Introduction
The presence of malignant gastrointestinal obstruction (MGO) implies a significant deterioration in quality of life and has a poor prognosis due to high short-term mortality rates, high recurrence, and refractoriness to palliative medical care. Furthermore, taste and the ability to ingest food are limited, which can have a negative impact on the quality of life of persons with advanced neoplastic disease.
The prevalence of MGO ranges between 3 % and 15 % among patients with advanced cancer1-3. Up to two-thirds of cases are caused by tumors in the small intestine, one-third by tumors in the large intestine, and 20 % by both. The presence of symptoms depends on the level of obstruction, and nausea, vomiting, pain, and abdominal bloating are the most common. On the one hand, the primary neoplasms that most frequently cause MGO are mainly ovarian (20 %-50 %), colorectal (10 %-29 %), gastric (6 %-19 %), pancreatic (6 %-13 %), bladder (3 %-10 %), endometrial (3 %-11 %), breast (2 %-3 %), and melanoma (3 %) cancers4,5. In turn, in 80 % of patients with advanced cancer, MGO may have multiple levels of obstruction associated with a previous diagnosis of peritoneal carcinomatosis. On the other hand, it may be secondary to its treatment, as is the case of the formation of fibrosis or radiation enteritis, a benign etiology such as occlusions by flanges in up to 20 %, and indeterminate in 12 %6-8.
Up to 30 % to 40 % of patients with inoperable MGOs achieve spontaneous resolution of the obstructive episode8 and life expectancy has been estimated at 1 to 9 months in patients with a history of intra-abdominal cancer9,10. Chakraborty et al. described the natural history of individuals with MGOs, treated with surgery, chemotherapy, or medical therapy alone. Patients treated with drugs alone had an Eastern Cooperative Oncology Group (ECOG) score of 3 and 4, while those who underwent surgery had an ECOG score of 0 to 2. Patients who were operated on had a shorter hospital stay compared with those who were not (15 vs. 27 days)11.
Cognitive impairment, cachexia, dyspnea at rest, palpable abdominal tumors, liver failure, upper bowel obstruction, and dehydration have been reported to be factors associated with non-resolution of MGOs8.
Treatment is based on an individualized interdisciplinary evaluation, the stage of the disease, overall prognosis, and patient functionality, so it is critical to establish the treatment goal and patient preferences. Generally, the management of MGOs includes the adjustment of palliative pharmacological treatment or surgery, taking into account the evaluation of poor surgical prognosis factors that could lead to greater morbidity and that contraindicate invasive intervention. In patients with peritoneal carcinomatosis and MGO, gastrointestinal decompression with nasogastric tube (NGT) remains the first line of treatment; however, prolonged use is associated with psychological stress and potential complications such as nasal ala necrosis, laryngeal disorders, gastroesophageal lesions, otitis media, and aspiration pneumonia. In general, patients report discomfort with a permanent NGT and that may cause difficulties in its management in non-hospital settings.
In case of refractory malignant obstruction with intractable nausea and vomiting, percutaneous endoscopic gastrostomy (PEG) may be considered. The clinical presentation of the malignant intestinal obstruction depends on the location of the obstruction (proximal or distal) and on the degree of obstruction (partial or complete). In proximal bowel obstruction, abdominal pain is abrupt, and nausea and vomiting are abundant; abdominal distention is rare, unlike distal intestinal obstruction, in which symptoms are more insidious, and abdominal distention and vomiting of fecaloid characteristics are common.
In 1986, Malone et al. reported the first case of radiologically inserted gastrostomy as a decompressive treat. Subsequently, the technique was adopted and modified by Stellato and Gauderer, who described the first 8 cases of PEG in 198712, in which the need for the use of NGT was eliminated in all patients, maintaining its efficacy for months and even years within this subgroup of patients. PEG is a tool that can be complementary to pharmacological therapy in MGO, is cost-effective, and reduces morbidity and mortality rates. Moreover, it allows for the removal of NGT and the consumption of liquid and creamy foods by mouth, preserving the desire and taste for food and allowing patients to return to their place of care.
Clinical case
This is the case of a 49-year-old female patient with a history of micropapillary serous carcinoma of the ovary suggestive of a Krukenberg tumor, associated with unresectable peritoneal carcinomatosis and involvement of the small and large intestines. She was treated with second-line chemotherapy (carboplatin and liposomal doxorubicin) until completing 8 cycles with palliative intent. She was admitted to the emergency room of our institution due to multiple emetic episodes associated with abdominal distension and absence of bowel movements. As a result, malignant bowel obstruction was suspected and, in principle, NGT placement was indicated, which allowed obtaining abundant bilious drainage. A contrast CT scan of the abdomen was performed, confirming the presence of areas of intestinal compression secondary to peritoneal carcinomatosis. The patient continued to have abundant NGT drainage, so she was evaluated by the palliative care service, which considered management associated with ondansetron, dexamethasone, and haloperidol; however, the symptoms persisted. Therefore, she was evaluated by the gastroenterology service to consider performing a PEG.
On the day following the gastroenterology assessment, the patient presented with upper gastrointestinal bleeding and significant anemization. An esophagogastroduodenoscopy was performed, which revealed retention esophagitis, a 4-centimeter hiatal hernia with herniary sac erosions, erosive gastritis and moderate erosive bulbar duodenitis, without translumination, so it was decided to perform a PEG placement by interventional radiology. During her hospital stay, the patient presented with sepsis of pulmonary origin, which required high-flow supplemental oxygen and antimicrobial management. Subsequently, the patient stated that she did not want any further invasive interventions and was assessed by the clinical ethics service, which respected the principle of patient autonomy. Consequently, the therapeutic effort was refocused, and the palliative care service continued with her treatment.
Discussion
MGO is defined as the presence of clinical, physical, or radiological findings of intestinal outlet obstruction, which should be located beyond the ligament of Treitz, and is associated with primary intra-abdominal cancer or primary extra-abdominal cancer with notable intraperitoneal spread13. Patients usually present with nausea, vomiting, abdominal pain, bloating, and no bowel movements. Although the diagnosis is clinical, contrasted abdominal computed tomography (CT) is the gold standard and, if unavailable, abdominal X-ray and ultrasound are other diagnostic options7.
From a pathophysiological point of view, there are 2 types of mechanisms involved in the pathophysiology of MGO: mechanical and functional. The first is related to compression of the gastrointestinal tract by a tumor mass or by metastases in an intrinsic or extrinsic manner, while the second is related to tumor infiltration of the nerves of the myenteric plexus, which generates motility disorders, electrolytic alterations associated with the underlying condition, or alterations derived from the side effects of the drugs used. This leads to an increase in digestive secretions, which have a large volume, generating a third space and deteriorating the intestinal epithelium; this promotes inflammation and alteration of motility2.
With regard to treatment, the pillars of MGO management are to achieve pain control for obstructive symptoms and control nausea and emetic episodes, besides considering an alternate nutrition route. Initial measures include decompression with NGT, maintenance of hydration and control of electrolyte alteration, bowel rest, and pain control7,14.
At this point, emergency surgery should also be considered, evaluating factors of poor surgical prognosis. Patients with advanced cancer usually do not present with MGO, but they may be faced with a single obstruction that could be surgically managed15.
Treatment goals should be individualized taking into account the prognosis of the disease, comorbidities, functionality, and treatment options, which implies clearly establishing treatment goals and patient preferences. In a patient with advanced neoplastic disease, treatment goals are focused on controlling symptoms and maintaining or improving quality of life in search of a return to the person’s site of care. In addition, PEG seeks to reduce the burden of gastrointestinal secretions generated by pharmacological measures and mechanical compression.
Pharmacological treatment is multimodal and includes analgesia, management of nausea and vomiting, management of intestinal secretions, administration of anti-inflammatory drugs (steroids), and parenteral hydration. Management algorithms are heterogeneous. Anticholinergics and antiemetics are the first-line medications that allow to reduce smooth muscle contraction and acid secretion (metoclopramide is contraindicated in complete malignant intestinal obstruction)14. Corticosteroids confer an anti-inflammatory state, reduce pain, and have antiemetic properties, without an improvement in survival when compared with placebo16. These drugs should be discontinued gradually if there is no resolution of the obstruction. Octreotide, a somatostatin analog whose mechanism of action is based on the control of intestinal vasoactivity, has been used as the second line as it favors the reduction of intraluminal intestinal secretions14.
For all patients with MGO, the palliative care team must be integrated to perform a multidimensional assessment and comprehensive management of this high-impact disease. The measures to be defined comprise the indication of supplementary nutrition according to the treatment goals for the patient, which translate into optimizing the quality of life and avoiding futile measures that may prolong suffering in the case of patients at the end of their lives.
Nutrition is not only a physiological need of the human being, but also a social, cultural, and satisfying event. In people with advanced cancer who have MGOs sand symptoms refractory to pharmacological therapy, if the patient›s clinical status and preferences allow it, a PEG could be considered to promote palliation of symptoms and provide the possibility of maintaining the oral route to preserve their quality of life and to favor the satisfaction of the taste for food3,17.
From an ethical point of view, the sensation of food restored through PEG may be controversial since, first and foremost, the universal principles of non-maleficence and justice must be protected, given that the potential risks and complications after PEG must be evaluated. As for the principle of justice, the interdisciplinary team accompanying the patient should consider the patient’s clinical condition, capacity to tolerate the intervention, and the possible benefit after the procedure, always hand in hand with the principles of autonomy and taking into account the patient’s decisions, all in the context of the principle of beneficence.
PEG
In recent years, PEG has emerged as a complementary tool in the management of MGO when pharmacological therapy fails to control symptoms and the withdrawal of NGT needs to be considered due to all the associated side effects3.
PEG allows to reduce gastrointestinal secretions and intestinal gas; it also allows for the removal of NGT because it is safer than chronic NGT usage, which favors the return to the place of care3,18.
Antibiotic prophylaxis and technique
The Society of Radiology guidelines recommend antibiotic prophylaxis with cefazoline 1g if the technique is the passage of the catheter through the oral cavity; however, prophylaxis is controversial when performed through the transabdominal route to avoid peristomal infection3,19.
Contraindications
Table 1 describes the contraindications for PEG as defined by multidisciplinary guidelines of different societies3,19.
Table 1 Contraindications for PEG19
| Absolute | Relative |
|---|---|
| Uncorrectable coagulopathy | Recent gastrointestinal bleeding (peptic ulcer with identified large vessel or esophageal varices) |
| Bacterial peritonitis | Varices associated with portal hypertension |
Taken from: Itkin M et al. Gastroenterology. 2011;141(2):742-65.
Percutaneous gastrostomy can be inserted endoscopically, or using CT, ultrasound, or fluoroscopy. The debate focuses on which method should be used, radiological or endoscopic. Most gastrostomy probes are placed endoscopically; one study reported that between 18 % and 35 % were placed using fluoroscopy20. Other series have shown that the radiological method is often more successful19,20, while other studies have reported a high incidence of obstruction of the probe and the need for its replacement. Silas et al. reviewed the indications, complications, and results of the radiological versus endoscopic method in 370 patients and reported that both are safe and effective. However, in this series, early complications such as infection were more frequent with the fluoroscopic method (23 % vs 11 %; p = 0.002)21. In a prospective, randomized, controlled study of radiologic gastrojejunostomy versus PEG, the average procedure time was significantly different (53 minutes vs 24 minutes; p = 0.013)22.
Peritoneal carcinomatosis, tumor infiltration, previous gastric surgery, and colonic or hepato-diaphragmatic interposition between the stomach and the anterior abdominal wall are not considered contraindications, although they may hinder the performance of the usual procedure, but may necessitate a modified gastrostomy technique, such as performing the procedure under fluoroscopy and using longer needles in the case of peritoneal carcinomatosis, or performing prior paracentesis when ascites exist3,19.
A clear benefit of PEG over the radiological method is the ability to directly visualize and evaluate the gastric chamber to identify other disorders. Lesions such as severe esophagitis, active gastric or duodenal ulcer, erosive hemorrhagic gastritis, or neoplastic infiltration of the gastric wall have been reported in the literature, with rates from 10 % to 59 %23.
Outcomes
The main impact of PEG is related to improved quality of life since resolution and survival have not shown significant results. There are studies evaluating PEG, including a retrospective cohort, in which 439 patients with MGO were assessed for gastrointestinal oncological conditions, in whom PEG was performed, and the median survival was 37 days24. In another retrospective cohort of 75 participants with MGO who underwent PEG, the frequency of daily emesis and nausea decreased significantly, while minor complications occurred in 69 % and major complications in 24 %; the most frequent were stoma leakage, mild pain, and obstruction of the probe25.
Table 2 summarizes 3 studies that specifically target primary neoplasms, technical success rate, clinical success rate, and obstruction resolution, which describe success with the use of PEG for the management of MGO.
Table 2 Efficacy of PEG in MGO secondary to different neoplasms
| Study | Patients (primary neoplasm) | Technical success | Clinical success | Diet tolerance | Resolution |
|---|---|---|---|---|---|
| Pothuri et al.26 | 94 (ovarian) | 94 total (100 %); 92 PEG, 2 Rx | 86 (91 %) | 3: none, 9: sips, 40: liquids, 40: soft/regular food, 2: unknown; 27/34 (84.4 %) | 29/94 (31 %) received chemotherapy; 4/94 (4.3 %) resolved after cancer treatment |
| Ryan et al.27 | 45 (ovarian) | 44/95 (97.8 %) Rx | - | - | - |
| Campagnutta et al.23 | 34 (29 ovary, 4 uterus, 1 cervix) | 32/34; 28/34 PEG, 4 ultrasound | 27/34 (84.4 %) | 27/34 (84.4 %) | 8/37 (23.5 %) received chemotherapy; 3/34 (8.8) resolved after cancer treatment |
Rx: X-ray.
In the study conducted by Pothuri et al.26, 94 patients with ovarian cancer required PEG for MGO; the mean age was 56 years. The mean interval since the initial diagnosis of cancer and the performance of PEG was 3.1 years. 89 % of patients received 3 or more chemotherapy regimens prior to the procedure. 22 of 77 patients who underwent CT before the procedure had an encapsulated tumor of the stomach; of these patients, 59 (63 %) had ascites and 25 were taken to pre-PEG paracentesis. 100 % of patients successfully underwent probe placement and 91 % had clinical improvement. The mean number of days to get improvement was 1.7 and all patients showed improvement after 7 days.
One of the questions is the management of patients with MGO who present with ascites, because the risk of complications is higher in this group of patients; however, technical success has been demonstrated in patients with MGO and ascites. In the study carried out by Shaw et al.28, in which a PEG was carried out to treat MGO in 93 patients, 13 were taken to paracentesis, 78 patients were managed with an intraperitoneal catheter and 2 patients did not require drainage; complications occurred in 13.9 %. Therefore, management with pre-procedure paracentesis was indicated and a drainage catheter was placed, which improved ascites and favored the performance of PEG, without increasing the risk of infection.
Another prospective study, which evaluated 25 patients with advanced gynecological and gastrointestinal cancer and MGO taken to PEG, described significant improvement in quality of life in 16 (64%) patients, non-significant worsening in 7 (28%), mainly due to the persistence of physical symptoms, and 2 (8 %) without changes in quality of life score29.
Complications
Complications may occur, both major and minor, ranging from 5.9 % to 7.8 %, respectively. In children, they include superficial bleeding, pericatheter stoma infection, excessive granulation tissue near the stroma, catheter obstruction, catheter dislocation, and catheter leakage. Other more serious but less frequent complications are bleeding, intestinal perforation, peritonitis, abscess formation, and deep tissue infection of the skin3.
Ryan et al.27 reported major complications in 6.7 % with a patient requiring emergent laparotomy 18 days post-procedure, after CT showed that intraperitoneal rupture of the tube with necrosis of the anterior wall of the stomach and abdominal wall. This patient died at 7 weeks following multiple organ dysfunction. In the study by Pothuri et al.26, complications associated with PEG occurred in 17 (18 %) patients, and the most frequent complication was fistula in 8 of 94 patients (9 %).
Based on the foregoing, a MGO management algorithm is presented in Figure 1.
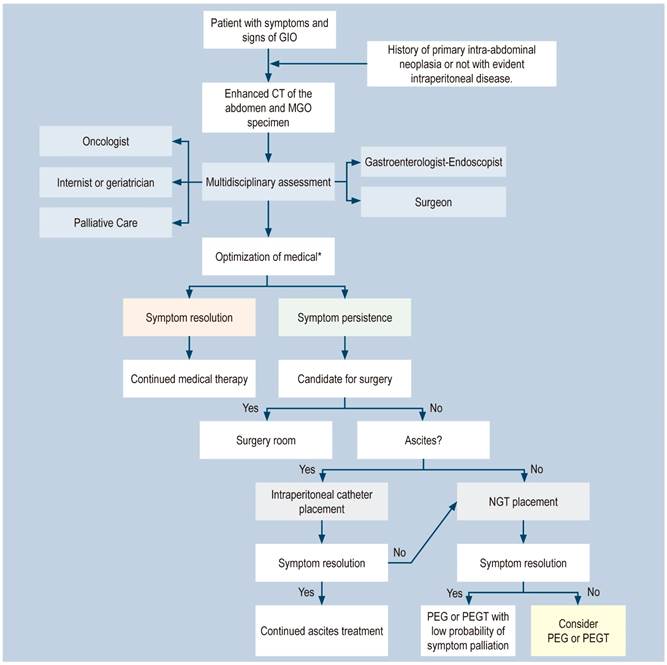
Figure 1 MGO Management Algorithm (28). *Optimizing medical therapy: proton pump inhibitors (PPIs), H2 blockers, anticholinergics, corticosteroids. Octreotide. GIO: gastrointestinal obstruction. MGO: malignant gastrointestinal obstruction; PEGT: percutaneous endoscopic gastrostomy tube. Adapted from: Shaw C et al. Ann Surg Oncol. 2013;20(2):497-505.
Conclusions
PEG may be a suitable tool to treat selected patients with malignant bowel obstruction because it supports symptom palliation, facilitates the return to the site of care, and may allow the possibility of restarting the oral route, which can lead to significant changes in emotional, dignity and autonomy aspects, as well as perception of well-being. The impact of MGO on the already compromised quality of life of these patients and their current treatment with NGT can be alleviated with PEG; the available evidence from retrospective case series suggests that it may be an effective and relatively safe alternative in the management of this severe condition.











 texto en
texto en 



