Introduction
Currently, the analysis of liver tissue obtained by biopsy is highly important for establishing a diagnosis, evaluating and staging the underlying disease, and directing treatment based on reported histology1; therefore, liver biopsy is still a fundamental test in the study of liver disease. Some consider it the gold standard and others the best existing test; however, the quality of the test depends on several factors: an adequate clinical indication, the physician performing the procedure (in our environment, a radiologist), the needle used, the number of successful punctures (cylinder of tissues obtained), the quality of the sample, and the pathologist who reads and reports it2-5.
Previous guidelines suggest what aspects make a good specimen and recall that a liver biopsy between 1 and 3 cm in length and between 1.2 and 2 mm in diameter represents 1/50 000 of the total liver mass6. A sample of at least 2 to 3 cm in length, taken with a 16-gauge needle, is recommended since it allows pathologists to evaluate 11 complete portal tracts plus a large part of the hepatic lobule, so that a definitive diagnosis or a suggestion of it can be made, taking into account that diagnosis, classification and staging can be incorrect due to an insufficient sample size. If cirrhosis is suspected, a larger needle with a cutting tip is recommended instead of a suction needle to obtain a better sample and avoid its fragmentation1,7-10; similar parameters have been formulated in the new British guidelines11.
Liver biopsy is performed in different hospitals in the city of Bogotá, Colombia, and differences in the final results may be observed. For this reason, the aim of this study was to evaluate the quality of percutaneous liver biopsies based on the frequency of definitive diagnosis in their reading in several hospitals in Bogotá and their relationship with the number of portal spaces and length of the biopsy in the final pathology report.
Methodology
Retrospective observational study based on patient records attending the specialized hepatology service at the Centro de Enfermedades Hepáticas y Digestivas (CEHYD) in Bogotá, Colombia. All medical records of patients treated between January 1, 2010, and July 30, 2017, were reviewed.
Exclusion criteria
Liver biopsy by surgical wedge,
Liver tumor biopsy,
Sample not suitable for reading according to the pathologist,
Pathology results not available,
Not knowing the center where the biopsy was performed.
The pathology result of the liver biopsy was evaluated, and the following variables were considered:
Sample size: the largest length in cm was considered and classified into 3 groups as follows: < 1 cm, 1-1.9 cm, and ≥ 2 cm. In case several fragments were reported, the largest was taken into account.
Number of portal spaces reported: it was classified into 3 groups: < 5, 6-10, and ≥ 11.
Information (yes or no) on the characteristics of the portal space.
Information (yes or no) on the characteristics of the hepatic lobule.
Diagnosis: the probability of certainty was considered when analyzing the final conclusions of the result issued by the pathologist, defined as follows:
Definitive diagnosis, 100 % certainty: diagnosis clearly stated; pathologist refers to “conclusive or highly suggestive of,” histologic description strongly suggestive of a disease.
Probable diagnosis, 50% certainty: “may be a...”, “it is suggested…”, “other differential diagnoses”.
No diagnosis, 0% certainty: no diagnosis is given or suggested. The description is incomplete.
Data associated with biopsy (number of punctures, cylinders for reading or fragmentation of the sample) or with pathologists (number per center or experience) were not tabulated since this information was unknown.
The analysis of the degree of fibrosis was carried out using the METAVIR scale; if the report was presented with another scale, it was compared with METAVIR.
Statistical analysis
The information collected was summarized descriptively. Qualitative variables were presented as absolute frequencies, while quantitative variables as central tendency and dispersion measures depending on their normal distribution (Kolmogorov-Smirnov test for normality). Because the data did not have a normal distribution, the difference between health centers was shown using the Kruskal-Wallis test. Univariate and multivariate analyses were performed with the type of diagnosis (definitive and non-definitive) and the values of the number of portal spaces and length of the biopsy by means of logistic regression. Spearman’s Rho correlation was used. All analyzes were carried out in the statistical software STATA version 13 and R-4.0.3.
Results
Of a total of 927 patients who required liver biopsy during the period analyzed, 268 were excluded because they did not meet the inclusion criteria (especially due to the unavailability of the final pathology report and, in some cases, incomplete medical records). Finally, 659 patients were included because their liver biopsy reports, performed in 10 different centers, were available. The number of patients in each center ranged from 14 to 314. Of the total number of patients analyzed, 601 reported biopsy length or portal spaces. The percentage of portal space reporting by center varied between 15 % and 87.4 % (Table 1). The vast majority of pathologists described portal spaces and liver lobules.
Tabla 1 Characteristics of biopsy outcome in health centers (n = 659)
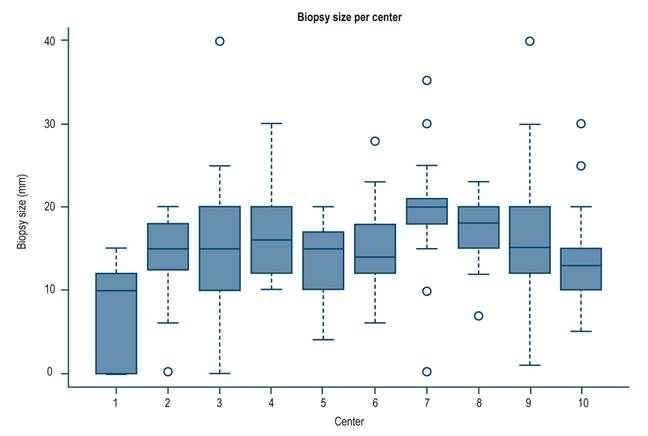
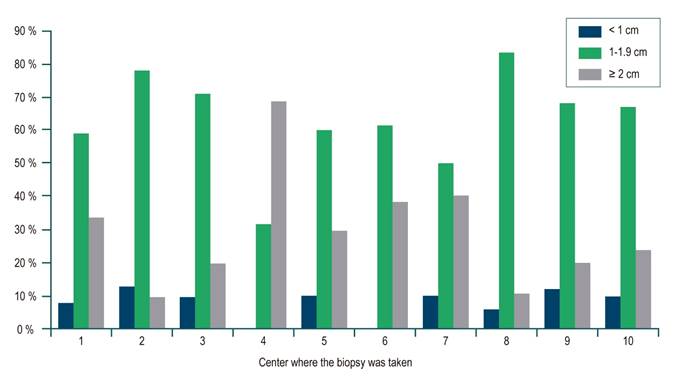
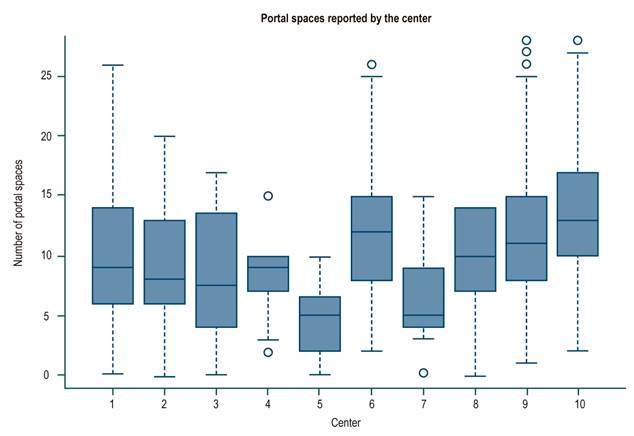
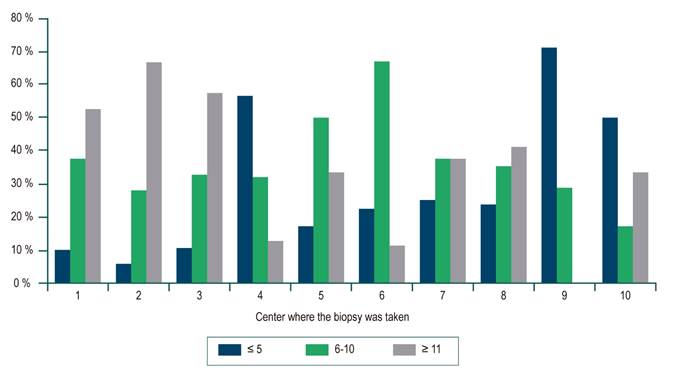
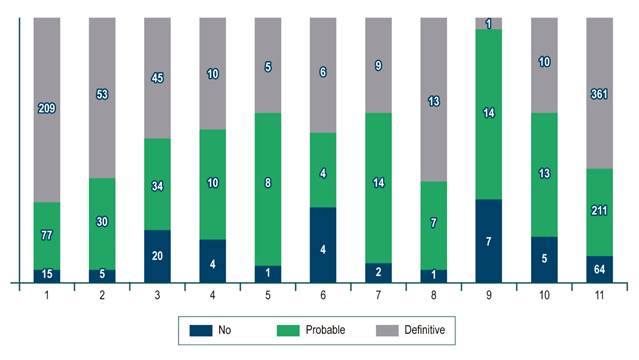
For the whole sample, the median biopsy length was 1.5 cm (interquartile range [IQR]: 1-2) and the number of portal spaces was 10 (IQR: 7-15). Figures 1 and 2 show the significant differences in length reported by the centers. Most centers obtained liver tissue samples of medium (1 to 1.9 cm) or good quality (≥ 2 cm), as in the case of the 4th center, where 70 % of biopsies are classified in the latter group. The distribution of portal spaces by center is shown in Figures 3 and 4, and centers 1, 2, 3 and 8 stand out with biopsies showing more than 11 portal spaces. There is a significant difference in the proportion of definitive diagnoses, probable diagnoses, or no diagnosis among centers. Figures 5 presents the proportion and number of samples processed by center. There was no correlation between the volume of samples processed by center and the number of portal spaces identified or the length of the biopsy (Spearman’s Rho).
At each center, definitive diagnoses ranged from 35 % to 69 %; probable diagnoses from 25 % to 63 %; and no diagnosis from 5 % to 32 %. A definitive diagnosis was reported by 3 centers in more than 60 % of the cases, while the other 7 centers reported it in less than 50 %. Center 9 had a low number of biopsies reviewed (only 24) but had the highest percentage of patients with fibrosis stage 0. 62 patients, representing 9.4 % of the total sample, had cirrhosis (F4) and there was no report of portal spaces in 77 % of them, ranging from 58 % to 100 %.
Regarding the logistic regression of the diagnosis (definitive and not definitive), the univariate analysis revealed that the number of spaces had an Odds ratio (OR) of 1.12 (95 % confidence interval [CI]: 1.05-1.19), while length had an OR of 1.74 (1.06-2.87); that is, each increase in a portal space increases the probability of a definitive diagnosis by 1.12 times, and each 1 cm increase in the length of the biopsy increases the probability of presenting a definitive diagnosis by 1.74 times. Concerning the multivariate analysis, sex-adjusted OR and obesity for definitive diagnosis are significant for the number of portal spaces (OR: 1.12[1.02- 1.22]). The length of the biopsy loses its significance (OR: 1.41 [0.7-2.84]) and the other variables are not significant (Table 2).
Discussion
Liver biopsy has made histologic analysis of the liver an essential test in the study of patients with liver disease1-5,11. Recently, Neuberger et al.11 issued recommendations on the deal conditions for a liver biopsy and several articles insist on criteria that would make a liver biopsy overall adequate and of good quality1,7-12. Based on the above, it was decided to assess the quality of liver biopsies involved in the management of our patients by analyzing 3 aspects of the definitive pathologist’s report: length of biopsy sample, number of portal spaces, and the definitive, partial or not, diagnosis, all of which are critical elements in the pathologist’s report.
The ideal size of a liver biopsy has varied12. In chronic hepatitis C, it was initially suggested that a biopsy of 1-1.5 cm length with 4-6 portal spaces was sufficient for staging7,13,14, while other authors mention a minimum size of 2 to 2.5 cm as the ideal, with at least 11 portal spaces10,15-17, bearing in mind that the larger the size, the greater the number of portal spaces17. These latter recommendations are currently suggested1,5,10,11,14. In our study, the median length and portal spaces were 1.5 cm and 10, respectively, as the lower limits of a good-quality biopsy, and the number of portal spaces available for reading are reported in 74 % of the samples. With respect to the available descriptions of portal spaces, the total percentage is greater than 90 %.
It should be noted that 4 centers with a low number of patients reported the number of portal spaces in less than 40 % of the cases and, on the contrary, the 8th center, with a similar number of patients, reported portal spaces in 76 %; this could be partly explained by the presence of cirrhosis. In fact, a total of 62 patients (9.4 % of the total number of non-portal space reports) were classified as F4 and explain some of these cases. On the other hand, it could be a matter of little experience or training of the pathologists of the first 3 centers, and more experience or training of the pathologist of center number 8.
The needle gauge and the number of successful punctures (or cylinders of liver tissue obtained by the radiologist) are directly related to the size of the biopsy sample and the number of portal spaces that the pathologist studies; therefore, a 16-gauge needle and at least 3 unfragmented samples of sufficient size are recommended18,19. Although a study comparing 2 with 3 punctures shows a higher risk when performing 3 samplings, mainly due to pain in 74 % of the cases and hemorrhage in 33 %20, in other studies (one with more than 15 000 biopsies) this risk was not demonstrated21,22. There was no access to these date for the present study, but a tendency in our environment to use thinner needles and usually with a single puncture (cylinder) was observed, possibly due to the fear of complications.
The pathologist’s experience or training in liver disease is a fundamental aspect of the quality of the liver biopsy report and may sometimes make up for the deficits of a sample. In contrast, some studies report diagnostic errors (clinically significant) made by inexperienced pathologists in more than 25 % of the cases in a university center23,24, therefore, in view of diagnostic doubt, a second opinion is recommended24,25. In our series, this aspect, which is inherent to the expertise of the pathologist, could explain the aforementioned findings of the 8 centers, while the lack of expertise would explain findings such as the low rates of definitive diagnosis in several centers with a good number of patients, such as the 3rd center, or with relatively good samples, such as centers 5 and 6, where the median samples were ≥ 1.6 cm and ≥ 9 portal spaces; moreover, it would also explain the non-description of portal spaces and lobules in some centers.
It was found that 3 centers, 1, 2 and 8, reported definitive diagnoses in more than 60 % of the cases: the first 2 had biopsies close to ideal in terms of size and number of portal spaces, while the third center had a low number of patients and medium-quality biopsies, a situation in which the pathologist’s experience or training is, once again, surely playing a key role.
Regarding the staging of the degree of fibrosis, the 9th center, with a low number of biopsies performed and the lowest percentage of portal space reporting and definitive diagnosis, had the highest percentage of patients with stage 0 fibrosis; this finding has been described in the literature and it is known that the lower the quality of the sample, the greater the likelihood of low staging10,15. Likewise, a poor sample generates a higher probability of sampling errors due to the heterogeneity of the liver in the different stages of the disease, an error that increases with a higher degree of fibrosis4,26-28. It should also be noted that patients with cirrhosis have a high probability of biopsy fragmentation, a finding demonstrated by Poynard, who considers that “fragmentation is a sign of cirrhosis, regardless of the length of the biopsy”29.
The limitations of this study include its retrospective nature and its focus on the analysis of the pathology report, which limits the analysis of other variables; however, the results suggest that an adequate sample size and reading from an expert pathologist, based also on clinical patient information, make the diagnosis issued much more reliable.
Conclusions
In our study, 3 hospital centers in Bogotá had adequate pre-analytical quality when obtaining biopsies and categorical diagnoses over 60 % that guided the physician. Definitive diagnosis in liver biopsy was associated in this series with the presence of a cylinder of liver tissue of adequate length and number of portal spaces. The multivariate analysis showed that the number of portal spaces was significant. Although these aspects were not analyzed, the relevance of the pathologist’s experience and training in evaluating the biopsy, as well as the number of patients treated at each center (which would imply higher experience), are emphasized as essential factors in reaching these results.











 text in
text in