Introduction
In the context of the pandemic caused by the coronavirus disease 2019 (COVID-19), a disease caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), it is clear that there is a threat to humanity that requires major public health efforts to reduce the risk of infection and avoid resulting complications. Due to the pathophysiology of the disease and the immunomodulatory treatments used, the management of patients with inflammatory bowel disease (IBD) who become infected with SARS-CoV-2 remains a challenge, raising many questions about the feasibility of vaccination in this population. The third peak of this pandemic in Colombia was surprisingly more accelerated than the previous two peaks; however, in the face of hopelessness and lack of control among individuals, the presence of a vaccine is a source of motivation and optimism.
Vaccination is the most powerful way to avoid the negative consequences of this pandemic. For the time being and in the short term, it is clear that vaccines will save lives and help prevent serious forms of the disease, making it possible to control it, as has been demonstrated by the thousands of people who have participated in clinical trials during the various phases of vaccine development, as well as data obtained from mass vaccinations.
The prevalence of IBD, which includes Crohn’s disease (CD) and ulcerative colitis (UC)1, has increased in Colombia2 and its treatment requires drugs such as high-dose corticosteroids (doses of ≥ 20 mg prednisolone), thiopurines, methotrexate and calcineurin inhibitors, anti-cytokine therapies including anti-tumor necrosis factor (anti-TNF) and anti-interleukin 12/23 (anti-IL-12/23) drugs, anti-integrin therapies (vedolizumab) and small molecule signaling inhibitors (Jak/Stat-tofacitinib), which may leave these patients susceptible to infection. Therefore, all patients with this type of immunosuppression should be considered a population at high risk of developing COVID-19 after infection3-5. For this reason, all patients should be vaccinated against SARS-CoV-2 infection; however, there are concerns about the impact that vaccination can have on their disease.
Consequently, this narrative review was carried out to address the main questions in relation to this issue. A search on the efficacy and safety of the vaccine in patients with IBD was made in Medline (PubMed) and Scopus bibliographic databases, using the following MeSH terms: (“COVID-19” O “SARS-CoV-2”) and (“Inflammatory bowel disease” O) and (“vacuna *” O “ARNm”).
Are patients with IBD at increased risk for SARS-CoV-2-induced infections?
Coronaviruses generally use receptors to reach their target cells; in this case, it has been documented that it binds to angiotensin-converting enzyme II (ACE-II) receptors and are constitutively expressed by epithelial cells in the lung, intestine, kidney, and blood vessels6. Specifically at the level of the digestive tract, these receptors have been found in higher concentrations in the terminal ileum and colon7. In patients with IBD who have chronic inflammation at the ileal and colonic levels, high concentrations of ACE-II receptors have been reported, which increase further their expression during acute inflammation. This was observed in a proteomic analysis of tissue samples from IBD patients, which revealed that CD had significantly higher levels of ACE-II expression than UC8,9.
Furthermore, an increased expression of proteases that process the spike protein10,11, which is critical in the process of binding to ACE-II receptors, has been documented in IBD patients, suggesting that the inflamed intestine of IBD patients is an optimal gateway for the virus to enter human tissues; however, it is paradoxical that reports of infected patient populations do not present significant findings suggesting increased severity in this condition12. Further studies are required to elucidate what additional factors may play a role in the binding of the virus to inflamed intestinal cells.
What are the main features of the vaccines currently available?
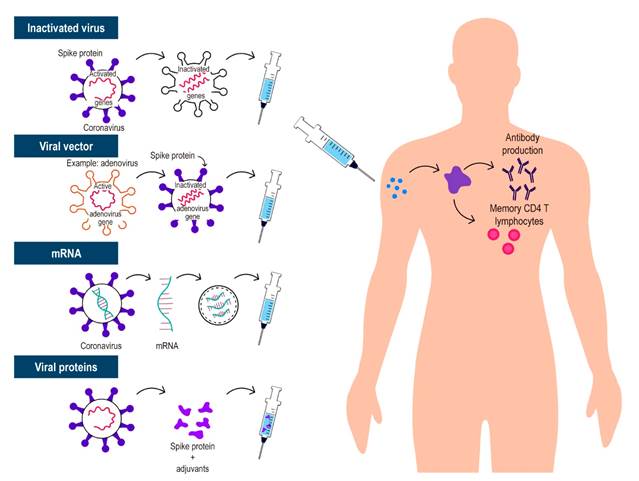
Given the urgent need for a vaccine, science has demonstrated that quick sequencing of SARS-CoV-2 allows for the rapid production of various types of vaccinations, which is unique in history. This led to a reduction in the development of these vaccines from a time that usually ranges from 10 to 15 years to less than 1 year. Throughout this process, several ways to generate immunity against SARS-CoV-2 have been described. The most relevant are 4 (Table 1 and Figure 1): whole virus (inactivated or attenuated), viral vector (replicating and non-replicating), nucleic acid (deoxyribonucleic acid [DNA] and messenger ribonucleic acid [mRNA]), and protein-based (protein subunit, virus-like particle). All vaccines attempt to introduce viral antigens to the immune system and thus generate effective immune responses that will eliminate or prevent the virus from entering cells and causing disease, or if infected, to generate mild forms of the disease13. Currently, despite the short time, there is accumulated information and data that allow understanding how they work. It is important to note that populations such as young children, very elderly people (> 85 years), and pregnant or breastfeeding women who are immunocompromised (either due to immunosuppressants or a disease that suppresses them) were excluded from these initial randomized clinical trials. Pharmacoepidemiology studies are being conducted to assess serious adverse events in more specific populations, including these important subgroups14,15.
Table 1 Characteristics of the main vaccines used against SARS-CoV-216-22
Complete virus-based anti-COVID-19 vaccines
These vaccines are based on viruses that have been weakened or inactivated using technologies that conventionally transmit the virus to animal or human cells, resulting in mutations or alterations through chemical or physical measures that inactivate or make the viruses less virulent. There are 2 types of these whole virus vaccines:
Live attenuated vaccine: this type of vaccine aims to mimic natural infection to trigger a strong immune response on its own, without the need for adjuvants. The use of the whole attenuated virus resembles natural infections; therefore, immunity includes all aspects of the immune response (innate and adaptive). However, although very efficient, these vaccines require more development time, which delays the process. These vaccines also contain microorganisms that have the potential to reactivate and cause disease, so they are contraindicated in patients with immune-related diseases and are generally contraindicated in patients receiving immunosuppressive therapy23.
Inactivated vaccine: in this vaccine, through physical or chemical means, the virus is inactivated, and the natural infection is imitated with its application, generating immunological memory. These vaccines do not raise concern in immunosuppressed patients since the virus is inactivated and does not have the capacity to generate disease and are, therefore, not contraindicated in patients with diseases associated with the immune system using immunosuppressants, such as IBD23.
Viral vector-based vaccines
This is a technology already used previously, in which the DNA of an adenovirus that normally infects chimpanzees or humans is modified through genetic engineering by adding the genetic sequences that synthesize the S protein or spike protein of SARS-CoV-2 and, with its administration, produce immunization. Viruses such as measles or adenovirus are used as vectors for these vaccines and are genetically modified to prevent them from causing the disease; these vaccines may be replicative or non-replicative, in the case of those using vectors such as measles or adenovirus, respectively. The immune response induced by this type of vaccine tends to be strong, with an excellent safety profile. Data on vector-based vaccines are available for immunocompromised individuals, with the possibility of disseminated infection by the vector being highly unlikely. It is important to note that viral vector replication is incompetent and viral-based vaccines are well tolerated.
Viral vector vaccines include Sputnik V (Gamaleya), AZD1222 (Oxford/AstraZeneca), Covi-shield (Serum Institute of India), Ad5-nCoV (Cansino), Ad26.COV2.S (Janssen), IIBR-100 (Israel Institute of Biological Research), and INS1-2019-nCoV-RBD-OPT (Wantaj). Regarding some of these viral vector vaccines, 3 cases of transverse myelitis have been specifically reported after the booster dose of ChAdOx1 nCoV-19 (AZD1222) vaccine; however, expert neurologists determined that these demyelinating findings were idiopathic or pre-existing findings of previously undiagnosed demyelinating disease. In addition, 11 cases of unusual thrombotic events and thrombocytopenia have recently been reported after vaccination with the recombinant adenoviral vector encoding the SARS-CoV-2 spike protein (ChAdOx1 nCov-19, AstraZeneca). These cases with thrombocytic events occurred between day 5 and day 16 after vaccination, and the presence of antibodies against the heparin-PF4 complex was documented in these patients, who experienced thrombotic events similar to heparin-induced autoimmune thrombocytopenia. However, it should be noted that their incidence remains very rare, and the benefit of vaccination will always outweigh the risk of presenting with these isolated cases of adverse events related to this vaccine23,24.
mRNA vaccine
This vaccine is based on mRNA fragments of the virus, but its administration does not generate SARS-CoV-2 infection in recipients. These vaccines that implement nucleic acids allow the synthesis of SARS-CoV-2 S protein (in the form of RNA or DNA) by means of a process in which lipid nanoparticles are included to favor the entry of the RNA into the host cells. The advantages of these approaches are their speed and ease of development; the disadvantages include logistical issues due to the need for special temperature conditions, as RNA is not very stable. In the timeline of COVID-19 vaccines, RNA-based vaccines are BNT162b2 (BioNTech/Pfizer), mRNA-1273 (Moderna) and CVnCoV (CureVac), while DNA-based vaccines are INO-4800 (Inovio) and AG0301-COVID-19 (AnGes). All studies on these vaccines generally show a good safety profile and adverse events comparable with the placebo group and other viral vaccines and are therefore considered safe in immunosuppressed patients16,17,23.
Protein-based vaccines
Protein subunit vaccines consist of fragments of viral proteins or proteins that mimic the outer layer of SARS-CoV-2. Most vaccines using protein subunits focus on the S protein, particularly its receptor-binding domain; these vaccines usually require adjuvants to improve immunogenicity. Moreover, they eventually require multiple doses to establish an effective and lasting immune response. Vaccines using this mechanism include RBD-Dimer (Anhui Zhifei Longcom), NVXCoV2373 (Novavax) and plant-derived virus-like particles - VLP (Medicago)23,25.
Should patients with ibd be vaccinated?
There are currently multiple articles that help guide the vaccination process in IBD patients, including the International Organization for the Study of Inflammatory Bowel Disease (IOIBD). This organization made a consensus of experts in which it is recommended to vaccinate all patients with IBD as soon as possible, regardless of the immunosuppressive therapy received, except for live attenuated vaccines or replicating viral vectors that reach the market in patients who are using immunosuppressive medication. A high percentage of the recommendations agree that the available vaccines are not associated with the onset or exacerbation of IBD, regardless of whether patients receive immunosuppressive therapy; additionally, patients with IBD can generate an immune response to all vaccines. No modification to the induction or maintenance scheme is required in the biological therapies used. Likewise, it was also stated in this consensus that the activity of IBD should not affect the date of vaccine application26-29.
Can the effectiveness of vaccination be modified in ibd patients?
IBD is currently managed with immunosuppressive therapies such as corticosteroids, immunomodulators, biological agents that include monoclonal antibodies such as tumor necrosis factor alpha (TNF-α) inhibitors, interleukin 12/23 (IL-12/23), integrins, and small molecules such as Janus kinase inhibitors (JAK). Previous studies have assessed the safety and effectiveness of different types of vaccines in patients with IBD, with special attention to the impact of immunological modification of these therapies on serological responses. It has been reported that these patients can generate an immune response, although it can be somewhat mitigated by immunosuppressive therapy; however, this is not considered a reason to delay vaccination or suspend immunosuppressive treatment26. Prospective records of IBD patients receiving SARS-CoV-2 vaccines are needed to measure the extent and duration of the immune response.
In patients with IBD, there are some reports on infliximab or adalimumab that show that they have decreased antibody titers and lower rates of seroconversion compared with controls in response to vaccination with inactivated influenza virus30, pneumococcus,31 and hepatitis B virus32; the response may be further attenuated by thiopurines, and methotrexate administered alone or in combination with anti-TNF therapy33. Patients with other immune-mediated diseases such as rheumatoid arthritis, who used the JAK inhibitor tofacitinib, had a normal antibody response to influenza vaccine and a decreased response to the pneumococcal vaccine34. IL-12/23 blockade with ustekinumab does not appear to alter the response to influenza 35 or pneumococcal vaccine36, and similarly, patients with IBD treated with vedolizumab do not have altered immune responses to the influenza vaccine37. However, vedolizumab does have a decreased impact on the efficacy of the cholera vaccine38, which is administered orally, and may reduce the effectiveness of mucosal vaccines, which may be relevant to some oral SARS-CoV-2 vaccines currently in development.
In view of the above, consensus and review articles on vaccination in patients with IBD recommend that vaccination should not be postponed if the patient is receiving oral or topical 5-aminosalicylic acid (5-ASA), systemic corticosteroids, thiopurines, methotrexate, biological therapy, or small molecules such as JAK inhibitors. It is also suggested that vaccination should not be delayed if these patients are in a clinical trial of an IBD drug26-28.
What other factors can modify the effectiveness of the vaccine?
It is important to keep in mind that, on the one hand, in addition to immunosuppressive drugs, there are other factors that can modify the response to vaccines in patients with IBD, such as age in older adult patients, and some studies suggest that the male sex may be associated with lower rates of postvaccination seroconversion39. On the other hand, comorbidities such as obesity, malnutrition, or damage to specific target organs can alter the response to the vaccine due to impaired T-cell function and reduced levels of interferon gamma and granzyme B40. It is also suggested that pre-existing infections or previous infections with other coronaviruses may generate what is known as immunological imprinting41, which affects the effectiveness of vaccines. In some autoimmune diseases, it has also been observed that when they have been present for a long time or are active, these factors may reduce vaccine seroconversion rates42.
Are COVID-19 vaccines safe for patients with IBD?
Initial clinical trials of vaccines that have been developed and approved did not include patients with conditions such as IBD; however, in general, inactivated vaccines are considered safe in these patients, and all vaccines currently being administered in our country are of this type. Specific adverse events must be investigated in studies of IBD patient populations. The IOIBD consensus recommends that vaccines for IBD patients are safe, that they are not related to the onset or exacerbation of IBD, whether they are receiving immunosuppressive therapy or not, and that live virus vaccines are always be contraindicated in patients with IBD who are receiving immunosuppressive therapy26. The British Society of Gastroenterology has recently published key recommendations strongly in favor of vaccination against SARS-CoV-2 in patients with IBD and emphasize that the main concern in patients treated with biological agents or small molecules is the theoretical risk of suboptimal vaccine responses instead adverse effects of the vaccine28. However, the risk of morbidity and mortality associated with complications of COVID-19 far exceeds the risk of vaccination.
About possible vaccine reactions, it is important to always distinguish between an adverse event and a side effect. Adverse event refers to a health problem following the administration of a vaccine that may or may not be caused by the vaccine, while side effects are health problems caused directly by the vaccine. Adverse events may require treatment, while most side effects resolve spontaneously over time. A good safety profile of current COVID-19 vaccines has been described in all clinical trials; the most reported side effects include local reactions at the site of application such as pain, pruritus, erythema, inflammation, and induration. Moreover, some systemic reactions such as fatigue, headache, myalgia, arthralgia, malaise, and loss of appetite have also been reported16-22. Most side effects have been mild to moderate and transient; therefore, it is critical to always give warning signs to all patients who will be immunized and to monitor patients for at least 30 minutes immediately after the administration of the vaccine to rule out immediate side effects. In conclusion, observations so far indicate that, in general, COVID-19 vaccines are safe. Currently, there is no evidence available to contraindicate the administration of vaccines in patients with immune-mediated diseases. However, pharmacovigilance programs should be established in which all IBD patients receiving the vaccine can participate.
Should pregnant IBD patients be vaccinated?
As mentioned above, clinical trials of available vaccines have excluded pregnant and breastfeeding women, so there is always a debate on this issue; however, the IOIBD consensus established that SARS-CoV-2 vaccines are considered safe in pregnant women without IBD, and there are no other factors that should exclude pregnant patients with IBD to benefit from the vaccine, so they are considered safe in this special population26. It should be noted that pregnant women are more likely to develop severe symptoms, with higher morbidity and mortality, affecting both the mother and the fetus; therefore, the risk/benefit of vaccination implies the possibility of this population having access to this preventive measure. This was reflected in a study that reported that pregnant women were significantly more likely than non-pregnant women to be admitted to an intensive care unit (ICU) and receive mechanical ventilation and extracorporeal membrane oxygenation (ECMO)43.
Is it possible for COVID-19 vaccination to cause autoimmune diseases?
Theoretically, autoimmunity can be potentially triggered by vaccines44, immunological mechanisms such as antigen presentation, cytokine production, anti-idiotypic networks, epitope activation and propagation, and polyclonal B cell activation, which are involved in both infectious immunity and self-reactivity45,46. The presence of antinuclear antibodies and autoimmune cell response has been reported during and after viral infections; however, this autoreactive positivity is usually transient and is usually not followed by symptoms or clinical outcomes47,48. Self-reactivity is not the same as autoimmunity because there are several and sufficient control mechanisms that regulate immune system responses; in addition, there is strong evidence that the development of autoimmune diseases depends on many other factors and occur in the absence of the stimulus generated by vaccination49. The possible positive association between vaccines and autoimmunity was based mainly on anecdotal cases, case reports, and uncontrolled studies. For example , Campylobacter was linked to Guillain-Barré syndrome (GBS), influenza virus with multiple sclerosis, Coxsackievirus associated with diabetes type 1, and human parvovirus B19 associated with rheumatoid arthritis, which shows that natural infection itself can cause autoimmune disorders49-54.
To date, clinical trial records provide reassuring results on the issue of vaccine-induced autoimmunity. Some events considered rare, such as those reported regarding the AstraZeneca vaccine, have been reported, which led to the temporary suspension of the Oxford study due to the presence of cases of transverse myelitis55. As explained above, expert neurologists carried out evaluations and considered that the cases were idiosyncratic and that some of them were associated with patients with silent demyelinating diseases. Recently, some rare cases of thrombotic events associated with antibodies against platelet proteins resembling autoimmune thrombotic events associated with heparin-induced thrombocytopenia were published, which the authors of the paper suggested to name vaccine-induced immune thrombotic thrombocytopenia (VITT) to avoid confusion with heparin-induced thrombocytopenia56. However, in the case of these viral vector vaccines, the benefit of the vaccine continues to outweigh the very low risks of side effects such as the generation of autoimmune diseases.
With respect to other vaccine technologies, mRNA-based vaccines may behave as damage-associated molecular patterns and activate TLR7 and TLR8 toll-like receptors, resulting in the production of type I interferon57. According to some studies of vaccines with this type of mechanism of action, they can also increase cytokine and chemokine production following intradermal application, stimulate dendritic cell maturation, lead to robust T and B cell responses, and activate transient self-reactive lymphocytes. In theory, this process may reactivate autoimmune diseases, but mRNA exhibits an inhibitory function on antigen expression, which may suppress the immune response58,59. Therefore, despite these reports (which, as stated above, are very rare), it is emphasized that COVID-19 vaccines are unlikely to have a direct and significant relationship with the generation of autoimmune diseases or other inflammatory disorders in the participants of the clinical trials so far conducted compared with placebo.
Should IBD patients be prioritized for COVID-19 vaccination?
It is not clear whether patients with IBD are at higher risk for SARS-CoV-2 infection than those without IBD with similar levels of viral exposure, but the risk is unlikely to be lower, as discussed above. Evidence to date suggests that patients with IBD with COVID-19, including those treated with long-term biologic therapy and nonsteroidal immunomodulators, may not be at increased risk of serious outcomes such as hospitalization and death12 compared with patients with COVID-19 without IBD. However, recent use of high-dose corticosteroids may be associated with an increased risk of serious COVID-19 outcomes. Risk factors for severe COVID-19 outcomes in IBD patients appear to be similar to those widely recognized in patients with COVID-19 without IBD. These risk factors include older age and multiple comorbidities12. Some reviews suggest prioritizing patients with IBD with additional risk factors, such as corticosteroid use of a prednisone-equivalent of ≥ 20 mg/day and moderate to severe disease activity, as well as IBD patients with an occupational risk such as health workers, teachers, or other essential workers.
According to the IOIBD consensus, people who are not health care workers and do not have risk factors for COVID-19 complications but have IBD should be vaccinated at the same priority level as those who are not health care workers and have no risk factors for SARS-COV-2. In turn, they state that individuals with IBD who are on immunosuppressive therapy but are not at risk for complications of COVID-19 should be vaccinated at the same priority level as those who are immunosuppressed due to other causes, in accordance with regional recommendations26.
In Colombia, the Asociación Colombiana de Infectología (Colombian Association of Infectious Diseases) establishes that patients with autoimmune diseases, including IBD, who are on immunomodulatory or biological treatment require vaccine prioritization at the same level as those with risk factors for poor prognosis. Likewise, it is worth noting that there is no contraindication to vaccination in this population and this prevention strategy must be accessed in the risk-benefit assessment60. The Asociación Colombiana de Gastroenterología (Colombian Association of Gastroenterology) also issued a statement to the Ministry of Health and Social Protection requesting the prioritization of the population with IBD and risk factors for severe disease due to COVID-19.
Multiple reviews and consensus26-29,61-63 addressing this issue in patients with autoimmune diseases, which have very heterogeneous bases in their manifestations, severity and intensity of immunosuppressive management, suggest that if patients suffer from SARS-CoV-2 infection, they may have a higher risk of hospitalization and worse outcomes than the general population, implying that this group of patients should be prioritized for vaccination before the non-prioritized population of similar age and sex. In conclusion, patients with IBD should be vaccinated according to their overall risk of exposure and risk of complications from SARS-CoV-2. These risks continue to be investigated in epidemiological surveillance records, such as SECURE IBD and others based on study populations.
Conclusion
The SARS-CoV-2 pandemic we are currently experiencing has had an unprecedented impact on public health and, unfortunately, there is no widely effective treatment available to date; for this reason, the acquisition of herd immunity through vaccination is necessary to break the chain of transmission. IBD patients are a special population, as it is an immune-mediated disease that requires immunosuppressive therapy to control it; therefore, the approval of the SARS-CoV-2 vaccine creates an urgent need to develop recommendations for IBD patients.
Based on the current reviews and consensus published worldwide on SARS-CoV-2 vaccination in patients with IBD, it is concluded that they should be vaccinated with any of the non-live vaccines currently on the market and the best time to do so is at the first opportunity that the patient has considering their risk factors. These vaccines are safe, and their administration should not be delayed, regardless of the treatment or activity of the disease. However, it is emphasized that the treating gastroenterologist should advise patients with IBD on their efficacy and the possible decrease in seroconversion, especially with the use of systemic corticosteroids. Further studies are needed to define whether the antibody titer should be controlled after vaccination in this patient population and, if so, how often and for how long. Finally, IBD patients receiving SARS-CoV-2 vaccines should be monitored to ensure the best preventive strategy and NOT forget that the top priority is the administration of the vaccine as soon as possible.











 text in
text in