Introduction
The infection caused by the SARS-CoV-2 coronavirus constitutes a serious current health problem and of great global impact. Its rapid spreading and evolution since its origin in December 20191,2 had it declared a pandemic by the World Health Organization (WHO) (3. Currently, more than 160 million confirmed cases and 3.5 million human losses are currently reported worldwide (4.
This situation has promoted the restructuring of hospital services around the world, including the working methodology in digestive endoscopy units (DEU). Due to aerosol generation, endoscopy inadvertently exposes health workers to contagion through inhalation, fecal-oral transmission, or conjunctival contact of contaminated micro drops or secretions (5,6. In this sense, global endoscopy societies and expert groups recommend limiting elective endoscopic procedures, giving priority to emergency situations (7-10.
Upper gastrointestinal bleeding (UGB) is a life-threatening condition that requires timely diagnosis and management (11. Endoscopy makes it possible to identify and treat the source of the bleeding, so it is usually recommended to perform it within 24 hours after the presentation of the clinical picture (12,13. However, the current pandemic has brought with it a dilemma regarding endoscopy in patients with UGB and SARS-CoV-2 infection (14, suggesting that the risk of transmitting the virus could outweigh the benefit granted by the procedure15.
In this context, the emerging experience suggests optimizing medical treatment, which includes hemodynamic monitoring, restrictive transfusion support and pharmacotherapy (16, which justifies endoscopy in cases of hemodynamic instability (7 or due to failure of medical therapy in the first 24 hours. To date, there are no specific guidelines with strong evidence-based recommendations that evaluate the role of conservative medical treatment and/or endoscopic approach with respect to the evolution in patients with SARS-CoV-2 infection and UGB (17.
The objective of the study was to evaluate the management of UGB in the context of the SARS-CoV-2 coronavirus pandemic in a reference hospital in Lima, Peru.
MATERIALS AND METHODS
An observational, descriptive and retrospective study was conducted between March and August 2020 at the Guillermo Almenara Irigoyen National Hospital, in Lima, Peru.
A census of patients diagnosed with UGB in our unit was performed, who met the inclusion criteria: age ≥ 18 years, patients with a positive rapid and/or molecular test result for SARS-CoV-2, with records of the indispensable variables in the clinical history such as: signs of UGB (hematemesis and melena) upon admission, serum hemoglobin, record of transfusion of blood products, treatment received and evolution. The following were excluded: pregnant women, patients who requested voluntary withdrawal before 24 hours or who did not authorize the transfusion of blood products nor endoscopic procedures when indicated.
Data from medical records were collected, and the end date of follow-up was the day of hospital discharge, referral to another facility, or death.
Patients with a reactive serological immunoglobulin M (IgM) test or positive real-time polymerase chain reaction (PCR) test of nasopharyngeal swab were considered positive for SARS-CoV-2; symptomatic or asymptomatic, the latter even with normal chest tomography due to the possibility of being within the infectious period of this virus (high transmissibility and pathogenicity) (18,19. These patients were isolated as were symptomatic patients.
The Glasgow-Blatchford Score (GBS) was calculated at admission; a value ≥ 3 was considered as an indication of hospital admission and ≥ 12 of high probability of requiring endoscopic treatment and risk of death (12.
SARS-CoV-2 disease was classified according to the criteria established by the WHO (20:
Mild: Symptomatic patient, but no pneumonia or hypoxemia;
moderate: Pneumonia with oxygen saturation (O2) ≥ 90% to ambient air;
severe: Respiratory rate ≥30/min, O2 saturation < 90% or blood pressure of oxygen/inspired fraction of oxygen (PaO2/ FiO2) <300 mm Hg;
critical: Acute respiratory distress syndrome, sepsis or septic shock.
Medical management for UGB consisted of hemodynamic support (administration of physiological saline solution with or without the need for blood products) and pharmacotherapy (which included a proton pump inhibitor (PPI) such as intravenous omeprazole 80 mg bolus, followed by an infusion of 8 mg/hour or boluses of 40 mg every 12 hours). On the other hand, those with clinical, laboratory or diagnostic suspicion of liver cirrhosis, as part of the management of varicose UGB, octreotide of 50 μg intravenous bolus, followed by 50 μg/hour and an antibiotic as prophylaxis with intravenous ceftriaxone of 1 g/24 h.
The indication for upper digestive endoscopy (UDE) was considered for the suspicion of active bleeding before or after 24 hours of admission, and was performed according to international recommendations and medical criteria. In addition, hemodynamic instability was considered as an indication of early UDE (between 6-24 hours) (Figure 1). The time up to the UDE was determined by the hours elapsed from UGB emergency room admission up to the procedure.
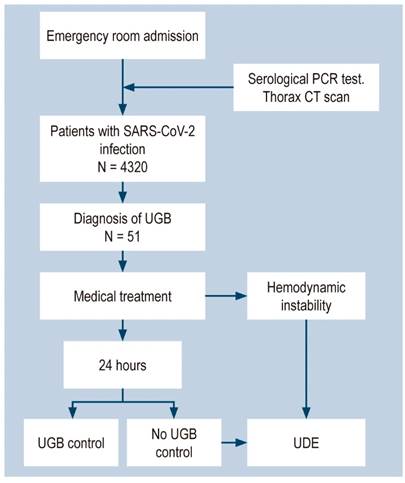
Figure 1 Flowchart of patients admitted to the emergency room due to SARS-CoV-2 infection and UGB. CT scan: Computed axial tomography.
Any new episode of UGB within the first 5 days after observing the absence of digestive (clinical and biochemical) bleeding was defined as rebleed. All patients who did not have an endoscopy at the initial hospitalization were discharged and instructed to return to the hospital 21 days after the initial positive test for further evaluation and scheduling of a diagnostic UDE; they were also instructed to return immediately if they showed signs of UGB. If this new episode reappeared within a month of hospital discharge, it was considered as readmission.
The study was approved by the Research Ethics Committee specific to COVID-19 of the Instituto de Evaluación de Tecnologías en Salud e Investigación (IETSI) from Seguro Social-EsSalud. Descriptive statistical measures were applied; the absolute and relative frequencies (percentages) were obtained from the categorical variables. The distribution of data was evaluated using the Kolmogorov-Smirnov test. For the quantitative variables, measures of central tendency such as median and interquartile range (IQR) were estimated. Variables such as: transfusion of blood products, hospital stay, rebleeding, readmission for UGB and specific mortality among patients who received conservative treatment versus endoscopic treatment were compared; for this we used the Mann-Whitney U test, the chi-square test (χ²) or the Fisher’s exact test, respectively. All statistical analyses were performed with the SPSS (Statistical Packages for the Social Sciences) version 25.0 program, and the tables and figures were built with Microsoft Excel, 2016. A p-value <0.05 was considered statistically significant.
Results
Of 4320 cases of patients with SARS-CoV-2 infection, 51 (1.18%) patients presented UGB upon admission to the emergency room. The median age of the population was 70 years. 30 (58.8%) patients were male. The most frequent comorbidities were hypertension (20 patients; 39.2 %) and liver cirrhosis (12 patients; 23.5 %).
According to GBS, 51 (100%) patients had an indication of hospital admission when admitted (GBS ≥ 3) and 29 (56.9%) had a higher probability of endoscopic treatment and death (GBS ≥ 12). With respect to SARS-CoV-2 infection, 20 (39.2%) patients had pneumonia; of these, 11 (21.6%) patients required oxygen support and only 1 (2%) received assisted ventilation (Table 1).
Table 1 Descriptive characteristics of patients with SARS-CoV-2 infection and UGB.
| Patient Characteristics | SARS-CoV-2 n = 51 |
|---|---|
| Age, years, median (IQR) | 70 (61-77) |
| Male n (%) | 30 (58.8 %) |
| Comorbidities, n (%) | |
|
|
|
| Outpatient medication, n (%) | |
|
|
|
| Signs of UGB, n (%) | |
|
|
|
| GBS, n (%) | |
|
|
|
| Hypotension/shock, n (%) | |
| Yes | 8 (15.6 %) |
| Saturation of O2, n (%) | |
| 92% | 11 (21.6 %) |
| Severity of SARS-CoV-2, n (%) | |
|
|
|
| Analytics at admission, median (IQR) | |
|
|
|
NSAIDS: Nonsteroidal anti-inflammatory drugs; INR: International Normalized Ratio; CRP:C-reactive protein; aPTT: activated partial thromboplastin time.
Regarding the management of UGB, 34 (66.7%) patients did not undergo UDE and received only medical treatment, and 17 (33.3%) received medical treatment plus UDE. As medical treatment, 47 (92.2%) patients received bolus PPI, 4 (7.8%) received infusion PPI, and 14 (27.5%) received intravenous octreotide. Of the patients who underwent UDE, only 6 (35.3%) required any therapeutic intervention. 16 (94.1%) patients underwent UDE after 24 hours of admission, with a median of 36 IQR hours (30-49). The most frequent finding was peptic ulcer disease in 7 (41.2%) patients, followed by esophagogastric varicose veins in 3 (17.6%). The other etiologies, as well as the endoscopic treatment received, are summarized in Table 2.
Table 2 Medical and/or endoscopic management of patients with SARS-CoV-2 infection and UGB.
| Management Characteristics | SARS-CoV2 n = 51 |
|---|---|
|
|
|
| Medical treatment + endoscopy, n (%) | 17 (33.3 %) |
| Time to endoscopy >24 hours | 16 (94.1 %) |
| Endoscopic findings, n (%) | |
|
|
|
| Endoscopic treatment, n (%) | |
|
|
|
APC: Argo plasma coagulation.
When analyzing between those patients who received only medical treatment and those who received medical treatment plus UDE, we observed no statistically significant differences between rebleeding rates (3 vs. 0, p = 0.542); UGB re-admittance at 30 days (3 vs. 0, p = 0.542); similarly, with the number of red blood cell transfusions [2 (0-2) and 2 (0-2), p <0.589]. The hospital stay was 7.5 IQR (3-15) and 5 IQR (2-14) days, respectively, without reaching a significant difference (p <0.352). Mortality secondary to UGB was 2 versus 0, p = 0.546. Mortality from a cause other than UGB was observed in 11 patients, 2 from decompensation of underlying chronic diseases and 9 from acute respiratory failure secondary to SARS-CoV-2 pneumonia. Overall mortality at 30 days was 25.4% (13 patients) (Table 3).
Table 3 Results according to the type of treatment received from patients with SARS-CoV-2 infection and UGB.
| Variables | Medical Treatment Only n=34 | Medical Treatment Plus Endoscopy n= 17 | p |
| Rebleed, n (%) | 3 (8.82 %) | 0 (0.0 %) | 0.542┼ |
| Re-admittance due to UGB, n (%) | 3 (8.82 %) | 0 (0.0 %) | 0.542┼ |
| Hospital stay, median (IRQ) | 7.5 (3-15) | 5 (2-14) | 0.352* |
| Number of red blood cell transfusions, median (IRQ) | 2 (0-2) | 2 (0-2) | 0.589* |
| Mortality secondary to UGB, n (%) | 2 (5.88 %) | 0 (0.0 %) | 0.546┼ |
*Mann-Whitney U; ┼ Fisher’s exact test
Discussion
Peru is one of the countries most affected by the current pandemic due to the large number of simultaneous infections and a significant number of health personnel infected. However, gastrointestinal bleeding continues to be a frequent reason for admission to our hospital.
There is growing evidence that very early UDE does not offer much benefit in terms of reducing mortality. A study by Laursen et al., with a cohort of 12,601 patients with UGB, found no significant association between endoscopy time and inpatient mortality in hemodynamically stable patients with no comorbidities. Only one benefit was seen on early endoscopy (between 6 and 24 hours) in those with hemodynamic instability (21. Lau et al. in a study of 516 patients with UGB described that there was no significant difference in mortality at 30 days between patients with GBS >12 and those who underwent an urgent endoscopy (before 6 hours) versus an early one (between 6 and 24 hours) (12.
There is currently a small number of reports assessing the impact of the SARS-CoV-2 pandemic on the management of UGB.
In our study, the median age was 70 years and 58.8% were male, data similar to those reported by Martin et al. in a study of 31 patients with UGB and SARS-CoV-2 infection in which the mean age was 68.7 years and 66% were male (22. Shalimar et al. reported that of 1342 patients with SARS-CoV-2 infection, 24 patients (1.8%) had UGB and 70.8% were male (14; however, the mean age was 45 years, significantly lower than that of our study.
In our series, the most frequent comorbidities were high blood pressure and liver cirrhosis, which coincides with other reports. In a study in Italy, Mauro et al., of 4871 patients with SARS-CoV-2 infection, 23 (0.47%) had UGB; of these, the most frequent comorbidity was high blood pressure (70%) (23. Martin et al. also found high blood pressure (66%) to be the most prevalent comorbidity (22. On the other hand, Shalimar et al. reported that almost all of their patients were cirrhotic (91%) (14. The large percentage of patients in our study with chronic liver disease could be because our hospital is a national referral center for patients with complex comorbidities.
We must highlight that 56.9% of our patients had an elevated risk of needing endoscopic therapy and death when presenting a GBS ≥ 12. Likewise, 39.2% had pneumonia and 21.6% required support with supplemental oxygen. Mauro et al. reported that of 23 patients with UGB, 82% required supplemental oxygen support, with a median IQR GBS of 13 points (10-16) (23. Martin et al. reported, in 31 patients with UGB, that 39% required intubation or admission to the intensive care unit (ICU), with a mean GBS of 10 points (22.
Some case reports have been published on the management of patients with UGB and SARS-CoV-2 infection. Cavaliere et al. reported 6 patients, 5 of them in need of oxygen support and 1 with assisted ventilation. All responded to conservative medical management and did not have a UDE (15. Gadiparthi et al. reported 3 cases, 2 of them were UGB with supplemental oxygen requirements and GBS ≥ 7, in which the clinical picture was resolved only with conservative medical treatment; they recommend considering this management if endoscopic intervention is unlikely to improve outcome (very stable or critical patients) (24. Barrett et al. reported 4 cases of patients with UGB, all were managed with conservative medical treatment and 1 underwent endoscopy without the need for therapeutic intervention (25. All agree that conservative medical treatment is a valid management option (15,24,25 under the premise that the risk of endoscopic treatment could outweigh the benefit, especially in the current context, where safeguarding health personnel is fundamental 24.
Kim et al., in a retrospective cohort study conducted in New York, analyzed 211 patients admitted for UGB (42% of them during the pandemic) and reported that only 28% underwent the procedure (26. In our series, 33.3% of our patients were prescribed UDE, with a time interval until the procedure was performed >24 hours in 94.1%. These findings are similar to those of Mauro et al., who performed UDE on 78.2% of their patients with an average time >24 hours (2-60 hours) (23. Martin et al. reported an average delay of 2.4 ± 2 days in 32% of patients who underwent the procedure 22. These variable results are probably due to the latter being a new disease with a high potential for contagion for health personnel, the indication would be limited to the only necessary selected cases, and the delay would be related to the preparation of all the necessary logistics recommended for the performance of the procedure (27.
Peptic ulcer disease and esophagogastric varices were the most frequent endoscopic findings, similar to what was found in recently published studies of UGB and SARS-CoV-2 infection. On the other hand, endoscopic therapeutic intervention was required in only one third of our patients, results similar to those reported by these authors (38%-40%) (22,23. This low therapeutic percentage is probably due to the time interval from the event to the procedure, where medical treatment also plays an important role in management.
During the evolution, 3 of our patients previously treated with medical management presented rebleeding. All had an endoscopy; 2 patients had peptic ulcer disease (received double endoscopic therapy) and 1 had advanced gastric cancer. Mauro et al. also observed rebleeding in 3 patients, of whom 2 were considered suitable for radiological embolization and 1 received endoscopic therapy (23. On the other hand, Martin et al. identified rebleeding in 4 patients during the same hospitalization, although none required endoscopic intervention; while Shalimar et al. (22 reported two rebleedings, one received argon plasma endoscopic therapy for having antral gastric vascular ectasia (GAVE) and the other had spontaneous resolution of bleeding and did not require UDE 14. On the other hand, those patients in our series who were readmitted for UGB and had received only medical treatment underwent an UDE in the new hospital admission, in which esophageal varices with stigmas of recent bleeding were found in 2 patients and peptic ulcer disease in 1; all of them underwent a therapeutic endoscopy with good evolution.
Kim et al. observed 2.86 times more probability of receiving a globular packet transfusion in patients with UGB hospital admission during the pandemic (p = 0.013), as well as 2.5 times more likely to stay in hospital ≥ 5 days (p = 0.004) compared to the pre-pandemic period (26. In turn, Shalimar et al. reported an average hospital stay of 7.5 (5-12.7) days, similar to our finding (14. In our study, when comparing between those patients who received conservative medical treatment and medical treatment plus UDE, we observed that despite there being a numerical difference between the variables studied (rebleeding, readmission due to UGB, hospital stay, number of red blood cell transfusions, mortality due to UGB), these did not reach statistical significance. Regarding this, Martin et al. found no difference on the need for transfusion of globular packages (3.75 ± 3.40 versus 3.33 ± 2.19; p = 0.777) among the medical treatment group plus UDE versus conservative medical treatment (22; similarly, another study noted similar mortality (3 vs. 2 patients) and rebleeding (2 vs. 1 patient), between both groups23.
Mortality secondary to UGB in our series occurred in 2 patients and was due to hypovolemic shock due to massive hemorrhage since admission. Despite attempting hemodynamic stabilization of patients to perform the procedure, they died within 6 hours of admission. Similar studies did not report secondary mortality to UGB (22-26, probably due to sample sizes with fewer cases, which would dismiss the occurrence of fatal outcomes.
Overall mortality at 30 days was similar to other reports (13% to 21%) (14,23 and was mainly due to respiratory involvement due to SARS-CoV-2.
Within the limitations of our study, it should be noted that it was carried out in a single center, with a small number of cases, in addition to being a retrospective study. Future studies should look at these aspects.
Conclusion
A reduction in the number of emergency UDE due to UGB was observed during the current pandemic, as well as a longer time than the standard one for its performance. Peptic ulcer disease continues as the primary etiology of UGB in patients with SARS-CoV-2 infection. More than 80% of patients who received medical treatment alone evolved favorably and only one third of the patients who underwent UDE required endoscopic therapy.











 text in
text in 



