Introduction
In December 2019, China publicly reported its first cases of coronavirus 2019 (COVID-19), a disease caused by the infection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and in March 2020, due to the global spread of the virus, the World Health Organization (WHO) officially declared the COVID-191 pandemic. SARS-CoV-2, the novel coronavirus, is characterized by being an enveloped single-chain ribonucleic acid (RNA) virus of the Coronaviridae family with an incubation period of around 14 days, and a median incubation period from 4 to 6 days (2,3. Person-to-person transmission of COVID-19 occurs due to contact or handshaking as well as to contact with droplets of saliva produced by sneezing or coughing infected individuals which are emitted through air (3.
The pathogenic mechanism of SARS-CoV-2 consists in using the angiotensin-converting enzyme II (ECA-II) as a receptor for its entry into the cell (2. ACE-II is not only present in the pulmonary alveoli, but also in cardiac myocytes, gastrointestinal tract, liver, kidneys, vascular endothelial cells and muscle cells in arteries, reflecting the involvement of various organs and tissues, justifying the variety of symptoms of this disease (4.
The clinical manifestation of COVID-19 is variable and may be asymptomatic, with mild to moderate symptoms, especially fever, headache, cough, nasal congestion and gastrointestinal symptoms; to severe manifestations, especially in a patient with an association of comorbidities and multisystem involvement (5,6. The pathophysiology of COVID-19 has not been fully elucidated yet; however, one of its mechanisms is the exacerbated release of inflammatory cytokines that ends up in multisystem involvement, which can trigger vascular and coagulative changes (7.
As for liver damage, due to the state of hyperinflammation by the “cytokine storm” and dysregulation of coagulation, there are laboratory analyzes of mild thrombocytopenia, prolongation of prothrombin time, elevation of ferritin and C-reactive protein (CRP)7. There are reports in the literature regarding liver damage associated with secondary sepsis to COVID-198,9, as well as a description of the use of hepatotoxic drugs in the symptomatic therapeutic treatment of COVID-19 and its complications (4,10.
In order to globally assess the physiology of the liver and identify liver lesions, biochemical markers are used: aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin and its fractions, alkaline phosphatase and γ-glutamyltransferase (GGT), which when above reference values indicate changes in liver function and possible hepatocellular damage (11.
Being still a very recent disease, COVID-19 lacks more studies to evaluate possible tissue damage, once the virus access to different organs has been reported. Given that the aim of this review is to correlate the development of alterations in liver function caused by SARS-CoV-2 and its impact on the clinical evolution of the patient with the infection, it is worth emphasizing that, so far, it is not clear whether the mechanism that causes liver damage in SARS-CoV-2 infection is the result of the disease and its pathophysiological mechanisms or the current treatment.
Material and methods
This study consists of a systematic review that mainly aims to correlate the hepatic alterations caused by SARS-CoV-2 infection with patient prognosis with this infection. The databases of PubMed Central® (PMC), the Latin American and Caribbean Health Sciences Literature (LILACS) and the Scientific Electronic Library Online (SciELO) were selected to investigate the topic related to this study. In addition, the following descriptors were defined to carry out the research aimed at the proposed topic: “injury” (D1), “hepatic” (D2), “coronavirus” (D3) and “COVID-19” (D4), in addition to the Boolean operator “Y”. In light of this, “D1” associations were used.
Then, the following filters were defined in order to be used in the searches: randomized trial, observational studies (cohort, cross sectional, and case-control), case series, articles published in the last year and full text, which were used as criteria to be included in the study. Also, as it is a recent topic, there was no language restriction. The exclusion criteria used were studies that did not address the topic or did not present sufficient data in order to achieve the objective of this review. Editorials, comments, meta-analyses and review articles were also excluded. It should be noted that, due to the fact that it is a recent disease, it was not possible to trace homogeneity in all the selected articles.
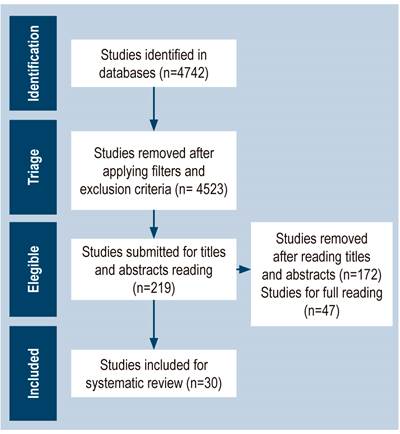
After applying the inclusion and exclusion criteria, 4742 articles were found in the databases, 4523 were excluded and only 219 articles were included. Subsequently, titles and abstracts were read in order to exclude studies that did not address the proposed topic; thus, 47 studies were chosen to be fully read. After reading, 17 articles that did not contain sufficient data to meet the objectives of this study were excluded; therefore, 30 articles were defined to make up the database of this systematic review (Figure 1).
No articles were selected from the LILACS database, as only two articles were found during the search, which did not address the proposed topic. In SciELO, there were four articles in the research, of which only one had sufficient data to achieve the objective proposed in this study. It is worth noting that two reviewers independently carried out the entire article selection process.
In order to apply the kappa index to analyze the concordance index between reviewers, authors María Adriely Cunha Lima (ML) and Tiago Costa (TC) did the article selection independently, and if the value determined, for instance, a lack of concordance, poor or moderate concordance, a third reviewer (Isadora Kniess [IK]) would be selected. Therefore, IK would choose an article at the point of intersection of the two previous reviewers with the purpose of eliminating the initial discrepancy (Table 1).
Table 1 Contingency Index
| Reviewer TC | ||||
|---|---|---|---|---|
| Cat. 1- Yes | Cat. 2- No | Total | ||
| Cat. 1- Yes | 28 | 13 | 41 | |
| Reviewer ML | Cat. 2- No | 17 | 4684 | 4701 |
| Total | 45 | 4697 | 4742 | |
Cat.: category. Source: Research data, 2020.
The kappa index was applied and a value of 0.648 was obtained, which, according to Landis and Koch12, is interpreted as a strong agreement, which is why the systematic review can take place (Table 2).
Table 2 Kappa Index
| Indexes | Category 1* | Category 2** |
|---|---|---|
| Category kappa | 0,648 | 0,648 |
| Standard category kappa error | 0,0641 | 0,0601 |
| 95% CI for category kappa |
|
|
*Articles included in the study. **Articles excluded from the study. CI, Confidence Interval; Inf.: Inferior; Sup.: Superior. Source: Research data, 2020.
Results
Table 3 shows that, in total, 19,234 cases of individuals infected with SARS-CoV-2 were analyzed, since 70% (n = 21) of the articles were written in China. In addition, it is worth mentioning that only one journal (3.33%) of those that published the studies used in this systematic review, has an impact factor below 1.0.
Table 3 Features of studies selected for review
| Article | Journal | Type of study | Place of study | Sample (n) |
|---|---|---|---|---|
| Asghar MS et al.13 | Cureus IF: 1.90 | Retrospective | Pakistan | 100 |
| Bahl A et al.14 | Internal and Emergency Medicine (IF: 2322) | Multicenter cohort | United States | 1461 |
| Cai Q et al.15 | Allergy (IF: 8706) | Retrospective | China | 298 |
| Chen J et al.16 | The Journal of Infection (IF: 4603) | Retrospective | Shanghai (China) | 249 |
| Chen LY et al.17 | Journal of Digestive Diseases (IF: 2937) | Retrospective | Wuhan (China) | 502 |
| Chen N et al.18 | Lancet (IF: 60 392) | Retrospective | Wuhan (China) | 99 |
| Davies P et al.19 | The Lancet Child & Adolescent Health (IF: 8543) | Multicenter observational | United Kingdom | 78 |
| Du RH et al.20 | Annals of the American Thoracic Society (IF: 4836) | Multicenter observational | China | 1017 |
| Falasca L et al.21 | The Journal of Infectious Diseases (IF: 5186) | Description | Italy | 22 |
| Fan Z et al.22 | Clinical gastroenterology and Hepatology (IF: 8549) | Single-center retrospective | Shanghai (China) | 100 |
| Hu W et al.23 | Annals of Translational Medicine (IF: 3297) | Single-center retrospective | Wuhan (China) | 16 |
| Jiang S et al.24 | Frontiers in Medicine (IF: 3.9 | Multicenter observational | Zhejiang (China) | 131 |
| Jin A et al.25 | Biosafety and Health (IF: 1972) | Descriptive | Beijing (China) | 45 |
| Lei F et al.26 | Journal of Hepatology AASLD (IF: 14 679) | Retrospective multicenter cohort | China | 5771 |
| Luo S et al.27 | Clinical Gastroenterology and Hepatology (IF: 8549) | Retrospective | Wuhan (China) | 183 |
| Monterde VB et al.28 | Biomedicines (IF: 4717) | Retrospective | Zaragoza (Spain) | 540 |
| Pan L et al.29 | The American Journal of Gastroenterology (IF: 10 383) | Descriptive and multicenter | Hubei (China) | 204 |
| Ramachandran P et al.30 | Journal of Clinical and Experimental Hepatology (IF: 1150) | Single-center retrospective | New York (United States) | 145 |
| Richardson S et al.31 | Journal of the American Medical Association (IF: 14 780) | Case series | New York (United States) | 5700 |
| Schattenberg JM et al.32 | United European Gastroenterology Journal (IF: 3549) | Case series | Germany | 44 |
| Shi M et al.33 | Journal of Clinical Laboratory Analysis (IF: 1.54) | Descriptive and multicenter | China | 161 |
| Teich VD et al.34 | Einstein (IF: 0.261) | Unicentric retrospective | Brazil | 510 |
| Wang Y unicentric et al.35 | Journal of Hepatology (IF: 20 582) | Retrospective | China | 156 |
| Xie H et al.36 | Liver International (IF: 5175) | Retrospective | China | 79 |
| Yu Y et al.37 | Critical Care (IF: 6700) | Multicenter observational | Wuhan (China) | 226 |
| Zhang B et al.38 | PLoS One (IF: 2740) | Retrospective | China | 82 |
| Zhang G et al.39 | Respiratory Research (IF: 3890) | Retrospective | China | 95 |
| Zhang S et al.40 | Critical Care (IF: 6700) | Retrospective multicenter cohort | China | 828 |
| Zhou F et al.41 | Lancet (IF: 60 392) | Retrospective multicenter cohort | Wuhan (China) | 191 |
| Zhou J et al.42 | Medicine (Baltimore) (IF: 2133) | Descriptive | Changsha (China) | 201 |
IF: Impact Factor. Source: Research data, 2020.
Regarding the characteristics of the sample, there is a higher prevalence of men, 9943 cases (54.1%), than of women, 8431 cases (45.9%). In addition, only 12 (40%) articles mentioned there were hepatic comorbidities in a percentage of the sample, and only 2 (6.7%) had a percentage greater than 9% of the sample. It is worth noting that 3 (10%) articles sampled only patients admitted to the Intensive Care Unit (ICU), while 2 (6.7%) articles analyzed only cases of COVID-19 deaths (Table 4).
Table 4 Features of studies selected for review
| Article | Age range (years) | Sex (M/F) | Hepatic comorbidities | Admission to the ICU | Deaths |
|---|---|---|---|---|---|
| Bahl A et al.13 | Median: 62 (18 to > 80) | 770/691 | - | 374 (25.6 %) | 327 (22.4 %) |
| Zhou J et al.14 | Between 1 and 84 | 102/99 | - | 45 (22.4 %) | 2 (0.99 %) |
| Jin A et al.15 | Average: 58.8 (between 7 and 94) | 18/27 | - | 9 (20 %) | 5 (11.1 %) |
| Asghar MS et al.16 | Average: 52.58 | 69/31 | Chronic liver disease: 9 (9 %) | 33 (33 %) | 22 (22 %) |
| Monterde VB et al.17 | Average: 70 (between 22 and 99) | 291/249 | - | 61 (11.5 %) | 109 (20.5 %) |
| Chen LY et al.18 | Average: 61 | 278/224 | - | - | 105 (20.9 %) |
| Wang Y et al.19 | Average: 51.2 | 82/74 | - | 9 (5.8 %) | 4 (2.6 %) |
| Du RH et al.20 | Average: 70.7 | 74/35 | Chronic liver failure: 2 (1.8 %) | 51** (46.8 %) | 109 (100 %) |
| Zhang G et al.21 | Average: 49 | 53/42 | - | 32 (33.7 %) | - |
| Chen J et al.22 | Average: 51 | 126/123 | Chronic hepatitis B: 2 (0.8 %) | 22 (8.8 %) | 2 (0.8 %) |
| Zhou F et al.23 | Average: 56 (between 18 and 87) | 119/72 | - | 50 (26 %) | 54 (28.7 %) |
| Luo S et al.24 | Average: 53.8 | 102/81 | - | - | 7 (3.8 %) |
| Pan L et al.25 | Average: 52.9 | 107/97 | - | 16 (7.8 %) | 37 (17.7 %) |
| Teich VD et al.26 | Average: 39.9 | 290/220 | Hepatitis B. C. HIV or other immunodeficiency: 2 (0.4 %) | 17 (3.4 %) | 1 (1.38 %) |
| Lei F et al.27 | Average: 56 | 2724/3047 | Chronic liver disease: 81 (1.4 %) | - | - |
| Chen N et al.28 | Average: 55.5 | 67/32 | - | - | 11 (11.1 %) |
| Yu Y et al.29 | Average: 64 (between 57 and 70) | 139/87 | Chronic hepatopathy: 3 (1.3 %) | 226 (100 %) | 9 (4 %)*** |
| Jiang S et al.30 | Average: 51.2 | 70/61 | - | - | - |
| Hu W et al.31 | Average: 44.1 | 6/10 | Steatosis: 3 (18.8 %) Dysfunction: 1 (6.3 %) | 0 | 0 |
| Zhang S et al.32 | Average: 62 | 447/381 | Chronic liver disease: 23 (3.02 %) | 100 (12.08 %) | 146 (17.63 %) |
| Zhang B et al.33 | Average: 72.5 | 54/28 | Liver disease: 2 (2.4 %) | 14 (17.1 %) | 82 (100 %) |
| Shi M et al.34 | Average: 59.38 | 104/57 | - | 161 (100 %) | 50 (31.06 %) |
| Ramachandran P et al.35 | Average: between 59.5 and 63 | 55/90 | - | - | 56 (38.62 %) |
| Schattenberg JM et al.36 | Average: 68 (23 and 86) | 30/14 | Pre-existing liver disease: 10 (23 %) | 13 (29.5 %) | 2 (4.5 %) |
| Falasca L et al.37 | Average: 27 and 92 | 15/7 | - | - | 22 (100 %) |
| Richardson S et al.38 | Average: 63 | 3437/2263 | Cirrhosis: 19 (0.4 %) Hepatitis B: 8 (0.1 %) Hepatitis C: 3 (0.1 %) | 373 (14.2 %) de 2634 pacientes | 553 (21 %) de 2634 pacientes |
| Davies P et al.39 | Average: 11 | 52/26 | - | 78 (100 %) | 2 (3 %) |
| Fan Z et al.40 | Average: 50 | 73/75 | - | - | 1 |
| Xie H et al.41 | Average: 60 | 44/35 | - | - | - |
| Cai Q et al.42 | Average: 47.5 | 145/153 | Alcoholic fatty liver: 5 (1.7 %) Non-alcoholic fatty liver: 9 (3 %) Chronic hepatitis B: 14 (4.7 %) | 30 (10.1 %) | 3 (1 %) |
Data is only from the sample that progressed to death. **All patients needed ICU admission, but there was availability only for 51 patients. ***At the end of the study, there were still 204 patients admitted to the ICU. F: Female; M: Male; HIV: Human Immunodeficiency Virus. Source: Research data, 2020.
The increase in AST (4695 cases) prevailed in relation to the total number of cases, when compared to ALT (3226 cases) (Table 5). Only one study did not mention changes in aminotransferase levels (39.
Table 5 Findings suggestive of liver injury in the studies included in the review considering increased enzyme activity (AST and ALT), proteins, albumin and bilirubin
| Article | AST | ALT | Other |
|---|---|---|---|
| Bahl A et al.13 | Elevated in 711 cases | Elevated in 434 cases | - |
| Zhou J et al.14 | Elevated in 31 cases | Elevated in 20 cases | - |
| Jin A et al.15 | - | - | Different degrees of abnormal liver function in 19 (42%) |
| Asghar MS et al.16 | Elevated in 32 cases | Elevated in 22 cases | Increased total bilirubin in 10 cases Increase in GGT in 40/77 |
| Monterde VB et al.17 | Abnormal* in 221 cases | Elevated in 141 cases | Abnormal liver test in 319 (64.3%) Abnormal GGT in 256 cases |
| Chen LY et al.18 | Elevated | - | - |
| Wang Y et al.19 | Elevated | - | - |
| Du RH et al.20 | Elevated in 50 cases | Elevated in 18 cases | - |
| Zhang G et al.21 | Elevated in 11 cases | Elevated | Decrease in total protein in 65 cases |
| Chen J et al.22 | Elevated | Elevated | Albumin reduction |
| Zhou F et al.23 | - | Increased in 59/189 | - |
| Luo S et al.24 | Slight increase, average: 65.8 ± 12.7 U/L | Slight increase, average: 66.4 ± 13.2 U/L | - |
| Pan L et al.25 | Elevated in 22 cases | Elevated in 27 cases | - |
| Teich VD et al.26 | Elevated in 15/70 | Elevated in 19/75 | Increased total bilirubin in 3 cases |
| Lei F et al.27 | Elevated | Increased | - |
| Chen N et al.28 | Elevated in 35 cases | Elevated in 28 cases | Decreased albumin in 97 cases and increased total bilirubin in 18 cases |
| Yu Y et al.29 | Elevated in 46 cases | Elevated in 85 cases | Decreased albumin in 145 cases and increased total bilirubin in 42 cases |
| Jiang S et al.30 | Abnormal in 41 cases | Abnormal in 45 cases | Risk factor: treating with several drugs concomitantly |
| Hu W et al.31 | Elevated in 5 cases | Elevated in 5 cases | Decreased albumin in 13 cases and increased total bilirubin in 3 cases |
| Zhang S et al.32 | - | Elevated | Increased total bilirubin |
| Zhang B et al.33 | Elevated in 44 cases | Abnormal in 42 cases | Decreased albumin in 56 cases and increased total bilirubin in 22 cases |
| Shi M et al.34 | Elevated in 82 cases | Anormal en 42 casos | Abnormal albumin in 141 cases |
| Ramachandran P et al.35 | - | - | Increased aminotransferases in 20 cases |
| Schattenberg JM et al.36 | Elevated in 26 cases | Elevated in 6 cases | Increased GGT in 7 cases |
| Falasca L et al.37 | - | - | Histopathological changes |
| Richardson S et al.38 | Elevated in 3263 cases | Elevated in 2176 cases | - |
| Davies P et al.39 | No changes | No changes | - |
| Fan Z et al.40 | Elevated in 32 cases | Elevated in 27 cases | Increased GGT in 26 cases and bilirubin in 9 cases |
| Xie H et al.41 | Elevated in 28 cases | Elevated in 25 cases | Increased total bilirubin in 4 cases |
| Cai Q et al.42 | Elevated | Elevated | Increased GGT |
The values regarding the total sample size, cited in each study presenting hepatic alterations, are described in Table 1. *Abnormal: alteration of reference values. Source: Research data, 2020.
Asghar MS et al. (13 recorded, in a sample of 100 patients, two cases of fatal liver failure, in those who had previous hepatic enzyme changes. Jiang S et al. (24, in a sample of 131 COVID-19 patients, mentioned that 76 (58%) of them had liver damage, of which 81.5% corresponded to the most severe cases.
Three studies mentioned death due to severe liver injury. Jin A et al. (25, with 45 cases, mentioned that only one patient developed severe liver failure (Child-Pugh class C) and evolved to death; that of Zhang B et al. 38),, in which out of the 64 patients with liver damage, only one evolved to death; and the study of Zhang S et al. 40 with a sample of 146 deaths, 15 (10.3%) of such cases died due to acute liver damage.
Pan L et al. (29, when evaluating digestive symptoms and changes in hepatic enzymes (ALT and AST), in a sample of 204 COVID-19 patients, observed that from a group of 49 patients with increased ALT and AST, 38 mentioned digestive symptoms. They concluded that those who had digestive symptoms were more likely to have hepatocellular damage and, consequently, enzymatic changes. Wang Y et al. (35 mentioned that the increased level of these enzymes was related not only to the severity of the disease, but also to a higher incidence of coughing.
Zhang G et al. (39 associated an increase in ALT and AST enzymes, and a reduction in the level of total proteins with an increased risk of ICU admission, mechanical ventilation, and death, in which near half of the patients had impaired liver function. This condition was also cited by Cai Q et al. 15, when they identified 298 COVID-19 patients, of which 44 (14.8%) had liver damage, and was more common in those who showed a more severe clinical picture. However, they did not clarify how many of them were considered serious cases.
Chen LY et al. (17 mentioned that, out of a sample of 502 individuals, 301 (60%) patients had abnormal liver function upon admission and this condition correlated with worsening in the clinical course of SARS-CoV-2 infection; this progression was also mentioned in the article by Xie H et al. 36. The condition worsening of these individuals was demonstrated by a greater drop in oxygen saturation, an increase in leukocyte and neutrophil counts, higher levels of CRP, D-dimer, lactate dehydrogenase (LDH) and others. Furthermore, 28.9 % of individuals with altered liver function died; whereas for those with preserved liver function, such rate was of 9 %17. This study also classified the liver injury severity as normal liver function grade 0 (201 patients); grade 1 (138 patients) hepatocellular or biliary type; and hepatocellular type grade 2 (163 patients) along with biliary type (17.
When analyzing the possibility of in-hospital death, Zhou F et al. (41 mentioned that elevated ALT was one of the factors increasing this risk, a condition also recorded in other studies (14,26. Lei F et al. (26 considered that the risk of all-cause mortality increased significantly 4.81 times when AST was altered. Chen J et al. 16 noted that low albumin levels are among the factors associated with the development of respiratory distress syndrome.
The authors identified histopathological changes in the liver of patients who died from COVID-19, such as inflammatory infiltrate, congestion, and steatosis (21.
Discussion
In this systematic review, there was a higher prevalence of men. As for COVID-19 laboratory alterations, sex differences are accentuated from the age of 13, with changes in liver function markers common in different age groups, whereas elevation of inflammation biomarkers stand out in older men (43.
In the scenario of hepatobiliary biochemical changes, hyperbilirubinemia and elevation of aminotransferases, especially AST, were observed, as well as elevation of GGT, which shows direct or indirect liver damage due to COVID-1944. Among the mechanisms that may justify changes in liver function due to SARS-CoV-2 infection is that the exacerbated release of cytokines and the consequent Acute Respiratory Distress Syndrome (ARDS) promote hypoxia and oxidative stress, which can cause liver damage (45,46. Furthermore, the ACE-II receptor, used by SARS-CoV-2 to enter the cell, is present in the epithelial cells of hepatocytes and the bile duct, which may set up another mechanism that justifies the elevation of observed hepatic enzymes and bilirubin (4,44.
Regarding the association between changes in hepatocellular markers and the severity of COVID-19 patients, Shi et al. 47 described AST at normal levels in asymptomatic patients with SARS-CoV-2 infection; whereas, for symptomatic patients, this marker was elevated, suggesting that changes in liver function reflect a worse prognosis of COVID-19. Among other factors reported as markers of SARS-CoV-2 infection severity, the reduction in albumin levels, observed in the studies analyzed, stands out, and its relationship with the acute inflammatory phase is worth mentioning (4,44.
The hepatic impact of COVID-19 on the patient can be measured with a histopathological analysis of the liver, which initially revealed microvascular steatosis and moderate lobular and portal activity (10,48. In critically ill patients, postmortem biopsy shows portal fibrosis, lobular inflammation, and significant vascular thrombosis (49. Other findings of the necropsy are hepatomegaly, leukocyte infiltration in zone 1, focal necrosis in zone 2 and congestion with mild thrombosis in zone 350.
In the case of chronic liver disease prior to COVID-19, as observed in some of the analyzed studies, these patients present an activation of the alternative pathway of the renin-angiotensin system that promotes a reduction in plasma levels of angiotensin II and also an elevation of ACE-II (51,52. However, in the literature it is described that, with the entry of SARS-CoV-2 into the cell, there is a decrease in ACE-II and, consequently, an increase in plasma levels of angiotensin II, and the increase in this hormone is related to the viral load and systemic complications of COVID-1953,54. Kulkarni et al. hypothesized that patients with chronic liver disease would experience a SARS-CoV-2 infection with fewer complications (44; however, more studies on the subject are needed to make such a claim.
Due to the absence, so far, of a defined treatment for infection by the novel coronavirus, since it is still necessary to demonstrate the effectiveness of what has already been proposed, the therapeutic approach to this disease involves the administration of drugs that favor the hepatotoxic potential, such as oseltamivir, hydroxychloroquine, paracetamol and acetaminophen (44,55. Therefore, the importance of studies on the relationship between the liver and COVID-19 is evident, so that health professionals, having this knowledge, can reduce the risk in their patients and thus avoid death due to liver damage from COVID-19, as stated by Zhang et al. 56 in their study.
Some factors may be considered limitations in this review study. There were studies published before the clinical outcome of all patients, as in Yu Y et al.37; consequently, it was not possible to fully assess the prognosis. Du RH et al.20 mentioned the unavailability of ICU beds for patients who needed it due to patient overload, which can directly interfere with the clinical outcome, given that the deficit in medical care is one of the interference factors.
In addition, several studies did not mention the sample size and described the findings subjectively, such as in the research of Ley F et al.26 and Zhang S et al. 40, who reported that only laboratory changes occurred without identifying the sample size of the patients studied. Regarding the proposed objectives, it is valid to report that, although there is data in the literature that report the hepatotoxicity of the drugs used to treat COVID-19, most of the articles did not address the condition in their reviews.
Conclusion
It is evident that there is a relationship between hepatic involvement from COVID-19 and its mortality, as described in the articles. However, there is still a limitation in terms of the number and, more importantly, homogeneity of the studies that performed such assessment. Therefore, it is of the essence to adopt hepatic enzymes as a parameter in the assessment of patients with COVID-19 in future studies, since, as explained in this review, there are reports of the association between their elevation and a higher mortality rate, even if the causing mechanism is not fully clarified, since it is a very recent disease











 texto em
texto em