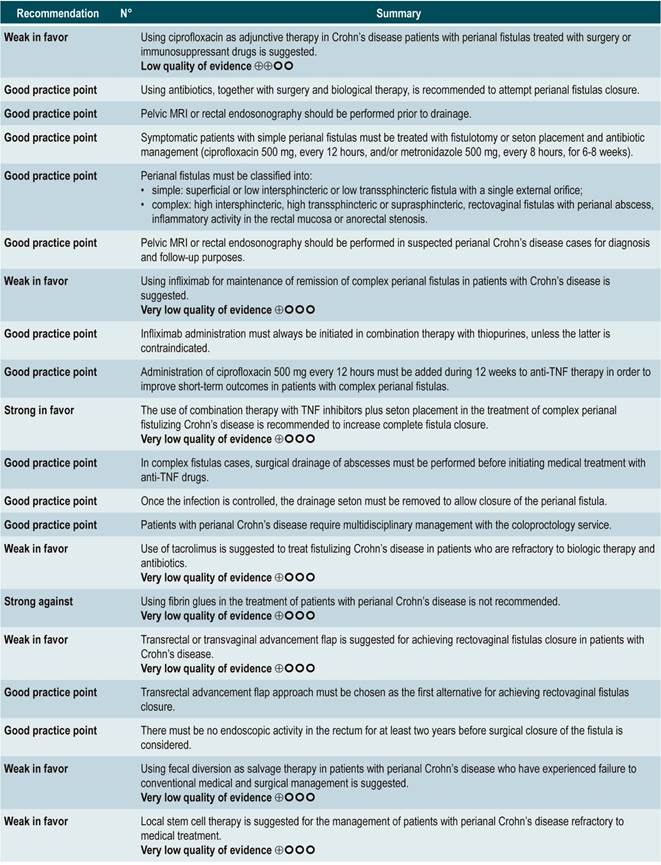
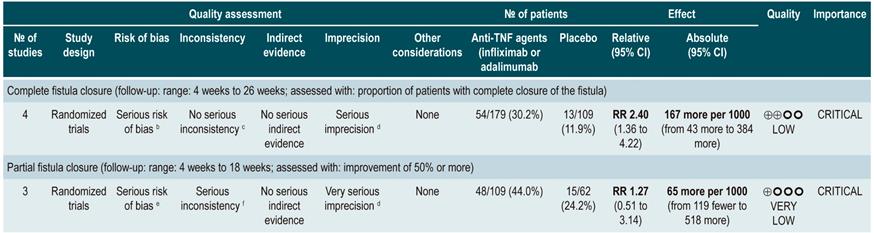
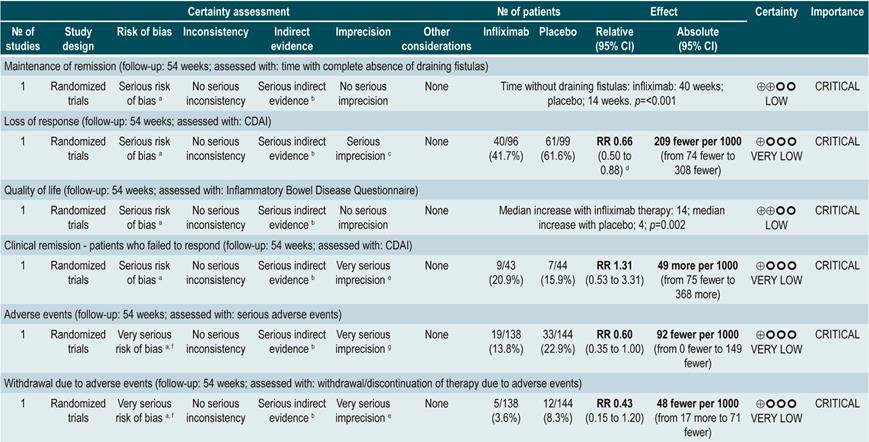
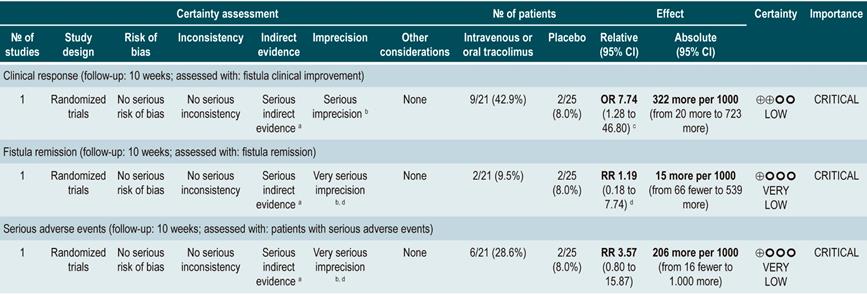
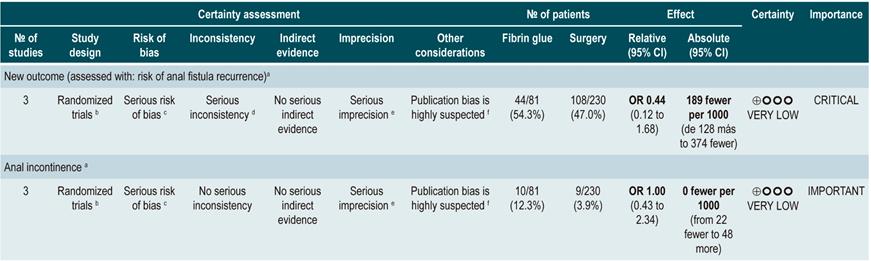
TNF inhibitors (infliximab or adalimumab) versus placebo in patients with fistulizing Crohn’s disease
A systematic review94 (AMSTAR score 8/11) evaluated the efficacy of using anti-TNF drugs (infliximab or adalimumab) to treat patients with CD and perianal or enterocutaneous or enteroenteral fistulas. Participants were administered infliximab 5 mg/kg or adalimumab 40 mg to 80 mg, and the outcomes assessed were complete or partial closure of the fistula. The review retrieved four randomized clinical trials conducted in 288 patients in total; when compared with patients in the placebo group, those in the anti-TNF therapy arm had a higher frequency of complete fistula closure (RR; 2.40, 95% CI: 1.36-4.22), but not of partial closure (RR: 1.27, 95% CI: 0.51-3.14)94.
Quality of evidence: very low ⊕ΟΟΟ
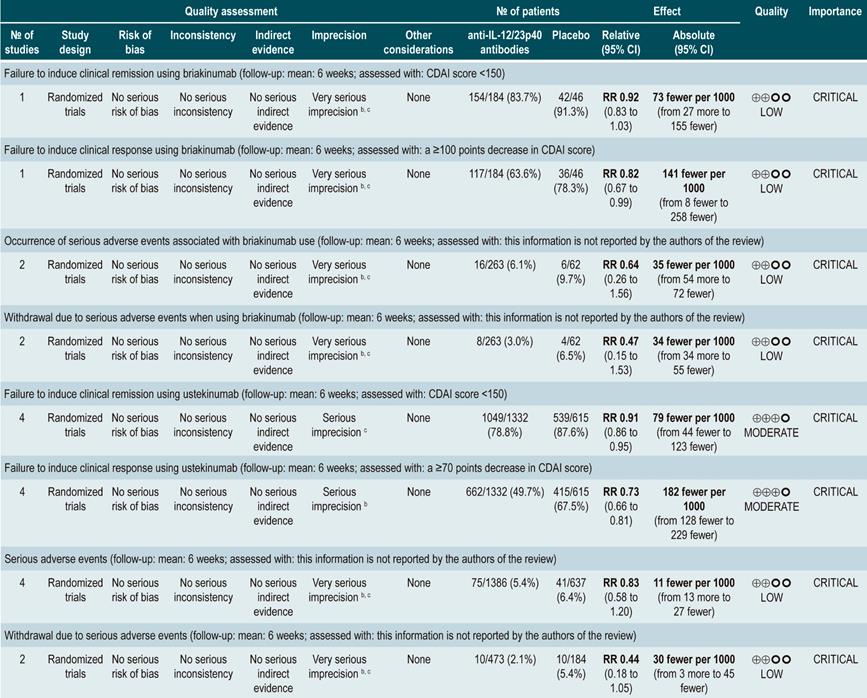
anti-IL-12/23p40 antibodies versus placebo for induction of remission in moderate to severe Crohn’s disease
A systematic review95 (AMSTAR score 9/11) evaluated the safety and efficacy, compared to placebo, of using anti-IL-12/23p40 antibodies for induction of remission in CD. Patients included in the ustekinumab or briakinumab therapy group had moderate to severe CD and prior failure to induce remission with anti-TNF agents or corticosteroids or immunosuppressants therapy. The following outcomes were assessed: failure to induce clinical remission (CDAI score <150) or clinical improvement (clinical response) (a ≥100 points decrease in CDAI score) and the frequency of serious adverse events or the frequency of withdrawal due to serious adverse events.
The review retrieved four randomized clinical trials (2023 participants in total). When compared with placebo, briakinumab was not associated with a lower frequency of failure to induce remission (RR: 0.92; 95% CI: 0.83-1.03). However, it was associated with a lower frequency of failure to induce clinical improvement (RR: 0.82; 95% CI: 0.67-0.99). There were no statistical differences between groups regarding the frequency of serious adverse events (RR: 0.64; 95% CI: 0.26-1.56) or of withdrawal due to adverse events (RR: 0.47; 95% CI: 0.15-1.53)95.
In addition, when compared with the placebo group, patients undergoing ustekinumab therapy had a lower frequency of failure to induce clinical remission (RR: 0.91; 95% CI: 0.86-0.95) or of failure to induce clinical improvement (RR: 0.73; 95% CI: 0.66-0.81). On the other hand, no significant differences were found between groups regarding the presence of serious adverse events (RR: 0.83; 95% CI: 0.58-1.20) or withdrawal due to adverse events (RR: 0.44; 95% CI: 0.18-1.05)95.
Quality of evidence: very low ⊕ΟΟΟ
A recent Cochrane systematic review evaluated the efficacy and safety of anti-IL-12/23p40 antibodies for maintenance of remission in CD. Three randomized controlled trials (646 participants) met the inclusion criteria, and all were assessed as having a low risk of bias. Two trials assessed the efficacy of ustekinumab (542 participants), while the other (n=145) compared subcutaneous administration of ustekinumab (90 mg) at 8 and 16 weeks with placebo. Failure to maintain remission at 22 weeks was reported in 58% (42/72) of the patients in the ustekinumab group and in 73% (53/73) of those in the placebo group (RR: 0.80; 95% CI: 0.63-1.02; moderate-certainty). A second study (n=388) compared subcutaneous administration of ustekinumab (90 mg) every 8 or 12 weeks for 44 weeks with placebo use, finding that 49% (126/257) of the participants in the ustekinumab group failed to maintain clinical remission at 44 weeks in comparison with 64% (84/131) in the placebo group (RR: 0.76; 95% CI: 0.64-0.91; moderate-certainty evidence). Moderate-certainty evidence means that, when compared to placebo, ustekinumab is likely to be effective for the maintenance of clinical remission and clinical response in patients with moderate to severe CD, without increasing the risk of adverse events (high-certainty evidence) or serious adverse events (moderate-certainty evidence)96.
Safety of biological therapy in patients with moderate to severe inflammatory bowel disease
A systematic review97 (AMSTAR score 10/11) assessed the risk of developing infections or malignancies in CD patients undergoing biologic therapy with adalimumab, certolizumab pegol, golimumab, infliximab, natalizumab, or vedolizumab. The outcomes reported in the study were the frequency of serious infections (i.e. those requiring hospitalization, use of intravenous antibiotics or resulting in death), opportunistic infections (presence of Mycobacterium tuberculosis; Nocardia, cytomegalovirus or Epstein-Barr infection; oral or esophageal candida infection; varicella-zoster or herpes zoster infection; Pneumocystis jirovecii or Histoplasma capsulatum infection or pneumonia caused by any species of Legionella bacteria, or herpes simplex infection, or any unspecified opportunistic infection), any type of infection, as well as the development of tuberculosis (confirmed diagnosis, TB reactivation, miliary or cavitary pulmonary TB or TB affecting any other organ) or malignancy.
This systematic review included 47 observational studies (14,440 participants), and based on the findings retrieved, the use of biologic drugs increased the frequency of opportunistic infections (OR: 1.90; 95% CI: 1.21-3.01) and of any other type of infection (OR: 1.19; 95% CI: 1.10-1.29), but did not increase the risk of serious infections (OR: 0.89; 95% CI: 0.71-1.12), tuberculosis (OR: 2.04; 95% CI: 0.71-5.89) or malignancy (OR: 0.90; 95% CI: 0.54-1.50). In addition, when subgroup analysis according to drug type was performed, no differences were found between anti-TNF agents and anti-integrins in the frequency of serious infections (anti-TNF: OR: 0.90; 95% CI: 0.69-1.17; vs. anti-integrin: OR: 0.87; 95% CI: 0.54-1.39; p-value for subgroup difference=0.89), opportunistic infections (anti-TNF: OR: 1.89; 95% CI: 1.15-3.12; vs. anti-integrin: OR: 1.99; 95% CI: 0.64-6.18; p-value for subgroup difference=0.94) or any other type of infection (anti-TNF: OR: 1.21; 95% CI: 1.10-1.33; vs. anti-integrin: OR: 1.14; 95% CI: 0.99-1.32; p-value for subgroup difference=0.49)97.
Quality of evidence: very low ⊕ΟΟΟ
A systematic review that included 15 observational studies evaluated the risk of infection in patients with inflammatory bowel disease treated with biologics and immunosuppressive agents. According to the findings of this study, compared with anti-TNF monotherapy, the risk of infection increased with the combination of anti-TNF therapy and immunosuppressants (RR: 1.19; 95% CI: 1.03-1.37; 6 cohorts), with the combination of anti-TNF therapy and steroids (RR: 1.64; 95% CI: 1.33-2.03; 4 cohorts) and with the combination of the three drugs (RR: 1.35; 95% CI: 1.04-1.77; 2 cohorts). On the contrary, the risk of infection was lower in patients undergoing monotherapy with an immunosuppressive agent than in those undergoing anti-TNF monotherapy (RR: 0.61; 95% CI: 0.44-0.84; 7 cohorts) or a combination therapy with an anti-TNF agent plus an immunosuppressant (RR: 0.56; 95% CI: 0.39-0.81)98.
Safety and efficacy of using CT-P13 (a biosimilar of infliximab) in patients with moderate to severe Crohn’s disease
A systematic review and meta-analysis99 (AMSTAR score 9/11) evaluated the safety and efficacy of using CT-P13 (a biosimilar of infliximab) in patients with CD located in the terminal ileum, the colon, the ileum and colon, or the upper gastrointestinal tract. None of the studies retrieved in the review performed a head-to-head comparison between the biosimilar and infliximab, and in all of them, patients were first treated with infliximab and then were administered the biosimilar. The following outcomes were reported: rates of clinical response (defined as a ≥25% decrease in the CDAI score or a ≥70 points decrease compared to the baseline score or, in case of fistulizing CD, a ≥50% decrease in the number or size of fistulas), and clinical remission (defined as having a CDAI <150 or, in case of fistulizing CD, complete fistula closure or a HBI <5 and absence of active fistulas), and, finally, the frequency of adverse events (assessed with infusion reactions, latent tuberculosis or development of infections)99.
The review retrieved seven observational cohort studies (225 patients combined). Regarding the clinical response rate outcome, 79% (95% CI: 65%-88%) patients with nonfistulizing CD and 67% (95% CI: 27%-92%) with fistulizing CD remained in remission in the short term (follow-up: 8-14 weeks). In the medium term (follow-up: 24-30 weeks), clinical response rates were 77% (95% CI: 63%-86%) and 67% (95% CI: 27%-92%) in participants with fistulizing and nonfistulazing CD, respectively. Finally, the long-term clinical response rate (follow-up: 48 to 63 weeks) was 75% (95% CI: 44%-92%) in patients with nonfistulizing CD; no information was retrieved for the fistulizing CD group99.
On the other hand, the clinical remission rates in the short (follow-up: 8 to 14 weeks) and the medium term (follow-up: 24-30 weeks) in nonfistulizing and fistulizing CD patients were 66% (95% CI: 53%-77%) and 33% (95% CI: 8%-73%), and 60% (95% CI: 49%-70%) and 50% (95% CI: 17%-83%), respectively. No information on clinical remission rates in the long term was retrieved. Finally, the overall frequency of adverse events was 10% (95% CI: 2%-31%)99.
Quality of evidence: very low ⊕ΟΟΟ
Safety and efficacy of different strategies to induce and maintain remission in patients with Crohn’s disease. Results of a network meta-analysis
A systematic review and network meta-analysis100 (AMSTAR score 7/11) evaluated the efficacy and safety of different strategies to induce and maintain remission in patients with CD. Patients included were characterized as having active disease (CDAI score between 150 and 450 points or HBI >7) or refractory or steroid-dependent disease, or refractory to azathioprine disease. The following interventions were assessed: the administration of azathioprine/6-mercaptopurine, methotrexate, infliximab, adalimumab, certolizumab and vedolizumab as monotherapy or as part of combination therapy. Natalizumab was not included because the available studies recruited patients who had experienced failure to induce remission using TNF inhibitors. The outcomes assessed were induction of remission (CDAI l ≤150 points or as defined by the author of each study), maintenance of remission (CDAI ≤150 or as defined by the author of each study), and withdrawals due to adverse events (assessed according to clinical judgment)100.
The review retrieved 24 randomized clinical trials (4694 participants in total). When compared to placebo, azathioprine/6-mercaptopurine or methotrexate use did not increase the probability of inducing remission (OR: 1.2, 95% CI: 0.76-2.1; OR: 1.5, 95% CI: 0.72-3.2, respectively) in patients with CD. On the other hand, infliximab monotherapy (OR: 2.8; 95% CI: 1.4-7.2), infliximab plus azathioprine (OR: 4.3; 95% CI: 2.0-9.8), adalimumab monotherapy (OR: 2.9; 95% CI: 1.6-5.5) and vedolizumab monotherapy (OR: 2.0; 95% CI: 1.2-3.3) increased the proportion of patients in whom clinical remission was achieved. The likelihood of these interventions being superior to placebo was >99%100.
Regarding the maintenance of remission, all interventions were superior to placebo: azathioprine/6-mercaptopurine (OR: 1.7; 95% CI: 1.3-2.6), methotrexate monotherapy (OR: 2.4; 95% CI: 1.1-4.8), infliximab monotherapy (OR: 2.8; 95% CI: 1.8-4.5), certolizumab monotherapy (OR: 2.0; 95% CI: 1.4-3.0), infliximab plus azathioprine (OR: 5.2; 95% CI: 2.8-11.0), adalimumab monotherapy (OR: 5.1; 95% CI: 3.3-8.1) and vedolizumab monotherapy (OR: 2.2; 95% CI: 1.3-3.7). The likelihood of these interventions being superior to placebo was >99%100.
Finally, the frequency of adverse events was higher in the following interventions: azathioprine/6-mercaptopurine (OR: 3.9; 95% CI: 2.4-6.4), methotrexate monotherapy (OR: 13; 95% CI: 3.2-109), infliximab monotherapy (OR: 2.7; 95% CI: 1.6-4.7), and infliximab plus azathioprine (OR: 3.2; 95% CI: 1.6-6.1). None of the other options, either used as monotherapy or as part of combination therapy, were associated with a higher or lower frequency of adverse events when compared to placebo100.
Quality of evidence: very low ⊕ΟΟΟ
From evidence to the recommendation
The expert panel expressed the need for appropriate use of biologics, since they must be used taking into account the preferences of patients and their medical record, as well as the monitoring of potential adverse events through clinical surveillance. The use of biosimilars will allow easier access to treatment.
Question N° 5. What are the safest and most effective interventions to treat perianal crohn’s disease in patients older than 16 years?
Summary of evidence: General considerations
Use of antibiotics
A systematic review73 (AMSTAR score 7/11) assessed the efficacy of using antibiotics to induce remission in patients with perianal fistulas secondary to CD. All the studies included in the review allowed concomitant use of other interventions (immunomodulators) and compared antibiotics use with either placebo, immunomodulatory monotherapy, or immunomodulatory therapy plus placebo. The efficacy outcome reported was the proportion of patients who achieved clinical improvement or remission (CDAI <150 and/or a ≥70 points decrease in CDAI score or a >50% reduction in the number of fistulas for at least 4 weeks) during follow-up. The review retrieved 15 randomized clinical trials conducted in 1407 participants altogether. In the perianal fistula subgroup, ciprofloxacin use was associated with short-term lesion improvement (RR: 1.64, 95% CI: 1.16-2.32; 3 studies, 63 patients)73.
Quality of evidence: low ⊕⊕ΟΟ
Monotherapy with biologics
The ACCENT II (A Crohn’s Disease) double-blind, randomized, placebo-controlled clinical trial101 evaluated the efficacy and safety of using infliximab compared to placebo in patients with fistulizing CD who had not been previously treated with infliximab. Participants were divided into two groups: those who had a response to the initial treatment and those who did not; in turn, in each group, participants were randomly assigned to receive an infusion of either placebo or infliximab 5 mg/kg every 8 weeks during 32 weeks. Efficacy outcomes were measured taking into account the Crohn’s disease activity index (CDAI), the period of time with complete absence of draining fistulas, and the quality of life (measured with the Inflammatory Bowel Disease Questionnaire), while safety was established based on the frequency of adverse events. Follow-up time was 54 weeks after initiation of therapy.
As a result, 282 patients were randomized with a similar frequency of fistulizing perianal CD between groups (82% to 87%). In the group of participants who achieved remission at 14 weeks, infliximab maintenance therapy increased the period of time with complete absence of draining fistulas (40 weeks vs. 14 weeks; p<0.001) and the quality-of-life score (infliximab maintenance therapy median increase 14 vs. placebo median increase 4; p=0.002) and reduced the probability of experiencing relapses (estimated RR: 0.66; 95% CI: 0.5-0.88)101.
On the other hand, in the group of patients who had no response, continuing infliximab therapy was not associated with an increased probability of achieving clinical remission (estimated RR: 1.31; 95% CI: 0.53-3.21). Finally, there were no differences between groups in the frequency of serious adverse events (estimated RR: 0.6; 95% CI: 0.35-1) and the proportion of patients who withdrew (estimated RR: 0.43; 95% CI: 0.15-1.2)101.
Quality of evidence: very low ⊕ΟΟΟ
Combination therapy with an anti-TNF agent plus seton drainage versus monotherapy in patients with perianal fistulas and Crohn’s disease
A systematic review94 (AMSTAR score 8/11) evaluated the efficacy of using combination therapy with anti-TNF drugs plus seton drainage for the treatment of patients with perianal fistulas and CD. The anti-TNF drug administered was infliximab and three of the four studies allowed the concomitant use of other medical therapies. The following outcomes were assessed in the review: complete or partial fistula closure and frequency of fistula recurrence, with a follow-up range of 4 months to 30 months.
The review retrieved three observational studies (293 participants in total). According to the findings of this systematic review, 45% to 100% of the patients in the combination therapy group had a complete closure of the fistula, in contrast with 17% to 70% and 63% to 82% in the seton drainage monotherapy and the anti-TNF monotherapy groups, respectively. On the other hand, regarding partial closure, the effect range in patients exposed to combination therapy was 14% to 88% versus 20% to 72% and 18% to 44% in those who underwent monotherapy with seton drainage and anti-TNF monotherapy, respectively. Finally, fistula recurrence was reported in 18% to 44% of patients who underwent combination therapy, in 42% to 78% of those who underwent anti-TNF monotherapy, and in 42% of those in the seton drainage monotherapy group94.
Quality of evidence: very low ⊕ΟΟΟ
Tacrolimus
A systematic review102 (AMSTAR score 7/11) evaluated the efficacy of using tacrolimus in patients with luminal or perianal CD. Studies addressing the intervention of interest, namely, oral, intravenous or topical administration of tracolimus were included, and subgroup analyses according to the location of CD were performed. Clinical improvement (defined as at least 50% closure of the fistula), remission (defined as complete closure of the fistula), and frequency of adverse events were reported as outcomes.
Regarding perianal CD, a randomized clinical trial comparing oral administration of tacrolimus with placebo was identified103. In this trial, differences in favor of tacrolimus use regarding the frequency of clinical improvement were found (adjusted OR: 7.74; 95% CI: 1.28-46.8); however, no differences in the frequency of remission (calculated RR: 1.19; 95% CI: 0.18-7.74) or serious adverse events (tacrolimus 5% vs. placebo 0%; p=0.46) were found102,103.
Quality of evidence: very low ⊕ΟΟΟ
Fibrin glue versus surgical treatment
A systematic review and meta-analysis104 (AMSTAR score 7/11) evaluated the efficacy of using fibrin glue for the treatment of patients with perianal CD. Patients included in the review were characterized by having simple, complex and transsphincteric anal fistulas; no additional information on their clinical characteristics was reported. The following interventions were included: fibrin glue injections (81 patients in total) versus any other surgical intervention (fistulotomy, seton drainage, advancement flaps). On the other hand, the outcomes reported were fistula recurrence and anal incontinence. The review included 3 studies (2 clinical trials and 1 non-randomized control group study) conducted in a total of 311 participants. No statistically significant differences were found between groups in terms of fistula recurrence (OR: 0.33; 95% CI: 0.03-3.66) or frequency of fecal incontinence (OR: 1.00; 95% CI: 0.43-2.34)104.
Quality of evidence: very low ⊕ΟΟΟ
Rectovaginal advancement flaps in patients with perianal fistulas
A systematic review and meta-analysis105 (AMSTAR score 7/11) evaluated the efficacy and safety of transrectal advancement flap compared to transvaginal advancement flap approach for treating rectovaginal fistulas in patients with CD; no further specifications are informed by the authors of the study. The following outcomes were reported: primary fistula closure, secondary closure, and fistula recurrence. The review included 11 nonrandomized studies for a total of 224 procedures. No significant differences were found between both approaches in relation to the rates of primary closure (estimated OR: 1.02; 95% CI: 0.33-3.21), overall closure (estimated OR: 1.14; 95% CI: 0.45-2.91) or recurrence (estimated OR: 0.36; 95% CI: 0.03-3.84)105.
Quality of evidence: very low ⊕ΟΟΟ
Fecal diversion in patients with refractory perianal Crohn’s disease
A systematic review and meta-analysis of proportions106 (AMSTAR score 9/11) determined the efficacy of temporary fecal diversion in patients with perianal CD. Adult and pediatric patients with perianal CD and with or without colonic disease were included. The proportion of successful restoration of bowel continuity was considered as the primary outcome, while early clinical improvement and the need for additional surgery, as secondary outcomes. The review retrieved 15 non-randomized studies. Of the patients who underwent temporary fecal diversion, 63.8% (95% CI: 54%-73%; 373 patients) had an early clinical response; 16.6% experienced successful bowel continuity restoration after the procedure (95% CI: 11.8%-22.9%; 15 studies, 545 patients); 26.5% required a re-diversion (without proctectomy) (95% CI: 14.1%-44.2%); and 41.6% needed to undergo a proctectomy after the procedure (95% CI: 32.6%-51.2%)106.
Quality of evidence: very low ⊕ΟΟΟ
Stem cell therapy
Use of adipose mesenchymal stem cells, bone marrow-derived mesenchymal stem cells, and hematopoietic stem cells
A systematic review and meta-analysis of proportions81 (AMSTAR score 10/11) evaluated the efficacy and safety of stem cell therapy in patients with active CD. The use of bone marrow-derived mesenchymal stem cells, adipose mesenchymal stem cells, or hematopoietic stem cells were considered as interventions; on the other hand, clinical efficacy (defined as clinical response or clinical remission), fistula healing, and the frequency of adverse events, endoscopic remission and clinical recurrence were considered as outcomes.
The review retrieved 13 prospective experimental studies reporting data on the efficacy of stem cell therapy for treating perianal CD. In the subgroup analysis performed according to the stem cell type used, the clinical response rate in patients who received bone marrow-derived mesenchymal stem cells was 59% (95% CI: 34%-80%; 6 studies), with a 60% probability of fistula closure (95% CI: 44%-75%). On the other hand, the clinical response rate in participants treated with adipose-derived stems cells was 56% (95% CI 13%-88%), with a 23% clinical remission rate (95% CI 7%-54%; 4 studies). Finally, in the group of patients who underwent hematopoietic cells therapy, a 73% clinical remission rate (95% CI: 36%-93%; 4 studies) and a 35% rate of adverse events (95% CI: 5%-86%) were found81.
Regarding the analysis of the route of administration, the fistula healing rate in patients who received systemic stem cell therapy was 29% (95% CI: 3%-85%), whereas in those who received local injection of stem cells, it was 60% (95% CI: 47%-72%)81.
Mesenchymal stromal cells versus placebo for the treatment of fistulas in Crohn’s disease
A systematic review and meta-analysis107 (AMSTAR score 7/11) evaluated the efficacy, compared to placebo, of using mesenchymal stem cells to treat fistulas associated with CD. The review included comparative studies conducted in patients with active disease (mean CDAI score <450 with presence of fistulas) and the outcome assessed was the frequency of fistula healing; safety outcomes were not considered. The review included 14 studies (477 patients in total). According to the findings reported in this systematic review, a lower proportion of persistent fistulas was found in the group of patients who underwent stem cell therapy (OR: 0.21; 95% CI: 0.09-0.32)107.
Quality of evidence: very low ⊕ΟΟΟ
In addition, a second systematic review that retrieved 11 studies that met the inclusion criteria and included only 3 studies with a control group in the meta-analysis, reports that mesenchymal stem cell therapy was associated with increased clinical healing of perianal fistulas when compared with controls at 6 and 24 weeks (OR: 3.06; 95% CI: 1.05-8.90; p = 0.04) and at 24 and 52 weeks (OR: 2.37; 95% CI: 0.90-6.25; p = 0.08). Also, there was no an increased frequency of adverse events (OR: 1.07; 95% CI: 0.61-1.89; p = 0.81) and serious adverse events (OR: 0.53; 95% CI: 0.28-0.98; p = 0.04) in patients treated with mesenchymal stem cells108.
Efficacy of medical therapy for the management of fistulizing Crohn’s disease
A systematic review that included 21 studies conducted in patients with fistulizing CD found moderate-quality of evidence supporting the efficacy of anti-TNF therapy (RR: 2.01; 95% CI: 1.36-2.97), particularly infliximab, ustekinumab (RR, 1.77; 95% CI: 0.93-3.37), and mesenchymal stem cell therapy (RR: 1.31; 95% CI: 0.98-1.73) for inducing fistula remission, and low-quality of evidence supporting the efficacy of vedolizumab and immunosuppressants. Regarding maintenance of fistula remission, moderate quality of evidence supporting the efficacy of anti-TNF therapy compared to placebo was found (RR: 1.94; 95% CI: 1.25-3.02; p = 0.003)109.
Question N° 6. What are the safest and most effective surgical and endoscopic interventions to treat crohn’s disease in patients older than 16 years?
Summary of evidence: General considerations
Biologic drugs and frequency of postoperative complications in patients with inflammatory bowel disease
A systematic review110 (AMSTAR score 9/11) assessed the frequency of postoperative complications in patients with CD who underwent open or laparoscopic surgery and who received anti-TNF therapy within the three months prior to the procedure. Of the studies included in the review, 11 recruited only patients with CD, and 3, patients with any type of inflammatory bowel disease (CD, ulcerative colitis, or indeterminate colitis). The outcomes reported in this review were the frequency of anastomosis-related complications (presence of dehiscence, fistula, intra-abdominal abscess or occurrence of enteric fistula), the proportion of patients who experienced a major medical complication (defined as life-threatening complication or a complication requiring hospitalization, including thrombotic, renal or cardiovascular diseases) or a minor complication (surgical site infection, prolonged ileus, adhesions, gastric bleeding or wound dehiscence), the need for reoperation and, finally, associated mortality.
The review retrieved 13 observational studies conducted in a total of 2046 patients. In nine studies, patients were administered infliximab, while in four they were administered adalimumab or certolizumab. All participants concomitantly received steroids and immunomodulators. According to the findings of this systematic review, the administration of biologic drugs during the months prior to the intervention was not associated with a higher or lower frequency of anastomosis-related complications (OR: 0.91; 95% CI: 0.56-1.47) or reoperation (OR: 1.09; 95% CI: 0.61-1.95), but it was associated with an increased risk of major complications (OR: 1.97; 95% CI: 1.23-3.14) and minor complications (OR: 1.40; 95% CI: 1.05-1.85). However, the frequency of these adverse events was not associated with increased mortality (OR: 4.80; 95% CI: 0.66-34.82)110.
Quality of evidence: very low ⊕ΟΟΟ
In a systematic review that included 27 studies, the effect of anti-TNF agent infusion timing prior to surgery and the risk of postoperative surgical site infection were evaluated. Compared with controls, no significant differences in terms of surgical site infections, abscess formation, or anastomotic leak were found in patients with CD when an anti-TNF drug was administered at 4, 8, or 12 weeks prior to surgery111.
Use of immunomodulators prior to bowel resection in patients with Crohn’s disease and risk of complications
A systematic review112 (AMSTAR score 7/11) evaluated the safety of using immunomodulators prior to performing bowel resection in patients with CD. In 14 of the studies included in the review, participants were administered an anti-TNF agent; in 13, corticosteroids; in 8, thiopurines; and in 6, two immunosuppressants were used concomitantly, within the 3 months prior to undergoing the surgical intervention. The outcome of interest was the proportion of patients with postoperative infection, defined as the presence of a focus of infection in soft tissues or the development of wound dehiscence, or having a diagnosis of intra-abdominal abscess, sepsis, pneumonia, peritonitis, or bacteremia.
This systematic review retrieved 21 observational cohort and case-control studies (648 patients in total). According to the findings of the review, prior use of anti-TNF drugs, as well as of corticosteroids, increased the proportion of patients who experienced postoperative infection (RR: 1.42, 95% CI: 1.05-1.92, and RR: 1.45, 95% CI: 1.01-2.08, for anti-TNF drugs and corticosteroids, respectively). However, this was not the case when thiopurines were used (RR: 1.23, 95% CI: 0.66-2.29). These findings remained constant when a subgroup analysis excluding studies that recruited patients with an inflammatory bowel disease other than CD was performed (RR: 1.31, 95% CI: 1.06-1.64, in the case of anti-TNF drugs, and RR: 1.45, 95% CI: 1.13-1.87, in the case of corticosteroids)112.
Quality of evidence: very low ⊕ΟΟΟ
Surgical management versus medical management in intra-abdominal abscesses
A systematic review and meta-analysis113 (AMSTAR score 5/11) compared the efficacy of medical management (antibiotics or antibiotics and percutaneous drainage) with that of surgical management (laparotomy with or without bowel resection) in patients with intra-abdominal abscesses secondary to CD. The location of CD, nor the type of antibiotic or the distribution of surgical techniques that were used were not specified in this review, and the following outcomes were assessed: resolution of abscesses, time required to show improvement, number of patients requiring stomas, and hospital stay. In all studies included in the review, the follow-up period was at least 1 year, except for one study, in which follow-up time was 3 months.
The review retrieved 9 retrospective observational studies. Differences favoring surgical treatment in terms of resolution of abscesses (OR: 3.44; 95% CI: 1.8-6.58) and clinical improvement before 1 year (OR: 4.58; 95% CI: 2.02-10.36) were found. Regarding the need for stoma creation, a higher frequency was observed in the group of patients who underwent surgical management compared with the medical management group (OR: 3.35; 95% CI: 1.43-7.87). Regarding hospital stay, it is descriptively reported that hospitalization length was shorter in the medical management group (quantitative data are not provided)113.
Quality of evidence: very low ⊕ΟΟΟ
Endoscopic pneumatic dilation in Crohn’s disease
A systematic review and meta-analysis of proportions114 (AMSTAR score 8/11) evaluated the efficacy of endoscopic pneumatic dilation in adult patients with stenosing CD. The review did not report the anatomic distribution of the disease and included a total of 1089 patients, 790 strictures and 2664 dilations. The following outcomes were reported: symptomatic response, endoscopic response, and the presence of postoperative complications. The median follow-up time was 83.5 months (range: 12 to 172 months). The review retrieved 25 studies, of which 16 included symptomatic response as an outcome, with a pooled frequency of 70.2% (95% CI: 60%-78.8%); the endoscopic response pooled rate was 90.6% (95% CI: 87.8%-92.8%; 22 studies), and the perforation pooled rate was 3% (95% CI: 2.2%-4%)114.
Quality of evidence: very low ⊕ΟΟΟ
Strictureplasty versus bowel resection in patients with small bowel Crohn’s disease
A systematic review and meta-analysis115 (AMSTAR score 8/11) evaluated the efficacy and safety of strictureplasty compared with bowel resection with or without dilation in patients with small bowel CD; no further specifications are provided. Surgical and medical recurrence were assessed as efficacy outcomes, and bowel obstruction, bleeding, septic complications, and overall complications, as safety outcomes. The review retrieved seven non-randomized studies that included pediatric and adult populations. Regarding recurrence-free survival time, differences in favor of strictureplasty were found (HR: 1.08; 95% CI: 1.02-1.15), but no differences were observed in terms of frequency of overall recurrence (OR: 1.36; 95% CI: 0.96-1.93) or overall complications (OR: 0.60; 95% CI: 0.31-1.16). Also, there were no differences between groups in the following outcomes: surgical and medical recurrence (OR: 1.36; 95% CI: 0.96-1.93), bowel obstruction (OR: 0.8; 95% CI: 0.09-1.93), hemorrhage (OR: 0.51; 95% CI: 0.13-2.0), and sepsis (OR: 0.67; 95% CI: 0.27-1.67)115.
Quality of evidence: very low ⊕ΟΟΟ
Laparoscopy versus open surgery
A systematic review116 (AMSTAR score 10/11) compared the efficacy of laparoscopy and the rates of reoperation and recurrence associated with it versus open surgery in patients with small bowel CD. The review included patients older than 16 years who underwent elective surgery with ileocolic resection and anastomosis or small bowel resection plus anastomosis or strictureplasty. Perioperative morbidity and reoperation due to disease recurrence were considered as primary outcomes, and postoperative pain, duration of postoperative ileus, postoperative hospital stay length, duration of the procedure, amount of blood loss, mortality, and conversion rates, as secondary outcomes. The median follow-up time of the studies included in the review ranged from 3 months to 10.9 years after surgery. The review found 4 randomized clinical trials conducted in a total of 120 patients. In the pooled analyses, no statistically significant differences were found between both groups regarding the rate of infections (OR: 0.25; 95% CI: 0.03-2.39), the rate of anastomotic leaks (OR: 0.97; 95% CI: 0.13-6.98), the rate of intra-abdominal abscesses (OR: 0.19; 95% CI: 0.11-74.12), and the rates of reoperation within the first 30 days (OR: 0.57; 95% CI: 0.07-4.46) or in the long term (OR: 0.85; 95% CI. 0,32-2,27). Also, there were no differences in hospital stay duration (OR: 0.7; 95% CI: 0.28-1.73), duration of postoperative ileus (OR: 0.55; 95% CI: 0.13-2.43), and mean blood loss (surgery: 133 ± 70 mL versus laparoscopy: 173 ± 123 mL; p = 0.25). A conversion rate <3% was found and none of the studies reported mortality cases116.
Quality of evidence: very low ⊕ΟΟΟ
Types of anastomosis in ileocolic resection
A systematic review and meta-analysis117 (AMSTAR score 8/11) compared the efficacy and safety of side-to-side anastomosis versus end-to-end anastomosis after ileocolic resection in patients with CD. The review included three randomized clinical trials and five non-randomized studies (821 patients in total) and the following outcomes were evaluated: frequency of complications, hospital stay, mortality, recurrence, and need for reoperation. Regarding the frequency of complications, differences in favor of side-to-side anastomosis were found (overall complications: OR: 0.54; 95% CI: 0.32-0.93), but these differences were not significant when complications were disaggregated into anastomotic leak, wound infection, pulmonary embolism, intra-abdominal abscess, and bowel obstruction or stricture. Regarding recurrence, statistically significant differences in favor of side-to-side anastomosis were found (OR: 0.20; 95% CI: 0.07-0.55), as well as regarding the frequency of reoperation (OR: 0.18; 95% CI: 0.07-0.45). No differences between both groups were found in terms of postoperative hospital stay length (weighted mean difference: -0.59; 95% CI: -1.87 to 0.68) or the risk of mortality (OR: 1.94; 95% CI: 0.30-12.48)117.
Quality of evidence: very low ⊕ΟΟΟ
Surgical resection in patients with colonic Crohn’s disease
A systematic review and meta-analysis118 (AMSTAR score 7/11) evaluated the efficacy and safety of segmental colectomy compared with subtotal or total colectomy in patients with colonic CD. Only pediatric or adult patients with a diagnosis of CD, as defined by the Price and Morson criteria, with initial colonic involvement or with active colonic CD were included. One of the studies retrieved included patients with ileocolonic CD. The review identified 6 comparative observational studies, and surgical recurrence, overall recurrence, postoperative complications, and the need for a permanent stoma were considered as the outcomes of interest. According to the findings of this systematic review, there were no significant differences between interventions in relation to surgical recurrence (OR: 1.08; 95% CI: 0.39-2.95), overall recurrence (OR: 1.01; 95% CI: 0.49-2.06), postoperative complications (OR: 1.43; 95% CI: 0.16-12.74) or the need for a permanent stoma (OR: 2.75; 95% CI: 0.78-9.71)118.
Quality of evidence: very low ⊕ΟΟΟ
Question Nº. 7. What are the safest and most effective interventions to prevent postoperative recurrence of crohn’s disease in patients older than 16 years?
Summary of evidence: General considerations
Using probiotics to prevent postoperative recurrence of Crohn’s disease
A systematic review71 (AMSTAR score 9/11) evaluated the efficacy and safety of using probiotics for maintenance of remission in CD. In this case, the following outcomes were analyzed: frequency of clinical relapse (CDAI >150 or a ≥70 points increase compared to the baseline score) or endoscopic relapse (Rutgeerts score >2) at 12 months. Compared to placebo, the administration of probiotics did not reduce the incidence of clinical relapse (RR: 1.06; 95% CI: 0.59-1.92) or endoscopic relapse (RR: 1.04; 95% CI: 0.82-1.31). It should be noted that this review did not assess the frequency of adverse events in this population (patients with CD).
Another systematic review88 (AMSTAR score 8/11) also assessed the efficacy of using probiotics in maintaining remission in patients with CD after undergoing surgical treatment. The outcome reported by this review was the incidence of endoscopic relapse (Rutgeerts score) with a follow-up time ranging from 3 to 24 months. Three studies comparing the use of this intervention versus placebo in 200 patients in total were included. No statistically significant differences were found between groups (RR: 1.08; 95% CI: 0.67-1.74).
Quality of evidence: very low ⊕ΟΟΟ
Using antibiotics to prevent postoperative recurrence of Crohn’s disease
A systematic review73 (AMSTAR score 7/11) assessed the efficacy of using antibiotics to induce remission in patients with postoperative recurrence of CD. All studies included in the review allowed concomitant use of other interventions (immunomodulators) and the outcome reported was the proportion of patients who achieved clinical improvement or remission (CDAI score <150 and/or a ≥70 points decrease in CDAI score or a >50% reduction in the number of fistulas for at least 4 weeks) during follow-up. The review retrieved one randomized clinical trial (33 participants). Compared to placebo, antibiotic administration did not increase the frequency of patients in which remission was maintained during the first 3 to 6 months (RR: 1.13; 95% CI: 0.43-2.98)53.
Quality of evidence: low ⊕⊕ΟΟ
A second systematic review119 (AMSTAR score 9/11) included two controlled clinical trials assessing the rate of severe endoscopic recurrence (3-month follow-up and assessed with a Rutgeerts score I2 or higher) and the rate of withdrawal due to serious adverse events when using 5-nitroimidazoles. Compared to placebo, 5-nitroimidazoles use decreased the frequency of severe endoscopic recurrence (RR: 0.44; 95% CI: 0.26-0.74), at the expense of a higher adverse events rate (RR: 3.00; 95% CI: 1.37-6.58)119.
Quality of evidence: low ⊕⊕ΟΟ
Use of azathioprine or 6-mercaptopurine to prevent postoperative recurrence of Crohn’s disease
A systematic review and meta-analysis120 (AMSTAR score 9/11) evaluated the safety and efficacy of using different interventions to maintain surgically induced remission of CD. This review assessed the following outcomes: the proportion of patients who experienced clinical relapse (defined as having a CDAI score >200 or requiring steroids or having a 60-point increase in CDAI score compared to the baseline score) or endoscopic relapse (defined as having a Rutgeerts score >2) and the frequency of withdrawal due to adverse events.
The review retrieved two controlled clinical trials (168 participants in total) comparing this intervention with placebo. According to the findings of this review, the use of azathioprine or 6-mercaptopurine reduced the incidence of clinical relapse (RR: 0.74; 95% CI: 0.58-0.94) and endoscopic relapse (RR: 0.40; 95% CI: 0.19-0.83) during the 3 to 12 months of follow-up, without increasing the frequency of withdrawal (RR: 1.33; 95% CI: 0.59-2.98). In addition, in this review, azathioprine or 6-mercaptopurine therapy was also compared with the administration of 5-aminosalicylates. In this regard, five studies conducted in 425 patients in total and comparing the safety and efficacy of these interventions were included. No differences were found between groups in terms of clinical relapse (RR: 1.14; 95% CI: 0.93-1.41) and endoscopic relapse (RR: 0.55; 95% CI: 0.23-1.32); however, a higher rate of withdrawal due to adverse events was observed in the azathioprine or 6-mercaptopurine arm (RR: 2.07; 95% CI: 1.26-33.90)120.
Finally, a comparison between azathioprine or 6-mercaptopurine therapy and anti-TNF therapy was also made. When infliximab was used, there were no differences regarding the rate of clinical relapse (RR: 2.00; 95% CI: 0.21-18.98), the rate of endoscopic relapse (RR: 4.40; 95% CI: 0.59-33.07) or the frequency of withdrawal (RR: 3.00; 95% CI: 0.14-66.53). However, azathioprine or 6-mercaptopurine therapy significantly increased the rate of relapse, both clinical an endoscopic (RR: 5.18; 95% CI: 1.35-19.83, and RR: 10.35; 95% CI: 1.50-71.32, respectively) when compared to adalimumab. Finally, withdrawal rates were similar between groups (RR: 1.88, 95% CI: 0.19-18.80)120.
Quality of evidence: very low ⊕ΟΟΟ
Using 5-aminosalicylates to prevent postoperative recurrence of Crohn’s disease
A systematic review121 (AMSTAR score 8/11) assessing the efficacy and safety of using 5-aminosalicylates for maintenance of surgically induced remission of CD was retrieved. This review included eight studies (1061 participants in total) and the outcomes reported were the frequency of clinical relapse during the first 24 months of follow-up (defined as a CDAI >150 or >200 or a >60 points increase compared to the baseline score) and the frequency of adverse events. Compared to placebo, the administration of 5-aminosalicylates reduced the frequency of relapse (RR: 0.71; 95% CI: 0.54-0.94) but did not reduce the frequency of adverse events (RR: 1.06; 95% CI: 0.61-1.85)121.
Quality of evidence: low ⊕⊕ΟΟ
Use of budesonide to prevent postoperative recurrence of Crohn’s disease
A meta-analysis, resulting from a systematic literature search119 (AMSTAR score 9/11), retrieved two controlled clinical trials assessing the rate of severe endoscopic recurrence (12-month follow-up and assessed with a Rutgeerts score I2 or higher) and the frequency of withdrawal due to serious adverse events when using budesonide. Compared to placebo, this intervention did not reduce the incidence of severe endoscopic relapse (RR: 0.87; 95% CI: 0.50-1.49), but neither did it increase the frequency of withdrawal due to adverse events (RR: 1.01; 95% CI: 0.37-2.78)119.
Quality of evidence: low ⊕⊕ΟΟ
Anti-TNF therapy versus conventional therapy to prevent postoperative recurrence of Crohn’s disease
A second systematic review122 (AMSTAR score 7/11) compared the efficacy of infliximab administration within the first 2 to 4 weeks after performing the surgical procedure to prevent histologic recurrence, which was defined according to the modified D’Haens histologic scoring system. This review retrieved a study conducted in 24 participants and in which this outcome was evaluated. When compared with the administration of mesalamine or 6-mercaptopurine, infliximab therapy increased the proportion of patients who did not experience a histologic recurrence episode during the first 54 weeks of follow-up (RR: 6.00; 95% CI: 1.02-35.37)122.
Quality of evidence: low ⊕⊕ΟΟ
Safety and efficacy of different pharmacological strategies to prevent postoperative recurrence of Crohn’s disease. Results of a network meta-analysis
A systematic review and network meta-analysis123 (AMSTAR score 8/11) evaluated the safety and efficacy of different pharmacological interventions to prevent clinical relapse (CDAI ≥ 150) or endoscopic relapse (Rutgeerts score I2-I4 or the combination of endoscopic and/or imaging relapse based on cross-sectional imaging or barium studies) in patients with CD after surgery. Patients with established CD, a history of successful bowel resection with removal of macroscopically visible disease, and who had started postoperative prophylaxis for CD within the first 3 months after undergoing bowel resection were included.
According to the results of this network meta-analysis, when compared with placebo (or no intervention), the use of mesalazine (RR: 0.60; 95% CI: 0.37-0.88), antibiotics (RR: 0.26; 95% CI: 0.08-0.61), immunomodulator monotherapy (RR: 0.36; 95% CI: 0.17-0.63), combination therapy with immunomodulators plus antibiotics (RR: 0.11; 95% CI: 0.02-0.51) and anti-TNF monotherapy (RR: 0.04; 95% CI: 0.00-0.14) were effective interventions in terms of decreasing the frequency of clinical relapse episodes. However, budesonide therapy did not appear to be superior to placebo (RR: 0.93; 95% CI: 0.40-1.84). On the other hand, regarding endoscopic relapse, this meta-analysis reported that the use of antibiotics (RR: 0.41; 95% CI: 0.15-0.92), immunomodulatory monotherapy (RR: 0.33; 95% CI: 0.13-0.68), combination therapy with immunomodulators plus antibiotics (RR: 0.16; 95% CI: 0.04-0.48) and anti-TNF monotherapy (RR: 0.01; 95% CI: 0.00-0.05) were superior to placebo, however this was not the case for mesalazine (RR: 0.67; 95% CI: 0.39-1.08) or budesonide (RR: 0.86; 95% CI: 0.61-1.22) (123).
Furthermore, this systematic review conducted a second analysis to determine which of these pharmacological interventions was the most effective in preventing clinical relapse. In this regard, the network analysis showed that anti-TNF monotherapy was superior to immunomodulator monotherapy (RR: 0.11; 95% CI: 0.01-0.40). On the other hand, anti-TNF monotherapy was also superior to antibiotic use, but this estimator was based primarily on indirect evidence (RR: 0.20; 95% CI: 0.01-0.84). Also, there were no significant differences between combination therapy with immunomodulators plus antibiotics and immunomodulator monotherapy (RR: 0.34; 95% CI: 0.05-1.20) or antibiotic monotherapy (RR: 0.48; 95% CI: 0.08-1.46). Immunomodulator monotherapy was not superior to antibiotic therapy (RR: 1.92; 95% CI: 0.93-4.00)123.
Finally, this systematic review also aimed to determine which of these pharmacological interventions might be the best in preventing endoscopic relapse. Based on the results of this network meta-analysis, anti-TNF monotherapy was superior to all other interventions: vs. mesalazine (RR: 0.02; 95% CI: 0.00-0.07), antibiotics (RR: 0.03; 95% CI: 0.00-0.15), immunomodulator monotherapy (RR: 0.04; 95% CI: 0.00-0.14), combination therapy with immunomodulators plus antibiotics (RR: 0.03; 95% CI: 0.00-0.49) and budesonide (RR: 0.005; 95% CI: 0.00-0.08). Also, there were no significant differences between combination therapy with immunomodulators plus antibiotics and immunomodulator monotherapy (RR: 0.54; 95% CI: 0.12-1.59) or antibiotic monotherapy (RR: 0.43; 95% CI: 0.10-1.19). Additionally, there were no significant differences between immunomodulator monotherapy and antibiotic monotherapy in terms of reducing the risk of endoscopic relapse (RR: 0.97; 95% CI: 0.26-2.53)123.
Quality of evidence: low ⊕⊕ΟΟ
A systematic review and meta-analysis that included 10 studies (751 patients in total) analyzed postoperative endoscopic recurrence of CD at 12 months of follow-up. Anti-TNF monotherapy was significantly better than placebo in preventing endoscopic recurrence (RR: 0.13; 95% CI: 0.04-0.39). The same outcome was obtained when combined with 5-ASA drugs (RR: 0.30; 95% CI: 0.12-0.75) or with nitroimidazoles (RR: 0.40; 95% CI: 0.23-0.69). Combination therapy with thiopurines plus metronidazole was also more effective than placebo (RR: 0.56; 95% CI: 0.40-0.80), as well as thiopurine monotherapy (RR: 0.84; 95% CI: 0.74-0.94). Somehow, nitroimidazoles and 5-ASA drugs were not superior to placebo in preventing endoscopic recurrence postoperatively124.
Anti-TNF agents versus immunomodulators for the treatment of postoperative relapse of Crohn’s disease
A systematic review125 (AMSTAR score 8/11) evaluated the efficacy of administering anti-TNF drugs to treat postoperative relapse of CD. The review retrieved two clinical trials conducted in a total of 50 patients, and the outcome reported was the frequency of endoscopic remission (Rutgeerts score <2) with a follow-up time ranging from 6 to 12 months. Compared to immunomodulators, anti-TNF therapy increased the proportion of patients who achieved endoscopic remission (OR: 16.64; 95% CI: 2.51-110.27)125.
Quality of evidence: low ⊕⊕ΟΟ
Values and preferences of patients
What are the needs of patients in relation to Crohn’s disease?
Values and preferences
Inflammatory bowel disease (IBD) has a major impact on the quality of life and psychological well-being of patients. An emotional component that may affect therapeutic outcomes in these patients has been identified.
A qualitative systematic review126 (AMSTAR 7/11) that included 36 studies conducted in adult population explored how to support improvement of these outcomes in IBD patients. According to this review, patients with psychological adaptation mechanisms to new situations (patients with a family support network and individual strength) showed faster improvement than those with psychological adaptation problems (i.e., patients who focus more on negative situations).
In addition, this review reports that, on average, 43% of IBD patients suffer some degree of depression according to specialized scales with emphasis on the “loneliness”, “resilience” and “disease activity” domains. In this regard, the systematic review included a study conducted in 49 patients reporting that the degree of depression is a predictor of the positive effect of the therapy. Also, a meta-analysis based on 50 patients found that psychological therapy had an effect on the disease at 12 months, but that it did not have any effect at 6 months. A qualitative study conducted in 31 patients with severe IBD found that eating habits changes, support networks, strategies to control IBD-related situations, positive attitudinal changes toward the disease, relaxation techniques, finding distractions from the disease, and disease awareness contributed to improved well-being and symptoms alleviation. The SR concludes that IBD patients should undergo psychological therapy for achieving emotional management of the disease126.
Values and preferences
Most patients with CD need to undergo surgery within 10 years after the initial diagnosis is made. Although it affects their quality of life, bowel resection is the surgical procedure most frequently used to treat CD.
A systematic review127 (AMSTAR 7/11) assessed the impact of bowel resection on quality of life using the HRQoL scale and identified predictors of postoperative health-related quality of life (HRQOL) and patient satisfaction with this surgery. Nine studies conducted in a total of 1108 patients with CD who underwent bowel resection were included. Patients’ median age range was between 29 and 41 years. Ileocolic resection was the most frequently performed bowel resection, and the most common indication for surgery were bowel obstruction, stenosing disease, perforation, and pharmacological therapy failure. Regarding HRQoL, it was found that all patients reported a postoperative improvement in their quality of life (at 30 days). Likewise, in general, the effect on HRQoL lasted from 2 to 5 years. Furthermore, the systematic review analyzed the predictors of HRQoL, finding that patients older than 49 years had less improvement in HRQoL at 9 months after surgery compared to younger patients. Also, a decreased improvement in HRQoL was observed in smoking patients compared to non-smokers. No differences were found in relation to gender and surgical technique. Regarding patient satisfaction, 80% of patients reported they were satisfied with the surgery and that they would undergo it again if necessary. Also, 92% expressed they preferred laparoscopic surgery127.
Values and preferences
Perianal CD can affect quality of life involving physical, functional, and psychological aspects.
A cross-sectional study128 determined the factors positively or negatively affecting quality of life in 69 patients with perianal Crohns’ disease, as well as their impact on symptoms. Participants’ mean age was 42.7 years and 62% were female. 80% of patients had undergone surgery prior to completing the questionnaire used in the study. Anal pain and discomfort were considered the most important symptoms by 41% of the patients, and 39% reported that managing sleeping difficulty, physical activity restriction, and feeling unclean were “very important to improve quality of life”. Likewise, 85% of patients expressed they would accept using a stoma to improve symptoms.
In addition, anal pain was the most important symptom for women compared to men (53% vs. 19%). The presence of drainage was reported as the strongest predictor of incontinence, feeling unclean, and confidence in going outside. Finally, the study concluded that physical symptoms are the most important symptoms for patients with perianal Crohn’s disease and that drainage affects their quality of life98.
Values and preferences
The management of CD poses challenges regarding the selection of the most appropriate therapy. Thus, patients’ preferences may guide therapy selection.
A qualitative study99 compared the preferences of 300 CD patients with those of 92 surgeons and 74 gastroenterologists in Australia. This study found that the preferences of patients differed from those of specialists. Patients were more interested in improving their quality of life given the risks and benefits of the therapies they were presented with. In this regard, 37% surgeons did not seek to perform ileocolic resection compared to 39% patients (p<0.01). Also, patients preferred using a permanent stoma instead of undergoing colorectal surgery (85% vs. 56%; p>0.01). However, when asked to choose between restorative proctocolectomy and permanent stoma, the same preference was observed in both, patients and surgeons (50%; p>0.05).
When the preferences of patients were compared with those of gastroenterologists, differences in surgical preferences were found. A higher proportion of patients preferred ileocolic resection. No differences were found regarding the selection of the pharmacological therapy (p>0.05)129.
Clinical aspect: prognosis
Question N° 1. What are the predictors of relapse of Crohn’s disease in patients older than 16 years?
Question N° 2. What are the safest and most effective non-biological interventions to induce remission of Crohn’s disease in patients older than 16 years?
Question N° 3. What are the safest and most effective non-biological interventions to maintain remission of Crohn’s disease in patients older than 16 years?
Question N°4. What is the safety and efficacy of using biological drugs to treat moderate to severe Crohn’s disease in patients older than 16 years?
Question N° 5. What are the safest and most effective interventions to treat perianal Crohn’s disease in patients older than 16 years?
Implementation module
Using the Manual for the implementation of clinical practice guidelines in the Colombian health insurance framework (MSPS, 2014) is recommended to support the implementation of this CPG.
The considerations that the different actors of the Colombian health system must take into account when implementing this CPG are presented below.
Actors responsible for the implementation of the recommendations described in the CGP
It is important to identify those responsible for supporting or executing the implementation activities of the CPG at the national level or in the institutions in charge of the provision of health care (ISPSS). The main actors in the process of implementing the CPG are:
Governmental agencies:
Colombian Ministry of Health and Social Protection.
Cuenta de Alto Costo Fondo Colombiano de Enfermedades de Alto Costo (Fund for Highly Expensive diseases)
Territorial bodies
National Superintendence of Health
Institutions:
Health Promotion Entities
Scientific associations
Colombian Association of Gastroenterology
Institutions responsible for the provision of health care
Patient associations and civil society representatives.
Key recommendations for the implementation of the CPG
In order to identify the recommendations of the CPG with the greatest impact and in which implementation efforts can be prioritized, the GDG selected the following recommendations:
Measuring C-reactive protein (CRP) levels and using erythrocyte sedimentation rate (ESR) to predict of Crohn’s disease is recommended.
Implementing mucosal healing as a therapeutic goal in patients with Crohn’s disease is suggested.
Clinical monitoring of opportunistic infections in patients with Crohn’s disease undergoing biological therapy is suggested.
Using systemic steroids for maintenance of remission in patients with Crohn’s disease is not recommended.
Using infliximab biosimilars to induce and maintain remission in patients with Crohn’s disease is suggested.
Using azathioprine, methotrexate, infliximab, infliximab plus azathioprine, adalimumab or vedolizumab to maintain remission in patients with Crohn’s disease is recommended.
The use of combination therapy with an anti-TNF drug plus seton placement in the treatment of complex perianal fistulizing Crohn’s disease is recommended to increase complete fistula closure.
Assessing the presence of signs of depression or worsened quality of life and, if necessary, referring the patient to support groups and specialized help groups is recommended.











 text in
text in