Introduction
Nodular lymphoid hyperplasia of the colon is defined as a group of > 10 lymphoid nodules observed during colonoscopy1, these have been reported most frequently in young children and without association with clinical symptoms, cases in which nodular lymphoid hyperplasia is considered to be physiological1-3; however, it has also been described in patients undergoing colonoscopy due to gastrointestinal bleeding, chronic abdominal pain4, refractory constipation, delayed growth, or anemia, in frequencies ranging from 12.8%5 to 49%1. In this regard, a study conducted in Cuba found that 63%, 33% and 4% of nodular lymphoid hyperplasia cases occurred in children under 6 years of age, children between 6 and 10 years old, and children older than 10 years, respectively6.
The clinical relevance and etiology of nodular lymphoid hyperplasia is unclear. For many years it was thought to be a casual finding during colonoscopy; somehow, it has been associated with infectious diseases such as Helicobacter pylori infection7, enterobiasis, amebiasis, Escherichia coli infection and Giardia lamblia infection8-11; Mediterranean family fever; immunodeficiency disorders such as common variable immunodeficiency12-14, IgA deficiency14,15, hypogammaglobulinemia12,13,16 and human immunodeficiency virus (HIV)17; food allergy; inflammatory bowel disease1,4,5,18; irritable bowel syndrome; celiac disease; and Behçet’s disease, among others5.
The diagnosis of nodular lymphoid hyperplasia is reached by means of an endoscopy, and histopathology studies are required for its confirmation19. Endoscopic features include nodules ranging from 2 to 10 mm and can occur in the stomach, the small intestine (most commonly in the terminal ileum), and the colon/rectum20. In the colon, they may look like red macules, circumferential target lesions, or raised papules21,22. When the large intestine is affected, the rectum is most frequently involved23,24. Their endoscopic appearance can be surprisingly similar to polyposis syndromes23, but histological findings can help reach a differential diagnosis. Histologically, it is defined by the presence of lymphoid follicles hyperplasia, germinal centers with mitotic activity, and well-defined mantle lymphocytes located in the lamina propria or the submucosa25; it may resemble a malignant lymphoma26, both clinically and histologically, but it can be differentiated by its polymorphic nature resulting from the infiltration, the absence of significant cytologic atypia, and the presence of reactive follicles within the lesion27.
Nodular lymphoid hyperplasa is considered a risk factor for the development of both intestinal28,29 and extraintestinal lymphomas30,31, adenomas, and carcinomas 32, and some authors recommend performing several barium swallows and capsule endoscopies for surveillance purposes24, although the duration and intervals of such surveillance studies have not been determined15. So far there are no studies evaluating the diagnostic validity of detecting nodules during colonoscopy compared to histological findings in the diagnosis of nodular lymphoid hyperplasia.
Objective
To determine the validity of detecting nodules during colonoscopy for the diagnosis of nodular lymphoid hyperplasia.
Materials and methods
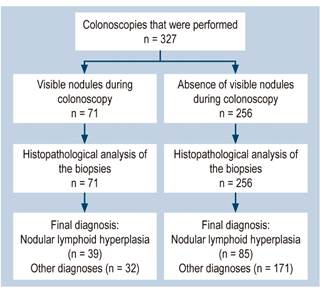
A prospective diagnostic test accuracy study was conducted in a pediatric referral hospital. A total of 327 colonoscopies performed consecutively between January 1, 2014 and December 31, 2018 were included. The sample size was calculated in Epidat 3.2 based on an expected sensitivity of 40 %-60 %, an expected specificity of 70 %-90 %, a disease prevalence of 30.9 % (average of the prevalence rates of nodular lymphoid hyperplasia described in the literature)1,5, with a confidence interval (CI) of 95 % and a statistical power of 80 %. All colonoscopies were performed by a pediatric gastroenterologist trained in this type of procedure; an Olympus PCF-Q150AI colonoscope was used in children over 10 kg, while an Olympus GIF-XP150N neonatal endoscope was used in those under 10 kg; in all cases, biopsies were taken during colonoscopy using cold biopsy forceps and removing at least 2 samples from the cecum, 2 from the ascending colon, 2 from the transverse colon, 2 from the descending colon, and 2 from the sigmoid colon and rectum, which were analyzed by 2 pathologists from the histopathology department of the institution; it should be noted that the analysis was blind and was carried out within a maximum of 1 week. The pathologists were previously validated by interobserver agreement with a Cohen’s Kappa coefficient of 1.0 (p = 0.000). There were no adverse events during the performance of both colonoscopies or biopsies.
The colonoscopic criterion was defined according to what has been reported in the relevant literature, that is, detecting a group of >10 nodules with a size ranging from 2 to 10 mm at any site in the large bowel20. The histology study was carried out using hematoxylin-eosin staining with a light microscope at 5X, 10X and 40X; the histological criterion was defined based on what has been described in the literature, that is, the presence of lymphoid follicles hyperplasia and well-defined lymphocyte mantles in the lamina propria or submucosa25.
Data were recorded in a database in the SPSS software, version 22, where they were coded for tabulation. In the age variable, the Kolmogorov-Smirnov test was performed, and since data distribution was not normal, medians and interquartile ranges were used. On the other hand, qualitative variables such as age interval, sex, indication for colonoscopy, colonoscopic findings, site of colonoscopic finding, and histological findings are expressed by means of frequencies and percentages. Colonoscopic findings were dichotomized based on the presence or absence of visible nodules, while histological findings, based on the presence or absence of nodular lymphoid hyperplasia and were crossed in 2 x 2 tables. The proportion of nodular hyperplasia according to age and indication for colonoscopy was compared using chi-square (χ2). The Epidat 3.1 software was used to obtain the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV), and positive and negative likelihood ratios with their respective confidence intervals, as well as the overall value of the diagnostic test.
Results
Of the 327 colonoscopies, 188 were performed in boys (57.5 %) and the rest in girls. Participants’ median age was 84 months (7 years) with an interquartile range of 111 months, a minimum value of 1 month, and a maximum value of 216 months.
Lower gastrointestinal bleeding (with or without anemia) was the most frequent indication for colonoscopy (n=127, 38.8%), followed by suspicion or surveillance of inflammatory bowel disease (17 %), suspicion or monitoring of food allergy (14.4 %), chronic diarrhea (11.3 %), polyposis syndrome surveillance (6.1 %) and suspected rectal polyp (3.7 %); the remaining colonoscopies were performed due to several reasons including suspected graft versus host disease, chronic abdominal pain, protein-losing enteropathy, vascular malformation, foreign bodies, and suspected colon cancer, among others.
The prevalence of colonoscopic detection of nodules was 21.7 % (n = 71). The most frequent location of the nodules was the total colon, with 33 cases (46 %), followed by rectum limited involvement (17 %), left colon limited involvement (14 %) and sigmoid colon and rectum limited involvement (13 %). The proportion of nodules detection was similar in boys and girls (21.3 % vs 22.3%). Nodules occurred more frequently at a lower age (p = 0.016) (Table 1 and Figure 1).
Table 1 Distribution of patients undergoing colonoscopy according to the detection of nodules, sex, and age (2014-2018)
*There is a statistically significant difference. Source: Data collection forms. Own elaboration.
The prevalence of nodular lymphoid hyperplasia according to the histopathology report was 38 % (n = 124). The lowest frequency was found in patients who underwent colonoscopy due to recurrence of intussusception, unexplained weight loss, and suspected intestinal pseudo-obstruction (0%), followed by, in ascending order, those who underwent it due to polyposis syndromes surveillance (5%) and suspected graft versus host disease (11 %). On the other hand, the highest frequency of nodular lymphoid hyperplasia was observed in the cases in which colonoscopy was indicated due to colon cancer, suspected intestinal tuberculosis, and refractory constipation (100%), followed by, in descending order, suspected rectal fistula and suspected enteropathy (50%), suspected or monitoring of inflammatory bowel disease (49 %), suspected rectal polyp (42 %) and suspected or monitoring of food allergy (40 %).
Statistically significant differences regarding the frequency of nodular lymphoid hyperplasia were found according to the indication for colonoscopy (p=0.038). With regard to the final diagnosis, the condition in which nodular lymphoid hyperplasia was most frequently reported was food allergy (47 %, n = 81), followed by chronic nonspecific colitis (52 %, n = 105), inflammatory bowel disease (22 %, n = 27) and polyposis (22%, n = 59); suspected cases of colon cancer and intestinal tuberculosis were ruled out.
A nodular pattern was observed during colonoscopy in only 32% of patients with nodular lymphoid hyperplasia, while such pattern was not present in 84% of children without this disorder; 56% of patients with a nodular pattern had nodular lymphoid hyperplasia and 67% of those without such pattern did not have this disorder. In addition, children with nodular hyperplasia were 2 times more likely to have a nodular pattern on colonoscopy, while in those without nodular hyperplasia not having a nodular pattern was 1.25 more likely (Table 2). The overall validity of nodular pattern was 64 %.
Table 2 Distribution of patients undergoing colonoscopy according to the nodular pattern finding on colonoscopy and the histopathological finding of lymphoid nodular hyperplasia (2014-2018)
| Nodular pattern by colonoscopy | Lymphoid nodular hyperplasia by histology | S (95% CI) | Sp (95% CI) | PPV (95% CI) | NPV (95% CI) | LR+ (95% CI) | LR- (95% CI) | ||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Total | |||||||
| Yes | 39 | 32 | 71 | 32,31 (23,8-40,7) | 84,13 (78,9-89,3 | 56 (44,1-67,9) | 66,5 (60,5-72,4) | 2,04 (1,37-3,04) | 0,8 (0,7-0,92) |
| No | 85 | 171 | 256 | ||||||
| Total | 124 | 203 | 327 | ||||||
CI: Confidence intervals; LR: Likelihood ratio; NPV: Negative predictive value; PPV: Positive predictive value; S: Sensitivity; Sp: Specificity. Source: Data Collection forms. Own elaboration.
Discussion
In our study, lower gastrointestinal tract bleeding was the main indication for colonoscopy (38.8%), a percentage similar to the 32% described by Colon et al.4.
The prevalence of nodules detection on colonoscopy in our study is similar to what Zahmatkeshan et al. have reported33; however, in the present study, the prevalence of this finding confirmed by means of the histopathology study almost doubled the one reported on the endoscopic procedure, so it was probably underdiagnosed.
Total colonic involvement was observed in most of patients in whom nodules were detected, a finding that differs from what has been described in the literature, as it has been reported that the number of nodules in the colon is greater in the anorectal region20,23,24.
Regarding age, it was found that the younger the patient, the greater the presence of nodules, which is similar to what is described in the study conducted in Cuba6. There were significant differences regarding the presence of nodular hyperplasia according to the indication for colonoscopy, which could be related to a higher frequency of this disorder in specific conditions, including food allergy; however, the association between nodular hyperplasia and its specific causes exceeds the objectives of the present study, and therefore should be addressed in future works.
The majority of patients who were diagnosed with lymphoid nodular hyperplasia based on the histology report did not show a nodular pattern during colonoscopy, which points out the different endoscopic mucosal lesion patterns of nodular lymphoid hyperplasia21-23, an aspect that should be studied in future works. The PPV of the detection of nodules was low; when analyzing the positive likelihood ratio, it was found that the probability of having visible nodules during colonoscopy was only two times higher in patients with nodular hyperplasia, so the detection of nodules has a poor diagnostic value.
The specificity of absence of nodules during colonoscopy was high, which means that most patients without nodular hyperplasia do not have a nodular pattern; somehow, the NPV and the negative likelihood ratio were low.
The usefulness of endoscopic findings obtained through new endoscopic techniques such as Narrow Band Imaging (NBI® by Olympus Medical Systems Corporation), Fuji Intelligent Color Enhancement (FICE® by Fujinon Corporation) or iSCAN (iSCAN® by Pentax) for the diagnosis of nodular lymphoid hyperplasia was not assessed, so further research is needed to determine their diagnostic validity.
Regarding the poor overall validity of detecting nodules during colonoscopy for the diagnosis of nodular lymphoid hyperplasia, we consider it appropriate to stop using the current definition of nodular lymphoid hyperplasia as the detection of > 10 nodules during the endoscopic procedure1, and start using a definition based only on histopathological criteria, being biopsies indispensable during colonoscopy.
Ethical considerations
This study complied with both international ethical standards and those in force in our institution. No experiments involving human beings or animals were performed. Besides, since this is a descriptive study, informed consent was not required. The identity of participants has been kept strictly confidential.











 text in
text in