Introduction
Endoscopic ultrasound (EUS) is used to approach to biliopancreatic diseases due to its good resolution to evaluate the extrahepatic bile duct and pancreas, as well as its ability to take samples under direct visualization in real time. Endoscopic ultrasound with fine needle aspiration (EUS-FNA) is the method of choice for sampling solid pancreatic lesions1-3. It is considered to be more sensitive than abdominal CT and MRI in lesions smaller than 10 mm. It is also a safe and cost-effective method, as it provides a high diagnostic performance 4. Diagnostic performance increases when FNA or fine needle biopsy (FNB) are added. Diagnostic accuracy is affected by lesion-specific factors such as location, size, and type of lesion; technical aspects such as number of passes, sampling technique (aspiration, slow-pull technique, fanning technique); endoscopist expertise; and the presence of a pathologist in the ward, which is one of the least studied factors.
Therefore, we propose to perform EUS-FNA with a pathologist in the room in all procedures to minimize the number of passes, the rate of inadequate or insufficient samples, and the need to repeat the procedure due to false negative results.
Materials and methods
Observational, retrospective study with prospective collection of information of patients over 18 years of age undergoing EUS-FNA in the hospital. EUS-FNA was performed by 2 operators (GMK, JJC), accompanied by a pathologist in the room (CE, JCP and RC). Fuji 580UT linear endosonograph was used, with Expect Needle or Acquire 22 G and 25 G needles.
Samples obtained from EUS-FNA were spread by a gastroenterologist and a pathologist and then evaluated by the pathologist in the rooms with Diff-Quick staining; when a sufficient sample was obtained, it was sent in a vial with formalin for cell block or biopsies (Figures 1A-D). The terminology used for the description and final diagnosis was the cyto-histological classification of the Papanicolau Society (Bethesda).
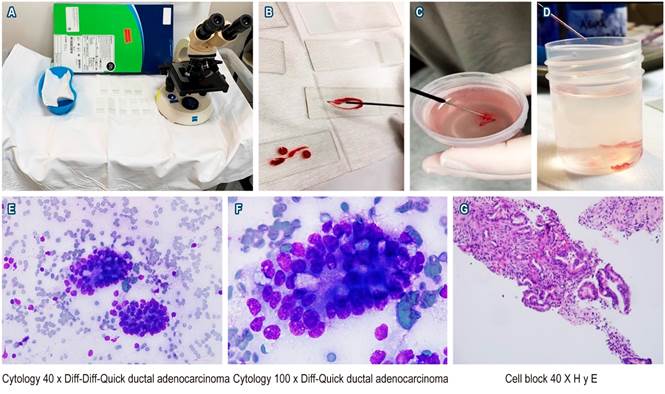
Figure 1 A. Workstation with materials used by the in-room pathologist to perform the initial evaluation of the samples (microscope, slides, kidney dish with Diff-Quick staining). B. Sample obtained on the slide. C and D. Cylinders in formalin flask for cell block and biopsy. E. Cytology 40 x Diff-Quick in which 2 clusters of cells of neoplastic appearance are identified. F. Cytology 100 x Diff-Quick showing evidence of ductal adenocarcinoma. G. Cell block with hematoxylin-eosin staining (H and E) in 40x accumulation of neoplastic cells.
The study variables were collected in the database by the study co-investigators (JVM, HSG) after reviewing the medical records of the hospital and the images from the Impax® or Agfa computer system.
The protocol was approved by the hospital’s ethics and research committee. Informed consent was obtained for all cases prior to the procedure.
Results
From January 2018 to July 2019, 48 biliopancreatic EUS-FNAs were performed with an in-room pathologist. Of the 48 cases, 25 were men (52.1 %) and 23 were women, with ages ranging from 20-88 years and a median of 64 years (interquartile range [IQR] between 55-71.7).
Indications for EUS-FNA are shown in Table 1. Most punctures were performed due to the presence of a pancreatic mass (solid or cystic) or chronic pancreatitis with pseudomass (which constitute 70.83 % of the punctures).
Tabla 1 Características clínicas y demográficas de la población
| Característics | Values (n = 48) |
|---|---|
| Age (IQR) | |
| - Median age in years | 62,0 (55,0-71,7) |
| Age distribution. n (%) | |
| - Male sex | 25 (52,1) |
| ASA classification. n (%) | |
| - ASA II | 3 (6,3) |
| - ASA III | 44 (91,7) |
| - ASA IV | 1 (2,1) |
| Study indication. n (%) | |
| - Mass in the pancreas | 30 (62,5) |
| - Periampullary mass | 4 (8,3) |
| - Bile duct or biliary mass | 4 (8,3) |
| - Peripancreatic retroperitoneal mass | 4 (8,3) |
| - Chronic pancreatitis | 2 (4,2) |
| - Kidney cancer (recurrence in surgical site) | 1 (2,1) |
| - Insulinoma | 1 (2,1) |
| - Gallbladder mass | 1 (2,1) |
| - Complex pancreatic cyst | 1 (2,1) |
ASA: American Society of Anesthesiologists.
The quality of the sample was considered sufficient in 97.9 % of the cases and insufficient in only 1 case (2.1 %); the minimum size of the lesions was 6 mm and the maximum size was 120 mm, with a median of 28 mm. The minimum number of passes was 2 and the maximum was 8, with a median of 3; the median number of positive passes for a sample sufficient for diagnosis or positivity was 3. The final diagnosis was divided into three categories based on the cyto-histological categorization of the Papanicolau Society (Bethesda): 97.9 % of cases were classified as group II (negative for malignancy) or group VI (positive for malignancy), 35 cases were classified as group VI (positive for malignancy), 12 cases were classified as group II (negative for malignancy), and 1 case was classified as group I (not diagnosed due to insufficient sample). Pancreatic cancer and pancreatic neuroendocrine tumor were diagnosed in 80 % of the 35 cases positive for malignancy, while cholangiocarcinoma was diagnosed in 3 (8.5 %) (see Figure 1E-F, where a case is presented in which a significant sample and confirmatory findings of adenocarcinoma of the pancreas are observed). When the location of the lesion was described in the pancreas (35 cases) the most common locations, in order of frequency, were head, body, uncinate, and neck (see Table 2).
Tabla 2 Sample characteristics and location
| Característics | Values (n = 48) |
|---|---|
| Quality of sample obtained, n (%) | |
| - Sufficient | 47 (97,9) |
| Location in the pancreas (n = 35) | |
| - Head | 13 (37,1) |
| - Body | 13 (37,1) |
| - Tail | 5 (14,3) |
| - Uncinate | 3 (8,6) |
| - Neck | 1 (2,9) |
| Extrapancreatic localization (n = 13) | |
| - Extrahepatic biliary lesions | 4 (31) |
| - Periampullary lesions | 4 (31) |
| - Retroperitoneal, peripancreatic mass | 5 (38) |
| Diagnostic sample, n (%) | |
| - Both | 46 (95,8) |
| style=”background-color:#B1CFD5”>- Block only | 1 (2,1) |
| - Cytology only | 1 (2,1) |
| Final diagnosis according to the the Papanicolaou Society, n (%) | |
| - Category I (non-diagnostic) | 1 (2,1) |
| - Category II (negative malignancy) | 11 (22,9) |
| - Category IV (benign neoplasm) | 1 (2,1) |
| - Category VI (positive malignancy) | 35 (72,9) |
| Accurate diagnosis (n = 43) | |
| - Pancreatic ductal adenocarcinoma | 25 (58) |
| - Biliary adenocarcinoma | 2 (4,6) |
| - Neuroendocrine tumor | 4 (9,3) |
| - Lymphoma | 1 (2,3) |
| - Plasmacytoma | 1 (2,3) |
| - Chronic inflammation and pancreatic fibrosis | 4 (9,3) |
| - Ectopic spleen | 1 (2,3) |
| - GIST | 2 (4,6) |
| - Other | 3 (7) |
Of the 13 punctures that did not show malignancy, 8 were non-malignant in the setting of lesions with a low pretest probability for malignancy (chronic pancreatitis with inflammatory pseudomass, solid/cystic lesion of the pancreas, intrapancreatic ectopic spleen, and retroperitoneal lesion near the pancreas) and 5 were false negative results (mass in the body of the pancreas, lesion in the common bile duct, mass in the neck of the pancreas, insulinoma in the body of the pancreas, and an extragastric GIST near the pancreas). Among the false negatives, EUS was repeated in 3 cases, that is, there were 43 diagnostic results (89 %), of which 35 detected malignancies (81 % compared with 19 % of results with benign mass). In addition, there were 5 cases (11 %) of false negatives.
Endosonographers described technical difficulties that limited puncture in 7 cases (14.6 %), corresponding to increased vascularization in or around the lesion.
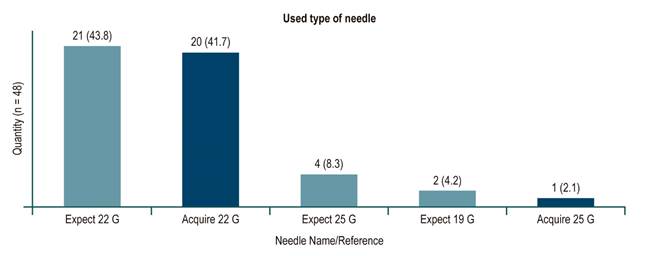
Regarding the needles used, punctures performed between January 2018 and February 2019 were done with a fine needle for puncture/aspiration (Expect Needle), while those performed between March 1 and June 2019 were done with a fine needle for biopsy (Acquire Needle). With regard to the latter biopsy needle punctures, 18 out of 20 were positive for malignancy, 1 was negative when the pretest diagnosis was chronic pancreatitis with pseudomass, and 1 was negative when an extragastric GIST was documented near the pancreas. This corresponds to a 95 % diagnostic efficiency in the latter EUS with biopsy needle (Figure 2 shows the number of cases related per needle and the diameter used).
There was 1 complication (2.1 %), namely, severe abdominal pain that required opioids and the performance of multiple studies and imaging scans, ruling out pancreatitis, perforation, and bleeding.
Discussion
In the present study, EUS-FNA with an in-room pathologist had a high diagnostic performance (89 % regardless of the type of needle used and 95 % when performed with biopsy needle). In most cases (almost 98 %), pathologists considered the samples sufficient to establish the histopathological diagnosis, with an average of 3 passes. These data are similar to those reported by Iglesias-Garcia et al.5, who described a significant association of in-room cytopathological assessment with a significantly low number of inadequate samples and a lower number of needle passes 5. This smaller number of needle passes could have an impact on reducing the risk of adverse effects during the puncture5,6. In our series, only a mild complication (abdominal pain) was observed, which coincides with the reports of multiple large studies with groups with high technical experience2,5,6. Moreover, there wee 5 false-negative results, corresponding to a mass in the body of the pancreas, a lesion in the common bile duct, a mass in the neck of the pancreas, an insulinoma in the body of the pancreas, and an extragastric GIST near the pancreas. Among these false negative cases, EUS-FNA was repeated in 3 of them (which were positive for malignancy). The other 2 cases corresponded to a patient with a common bile duct lesion, who underwent surgical resection with curative intent and whose pathology report demonstrated malignancy, and to a patient with a symptomatic insulinoma in the body of the pancreas who underwent USE-guided ethanol ablation. In all patients in whom the result of the puncture/biopsy was positive for malignancy, histopathology allowed definitive treatment of the cases.
We believe that acquiring sufficient and representative material of the lesion, as well as properly transferring the lesion to the pathology laboratory when there is no pathologist on the ward, is one of the keys to obtaining better cytology results using EUS-FNA. In highly experienced centers, with samples obtained with adequate cellularity, histological diagnosis can be established in up to 75 %-95 % of cases and with minimal variability in interpretation7. The present study emphasizes that the experience of the group of endosonographers is less than 3 years, so we consider that, despite the little experience of the group, the objectives of good diagnostic performance and minimum need to repeat procedures to obtain an accurate diagnosis were achieved. In a retrospective study conducted at a hospital in France, 106 cases with pancreatic lesions in whom EUS-FNA was performed were reviewed. The results were read by 2 cytopathologists, who evaluated the quantity and quality of the sample to be processed and had a third cytopathologist who was considered an expert. Inadequate samples were taken from other tissues or were related to a not representative sample. The average number of passes was between 1 and 5, with an average of 3, as described in our work. The results showed that sensitivity for detecting malignant pancreatic lesions improved with an experienced cytopathologist (72 % vs. 89 %). Having an experienced cytopathologist reduces the number of indeterminate samples, increases the recognition of malignant lesions, and provides accurate histology for suspicious or malignant diagnoses8,9.
Finally, although our study did not have the comparison of the type of needle used as its primary outcome, our pathologists had a perception of remarkable improvement in histopathological evaluation since they were able to perform the study of the cases with greater depth (immunohistochemistry, differentiation of neoplastic tissue, number of mitoses, among others), as described in the study by Rodrigues-Pinto et al., in which they compared EUS-FNA with an in-room pathologist with biopsy needle, showing that the acquisition of samples was technically successful in all patients. Furthermore, the pathology reports described the central tissue of all FNA lesions with a mean length of 15 mm, as well as the performance of biopsy needles used in all primary neoplastic lesions with respect to diagnosis, the degree of differentiation, metastatic origin, and proliferation rate; it also provided a statistically significant advantage for a definitive benign diagnosis (87.9 % [76 % -100 %] vs 27.3 % [11 % -43 %] , p < 0.001)10. In conclusion, we consider that the contribution of an in-room pathologist and the use of EUS-FNA could be a good strategy to improve performance, characterize lesions better, and minimize the need for repeating examinations and sampling.
The limitation of our study is its retrospective design. Therefore, in an attempt to reduce information biases, the quality of the data entry was controlled by double-entering it, although we recognize that the group had no control over the process of completing the clinical history. The data obtained in our cohort come from a single tertiary care university hospital that have pathologists who can perform these punctures, so the results may not be applicable in all centers in the country. Further multicenter analytical studies comparing the diagnostic performance of EUS-FNA with and without in-room pathologist should be conducted.
Conclusion
EUS-FNA is a promising method that significantly improve diagnostic performance in biliopancreatic lesions. Significant sampling is essential in a biliopancreatic center of excellence. This could help establish a faster and more accurate diagnosis. The presence of an in-room pathologist could improve diagnostic performance, objectify the presence of an adequate tissue sample, and require fewer needle passes, which reduces the risks associated with punctures. It could also contribute to shortening diagnosis times by eliminating the need for repeating tests, allowing for early clinical care and cost savings.











 text in
text in