Introduction
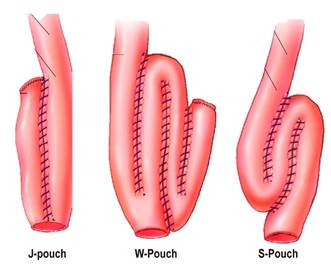
Ulcerative colitis (UC) is an autoinflammatory, multifactorial disease that produces chronic inflammation of the colon mucosa, with frequent extraintestinal manifestations; besides it has a variable clinical course and relapse is common in these patients1. The current therapeutic approach has reduced the need for surgery, somehow 20 %2 to 30 %3 of patients with UC must undergo surgery, either because of intractability, fulminant course, development of dysplasia or colon cancer2,3. Proctocolectomy and ileoanal pouch is the surgery of choice4. This procedure was first described by Park et al. in 19785. A functioning pouch is observed in 93% of patients 30 years after surgery6. “J-pouch”, “W-pouch” and “S-pouch” are the main types of pouch (Figure 1)7, being “J-pouch” the most frequently performed7.
After surgery, a persistent inflammation of the pouch, known as pouchitis, may occur, as well as non-inflammatory complications (Table 1)4-6. The review conducted in this paper specifically focuses on pouchitis, as it is the most frequent complication associated with ileoanal pouch surgery5,6.
Table 1 Differential diagnosis of pouchitis
| Inflammation of the remaining rectal mucosa (cuffitis) |
| Infectious diarrhea due to CMV or C. difficile |
| Pouch stricture |
| Pouch ischemia |
| Crohn’s disease of the pouch (there will be inflammatory alterations in proximal areas) |
| Adhesions |
| Pelvic floor dysfunction |
| Poor absorption of bile salts |
| Pouch emptying alteration |
| Autoimmune pouchitis |
Epidemiology
The incidence of pouchitis varies over time. At 1, 5 and 10 years, its incidence is 20%, 40% and 50%, respectively8. At 30 years after surgery, it may affect 80% of patients6. These figures contrast with the low incidence of pouchitis (0-10%) described in patients who underwent the same surgery but due to familial adenomatous polyposis9.
Pathogenesis
The exact pathophysiological mechanisms that cause pouchitis are unknown. However, it is more likely to be a multifactorial disease involving genetic predisposition, bacterial overgrowth and dysbiosis in the ileal pouch, among others9. Recently, it has been found that in acute pouchitis cases there is an increase of species of the Clostridia genus and, reciprocally, its decrease is associated with response to antibiotic treatment10. In contrast, in chronic pouchitis cases there is an increase in the amount of Staphylococcus aureus10. Other factors involved in its development may include decreased short-chain fatty acids, nutritional deficiencies, ischemia or immune response alterations10-13. In addition, other authors have considered that pouchitis may be a different inflammatory bowel disease14 and that several factors increase the risk of developing it15-18. The use of nonsteroidal anti-inflammatory drugs (NSAIDs) increases this risk 3.24 times (95 % confidence interval [CI] 1.71-6.13)15.
Similar to ulcerative colitis, smoking has been found to have a negative association and smoking cessation increases the risk of pouchitis, however this does not mean that people should start smoking to avoid developing it, as smoking has multiple harmful effects on humans and recently it has been reported that active smoking does not prevent the occurrence of this complication16. When primary sclerosing cholangitis and UC occur concurrently, there is a higher risk of pouchitis than when UC occurs alone17. High levels of perinuclear fluorescence pattern anti-neutrophil cytoplasmic antibody (p-ANCA) represent a risk of pouchitis of 8.517. According to a recent meta-analysis, the presence of p-ANCA was associated with increased risk of chronic pouchitis (Odds ratio [OR]: 1.8; 95 % CI: 1.2-2.6), but it was not associated with acute pouchitis18. However, it should be noted that p-ANCA determination was performed after surgery in most of the studies included in said meta-analysis.
Diagnosis
Pouchitis diagnosis is made based on the combination of clinical manifestations, endoscopic alterations (pouchoscopy) and histological findings19,20. At least 7 points are required for its diagnosis21. In terms of severity, it may be mild, moderate or severe19. In some cases, imaging studies of the abdomen and pelvis are necessary20,22. There are no specific clinical manifestations of pouchitis12. Clinically, there is an increase in the number of bowel movements, along with the presence of mucus, abdominal pain, tenesmus, incontinence and nocturnal symptoms12,22. Rectal bleeding is more suggestive of inflammation of the remaining rectal mucosa (cuffitis)20. However, the inflammation of said rectal mucosa may actually be a recurrence of ulcerative colitis20. Systemic manifestations such as fever, chills and weight loss most frequently suggest an infection of the pouch (infectious pouchitis) by Clostridium difficile or cytomegalovirus (CMV)12,20. Usually, patients with ileoanal pouch have 4 to 7 bowel movements per day12,22, and some even up to 20, so that the increased frequency of bowel movements alone is not suggestive of pouchitis. Its differential diagnosis includes several diseases (Table 1)12,20,22.
Endoscopic findings include erythema, edema, friability, granularity, spontaneous bleeding or bleeding on contact with the endoscope, ulcers, inflammatory polyps, mucosal bridges and decreased distensibility22. The presence of ulcers or erosions on the anastomosis alone is not suggestive of pouchitis20. Biopsies should be performed by taking samples from the pouch and the proximal loop of the ileum; samples from the suture line must be avoided20. Although the inflammation is located in the ileal pouch, sometimes it extends more proximally and affects the ileum proximal to the pouch12,20. The involvement of the ileum proximal to the pouch is known as pre-pouch ileitis, which was first described by Bell et al.23 in a retrospective study conducted in patients with UC who had been treated surgically and had undergone total colectomy with ileoanal pouch. In said study, concomitant pouchitis was reported in half of the patients, and granulomas were found in one case according to the histopathology reported, which is a very important finding, since Crohn’s disease can be erroneously diagnosed23.
Inflammatory infiltrate with polymorphonuclear infiltrates, crypt abscesses and ulcers are found in biopsy reports23,24. Additionally, chronic changes, flattening of the ileal mucosa villi leading to villous atrophy (intestinal metaplasia), and chronic inflammatory infiltrate are also found12,20,22. If there are no acute inflammatory changes, pouchitis cannot be diagnosed, since chronic stasis of fecal matter in the pouch produces chronic inflammatory changes25,26. Basophilic and eosinophilic inclusions are findings suggestive of CMV infection12,20. The final diagnosis of this infection is based on the positive detection of CMV by immunohistochemistry20,22. Apoptosis in the crypts is suggestive of autoimmune pouchitis27. Non-homogeneous or asymmetric inflammation suggests ischemic pouchitis12,20,27. When pouch stricture is identified, endoscopic balloon dilatation is the treatment of choice due to its efficacy and safety28.
Risk of dysplasia and cancer
The risk of dysplasia in the pouch is extremely rare when there is no neoplasia or dysplasia before undergoing this surgery29. In this regard, a study reported that dysplasia occurred only in 1 % of patients 10 to 15 years after surgery29; besides, according to a more recent research, the risk of cancer was 1.3%30. On the other hand, when there is dysplasia or cancer before surgery, the risk of neoplasia increases substantially as follows: in the case of perioperative dysplasia, the hazard ratio (HR) is 3.76 (95 % CI: 1.39-10.19), while in patients with previous cancer, it is 24.69 (95 % CI: 9.61-63.42)30. In these patients, performing endoscopic surveillance of the pouch annually is recommended. On the other hand, the performance of endoscopic surveillance of the pouch, along with biopsies, every 1 to 3 years is recommended in patients with a 10-year history of UC before undergoing ileoanal pouch surgery and with one of following risk factors: chronic pouchitis, cuffitis, family history of colon cancer in first-degree relatives, and primary sclerosing cholangitis20. If the aforementioned risk factors are not present, surveillance is performed every 3 years, starting 10 years after the UC diagnosis is reached20.
Clinical course and classification
The classification of pouchitis based on endoscopic examination, histology, clinical course, duration, response to antibiotics, and number of episodes per year is shown in Table 2 22,31,32.
Treatment
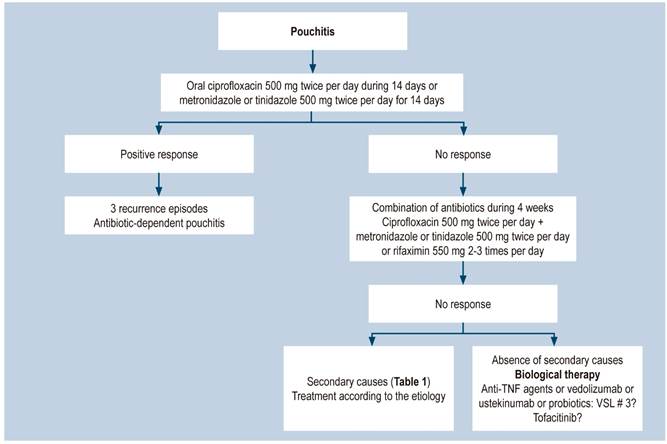
Drugs typically used to treat ulcerative colitis do not have similar efficacy when used in the treatment of pouchitis7,22,33,34. Antibiotics are the cornerstone of the management of pouchitis, and the implementation of additional strategies depends on failure of this first-line therapy7,12-14,20,22. Considering its high incidence and clinical course, management can be divided into primary prophylaxis with probiotics, on the one hand, and treatment of acute pouchitis with antibiotics, on the other7,12-14,20. According to their response to antibiotic therapy, patients are classified as antibiotic responders, antibiotic dependent (3 episodes per year) and refractory to antibiotics12-14,20,22, being the latter treated with a step up combination of antibiotics: budesonide, biological therapy with anti-tumor necrosis factor agents (anti-TNF: infliximab, adalimumab) or anti-integrins (vedolizumab), and even long-term high-dose probiotics. 60-80 % of refractory patients benefit from this step up strategy. However, 1-2% end up with a permanent ileostomy22.
Pouchitis management can be divided into primary prophylaxis, treatment of acute pouchitis, treatment of antibiotic-dependent pouchitis and treatment of chronic pouchitis.
Primary prophylaxis
Several studies have looked into the efficacy of probiotics to prevent the occurrence of pouchitis. However, results are inconsistent and, therefore, there is no definitive evidence about their efficacy31,35-39. In this sense, two studies reported a lower incidence of pouchitis in the group of patients who were administered probiotics34,35,40. In the study by Gionchetti35, conducted in 40 individuals, pouchitis occurred in 10 % of patients receiving probiotics, compared to 40 % of those in the placebo group. In the study by Yasueda40, conducted in 17 patients, 1 patient out of 9 in the probiotic group developed pouchitis after a 2-year follow-up period, compared to 4 out of 8 in the placebo group. On the contrary, in another study conducted in 30 patients there were no significant differences between both groups40.
Treatment of acute pouchitis
Antibiotics are the cornerstone of the treatment of this type of pouchitis7. Initial use of ciprofloxacin (500 mg every 12 hours for 14 days) is recommended12,14,20,22. Metronidazole or tinidazole (500 mg every 12 hours) are other options12,14,22,40. Most patients respond rapidly to this therapy. Ciprofloxacin use is preferred because of its greater efficacy and the fact it causes fewer side effects12,22. Most patients respond to this initial empirical therapy; however, at least 60% experience one episode of recurrence22. Ciprofloxacin is more effective than metronidazole (100 % vs. 33 %)22. Response to antibiotics supports the idea that dysbiosis may be involved in the development of pouchitis. Patients who fail to respond to the initial antibiotic therapy may receive a new course of the same antibiotics for 4 weeks, either based on culture results, or empirically by combining ciprofloxacin (500 mg every 12 hours) with metronidazole or tinidazole (each 500 mg every 12 hours), or with rifaximin (550 mg every 12 hours)12,20,22,40. Depending on their response or failure to respond to antibiotics, patients can be prospectively classified as described below.
Antibiotic-dependent pouchits
Patients who have an initial response to antibiotics, but have 3 or more relapses per year are considered to have this type of pouchitis. The recommended treatment for these patients consists of the permanent administration once or twice per day of antibiotics at low doses, such as ciprofloxacin 200 to 500 mg/day or rifaximin 200 to 550 mg. In a recent study, only 21% of patients who chronically received antibiotics experienced an improvement at one year of treatment, 28% experienced adverse effects associated with the use of antibiotics, and antimicrobial resistance was identified in 78% of patients33. This low efficacy raises the need for different treatments, including the use of biologics and even probiotics with VSL # 3 at 6-9 g/day doses12,20,22.
Chronic antibiotic-refractory pouchitis
There is no unanimous definition of chronic antibiotic-refractory pouchitis. However, it is widely considered that patients with symptoms for at least 4 weeks and who have failed to respond to antibiotic management have this type of pouchitis. Chronic antibiotic-refractory pouchitis occurs in 10-15% of patients with pouchitis. In these cases, ruling out secondary non-inflammatory causes, including mechanical ones (ischemia, stenosis, fistulas, lack of the “J-pouch” tip, among others), is required22. In addition, NSAIDs administration must be always suspended. In cases of CMV infection, treatment would consist of ganciclovir administration22,31. If there are no secondary causes, chronic antibiotic-refractory pouchitis can be managed with immunosuppressants such as budesonide or betamethasone12,22; biological therapies such as anti-TNF (infliximab or adalimumab)41 or vedolizumab therapy42 are another option for treating these patients, as well as those with antibiotic-dependent pouchitis. Some case reports have described benefits derived from the use of ustekinumab43 and recently, benefits associated with tofacitinib use have been reported in antibiotic-refractory pouchitis cases44.
When inflammation occurs in both the pouch and the proximal ileum, oral budesonide is the treatment of choice12,22. The clinical manifestations of cuffitis or inflammation of the remaining rectal mucosa are similar to those of pouchitis, with the exception that bleeding is more frequent12,20. The treatment of choice consists of topical 5-aminosalicylic acid (5-ASA) (suppositories) and, if the patient fails to respond to this therapy, adding oral mesalazine is recommended20,22. An algorithm of the general management of pouchitis is shown in Figure 2.
Surgical treatment is a salvage therapy option when medical treatment fails 34. The purpose of surgery is to resect the pouch (pouchectomy) and perform a permanent ileostomy7,22,34. The proportion of patients undergoing this surgery due to the loss of pouch is unknown, but it has been estimated that it may be necessary in 25% of patients45,46.
Cuffitis refractory to medical treatment can often be accompanied by ischemia and patients with these two diseases, as well as those with collagenous cuffitis, are considered candidates for transanal mucosectomy or even dysfunctionalization of the pouch7,22,34.
Fecal transplantation has been used in cases of chronic refractory pouchitis; however, so far the available evidence is scarce and limited to case series47.











 text in
text in