Introduction
When clinical signs and symptoms of a disease appear, there is little room for curative intervention and reversing the disease is unlikely. To date, global preventive medicine has focused on primary prevention, whose overall goals are to prevent the disease by promoting healthy habits and controlling risk factors (1. Another approach, perhaps more useful in specific pathologies such as cancer, is to actively identify individuals at high risk of developing the disease and intervene with preventive strategies (2.
In medical and surgical work, gastric cancer (GC) is a disease that continues to pose a challenge. Despite the global decrease in its incidence, it remains a serious health issue and is commonly diagnosed in advanced stages (3.
Every time a patient is diagnosed with advanced GC, the odds of being taken to surgery with curative intent are very low, just like their survival in 5 years. In this large group of patients, there are those who never attended consultation, those who consulted for non-specific gastrointestinal symptoms and received a clinical diagnosis of “gastritis” followed by the typical symptomatic treatment; however, also curiously and sadly up to a quarter of patients newly diagnosed with GC had been taken to an endoscopic study in the previous 3 years (3.
For the understanding of a disease, it is very important to know its natural history. Dr. Pelayo-Correa described the progression from normal mucosa to GC as a step-by-step process (4, with an extremely long interval between the onset of gastritis and its malignancy. All of the above offers time and opportunity to disrupt the oncogenic cascade and constitutes a reasonable justification for the development of primary and secondary prevention strategies (5.
Not all GC follow this sequence, and we know that a much faster progression has also been described for some diffuse-type cancers, particularly in young people with genetic susceptibility (6. However, the Pelayo-Correa sequence (4 still explains most GC. We then identified a first challenge referring to the quality of routine endoscopic studies and their efficiency in identifying premalignant lesions and early cancers.
In addition, clinical decisions are made based on endoscopic and histopathological reports that, most of the time, do not actively seek premalignant conditions and are limited to taking biopsies of the antrum, forgetting the natural history of the disease and not classifying the real risk for GC or its pertinent follow-up (7. A conscious training aimed at evaluating subtle mucosal changes would most likely increase the diagnosis of early lesions.
We know that the carcinogenesis process is multifactorial and sequential, and it is well known that not all subjects with preneoplastic lesions will develop GC, but many others will. In this complex process, which we do not fully understand, multiple risk factors are also involved. One of them is any trait, characteristic or exposure of an individual increasing the likelihood of suffering a disease or injury. Identifying the risk factors involved in each step and properly managing them could help reduce the incidence of GC. At the population level, knowledge of these risk factors is essential to plan, monitor and assess plans, policies and strategies for the control of this cancer (8.
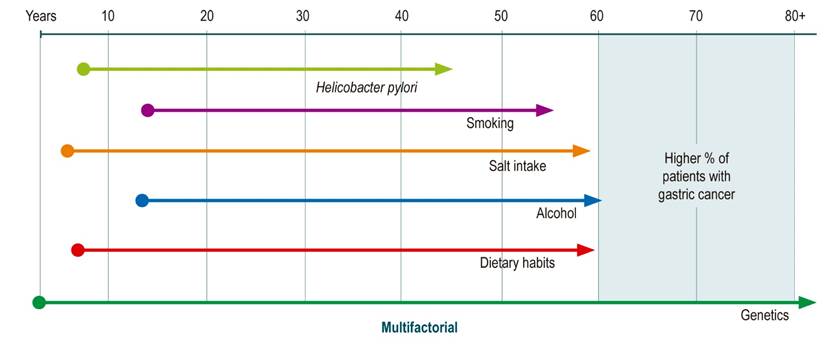
Probably the most well-known and discussed risk factor is Helicobacter pylori (HP) infection, whose eradication has reduced the incidence of GC. It is currently considered that the best option to reduce mortality from this disease is the eradication strategy in combination with endoscopic follow-up programs for high-risk individuals, which allow the adequate identification and treatment of early lesions (5. However, there are other factors directly related to lifestyle that are also involved in the development and incidence of this type of cancer and that, as stated above, have a wide time window to exert their influence (8,9 (Figure 1).
Different management guidelines for the prevention and early detection of GC focus on the identification of lesions and premalignant gastric conditions confirmed in histopathological findings, and according to their severity and extent propose follow-up. On the other hand, epidemiological risk factors do not have a defined importance when establishing high or low risk groups (10.
At this time, the question is whether an obese, smoking, alcohol-consuming patient with a GC family history should have the same follow-up interval as an athletic patient, with good eating habits and no cancer family history, but with the same endoscopic and histopathological findings.
Strategies aimed at creating a lifestyle that does not favor cancer have the potential to be massively applicable in the population and to impact people from a young age. We believe that It is necessary to develop a study tool based on risk factor identification that can be added to endoscopic and histological findings and used in clinical practice for GC risk classification (11.
Epidemiological contribution - risk factors
Different factors that increase or reduce (protective) the risk of GC have been studied and described (9. Risk factors have been classified as non-modifiable and modifiable: among the former are advanced age, male sex, ethnicity and genetic aspects; among the modifiable ones are the consumption of an unhealthy diet, smoking, alcohol intake, salt intake and HP infection. The latter, being modifiable, should be the focus of attention when designing prevention programs (9,12,13.
The challenge at this point is to identify and investigate GC risk factors with statistically significant value. There are multiple studies that describe the magnitude in which GC risk increases or decreases in relation to each factor (8,9.
Yusefi et al. (8, in a systematic review of 43 studies, identified 52 risk factors classified into: diet, lifestyle, genetic predisposition plus family history, infectious, demographic characteristics, occupational exposure, and ionizing radiation. The authors reported an association between HP infection and GC with an Odds ratio (OR) of 3 (95% confidence interval [CI] 2.42 to 3.72). 12 of the papers reviewed in this same study identified smoking as a risk factor for GC and is thus recognized by the International Agency for Research on Cancer (IARC).
The causal association between HP and GC is firmly established by epidemiological and clinical studies. GC develops only in 1% of infected patients, but at the same time more than 90% of patients with GC have or have had infection with this bacterium, so HP is the main risk factor for GC (3. This infection is one of the most common in humans and affects more than 50% of the population in the world; it can be acquired in childhood, its prevalence is directly related to age and most cases are asymptomatic (8,13. Many countries with high HP infection do not have a high incidence of GC. The interaction between HP, genetics, and diet may explain these discrepancies (14.
In a meta-analysis (15 of 19 cohort or case-control studies, which included 2491 patients and 3959 controls, the OR for GC in patients with serologically diagnosed PH infection was reported as 1.92 (95% CI 1.32 to 2.78).
HP acts indirectly on gastric epithelial cells causing inflammation directly on them, modulating their function through agents of the bacterium such as CagA9,13.
A UK case-control study estimated that 22% of GC cases are related to smoking and 32% are related to HP (8,9.
The consumption of alcoholic beverages is another risk factor that has been evaluated with non-homogeneous results. This finding was reported in 7 of the studies included in the report by Yusefi et al. (8. In their meta-analysis, Ma et al. (16 found that alcohol consumption may increase the risk of GC (OR of 1.39). Tramacere et al. (17 found an association between GC and alcohol consumption only in heavy drinkers (>4 drinks x day). Other studies showed that people who consume alcohol (>50 g a day) have a 24% higher risk for GC compared to others who do not consume or consume less (8,9.
Nemati et al. (18 reported that low intake of fruits and vegetables was a risk factor. In contrast, high intake of fruits and vegetables is a protective factor (9 studies). The protective role of fruit and vegetable consumption was also observed in a Dutch study with the consumption of 156 g x day (7,8. On the other hand, a cohort study with vegetarians found that the risk was low compared to those who eat meat (8. Excessive consumption of red meats, smoked food, processed meats, and salted meats was also reported as a risk factor for GC in 8 studies (8.
IARC has ranked salt intake as one of the most important risk factors for GC due to findings reported by multiple studies (13. It is estimated that 24% of cases in the UK were associated with the intake of more than 6 g of salt per day. The results of the meta-analysis by D’Elia et al. (19 reveal that GC risk was higher in people with high salt intake than in those with low intake. The systematic review published by Ge et al. (20 reports that salt intake increases the GC risk by 22%8,9.
Gómez et al. (21, in a prospective analytical observation paper, compared food consumption and related habits in two groups: one with GC and one with duodenal ulcer. The authors found significant associations between dietary intake patterns and GC, for instance, high salt intake and consumption of smoked foods. While HP is an established risk factor, it is also clear that it is not a sufficient cause for the development of GC; in this sense, salt intake may have a synergistic role with infection in the causality of this tumor. It was also found that familial cancer, as a GC risk factor, had an overall OR of 4.2, which is consistent with other studies.
Identifying risk factors can provide insight into the etiology of the disease and may suggest prevention strategies. This Latin American meta-analysis identified that the increased risk for GC was associated with smoking, alcohol consumption, high consumption of red meat and processed meats, high salt intake, and a decreased risk with higher education levels and high consumption of fruits and vegetables (22.
The same papers of Yusefi et al. (8 and Karimi et al.13 show evidence of the effect of family history and heredity on the incidence of GC. They find that having a family history of GC is a risk factor for developing GC. Likewise, Yaghoobi et al. (23 reported that the risk of GC was 2 to 10 times higher in people with a family history of GC.
Socioeconomic factors were reported as relevant in 10 studies. The higher prevalence of HP infection is known in lower socioeconomic strata, populations or human groups with low education levels and less access to basic health facilities, as well as in developing countries (8,9,13.
Nishimoto et al. (24, in a study in Iran, found that the higher incidence of GC was associated with low annual economic income, lower annual food expenditure, lower fruit and vegetable consumption, and higher unemployment figures.
Another meta-analysis12 explored the association between GC and 14 potentially modifiable risk factors. 231 studies were included, with a total population of 33,831,063 patients, and risk was expressed in OR values. The factors significantly associated with the GC risk are shown in Table 1.
Table 1 Risk factors for GC
| Risk factor | OR |
|---|---|
| Helicobacter pylori | 2.56 (2.18-3.0) |
| Smoking | 1.61 (1.49-1.75) |
| Smoking history | 1.43 (1.29-1.59) |
| Alcohol consumption | 1.19 (1.10-1.29) |
| Alcohol intake history | 1.73 (1.17-2.56) |
| Salty food | 1.28 (1.09-1.51) |
| Salt | 3.78 (1.74-5.44) |
Adapted from: Poorolajal J, et al. Epidemiology and Health. 2020;42: e2020004.
In summary, HP infection, smoking, high alcohol consumption and salt intake are found to significantly increase the risk of GC (7,12.
The origin of geographical areas with high GC incidence, pernicious anemia, history of partial gastric resection, Epstein-Barr virus (EBV) infection, blood type (type A), occupational exposure to toxic substances such as fumigation agrochemicals, cement work, rubber manufacturing, and coal and chromium mining have also been mentioned as risk factors. The latter in relation to the dose, duration and time of exposure (8,9,14.
Returning to several of the concepts mentioned, we can say that GC is a devastating and potentially lethal disease, with a long natural history and in which multiple factors are involved, without us knowing a single or ultimate cause of this cancer so far. Our focus has been largely on HP infection as the most important risk factor, as it can be related to up to 90% of cases; however, only 1% of infected individuals will develop GC, so HP is necessary, but not sufficient (7.
All this leads us to think that other risk factors may be as important as HP infection, and most likely play their role by modulating the tissue impact of chronic infection on the gastric mucosa (11. In that host-guest interaction, unique and different for each subject, which opens the way to chronic gastritis, peptic ulcer disease or cancer, may be at least one of the missing pieces of our puzzle.
Risk Prediction Models
Multiple authors have developed risk prediction models based on risk factors. Buckland et al. (25 evaluated a cohort to measure the impact of lifestyle factors on the occurrence of GC; Tata et al.26 developed a diagnostic system with a score based on clinical variables.
However, these models do not involve the presence or absence of HP infection or the presence of lesions or premalignant conditions such as atrophic gastritis (AG) (11. On the contrary, other papers neglect the clinical and lifestyle aspects to focus only on the evaluation of the first two factors mentioned and combine the detection of antibodies against HP and serum levels of pepsinogen I and II, which can be considered as the biochemical expression of the histological phenomenon that is AG. Both types of pepsinogen are produced in the mucosa of the fundus and gastric corpus, and type II mainly in the antrum; as gastric atrophy progresses from its initial territory (which is usually the antrum) and progressively compromises the body, pepsinogen II production increases while type I production decreases, and the I/II ratio decreases. In that same sense, the atrophic mucosa produces less acid and serum gastrin rises (27.
When the measurement of HP antibody levels and serum pepsinogen I and II levels is used, only two risk factors are involved. Since the GC is multifactorial, we believe that the risk prediction tool should also be multifactorial.
Iida et al. (28 conducted a study in the city of Hisayama (Japan) with the aim of developing and evaluating a tool for measuring individual GC risk. Asymptomatic residents over the age of 40 were chosen and followed from 1988 to 2002 as the first study cohort, which had 2444 individuals, with a 14-year follow-up, and from 2002 to 2007 the validation cohort, which had 3204 subjects, with a 5-year follow-up. During follow-up, 90 GC cases were found in the study cohort and 35 cases in the validation cohort. With the study cohort, they developed the risk prediction model using significant risk factors; subsequently, this tool was validated internally. In the univariate analysis, age, sex, combination of HP antibodies and serum pepsinogen I and II values, glycosylated hemoglobin level (HbA1c), smoking, and alcohol intake were significantly associated with the incidence of GC. In the multivariate analysis, with the exception of alcohol consumption, all factors were still significantly associated with the incidence of GC. A score was assigned to each risk factor. The importance of each factor (score assigned) to predict GC risk was determined with the evaluation tool based on the coefficients of a Cox proportional risk model in the study cohort.
In this paper, the total score was calculated as the sum of the risk scores for the different factors. The cut-off point for GC risk prediction was 8. In subjects with a score of 8 or higher, the risk for developing GC was increased by 5.3 times (95% CI 3.4 to 8.2) compared to those who scored 7 or lower. The recommendation was to study with upper digestive endoscopy (UDE) those who had 8 or more points (Table 2).
Table 2 Risk Prediction Models for GC
| Risk factor | Authors | ||||
|---|---|---|---|---|---|
| Variable | Range | Iida Score | Cai Score | Chavart Score (Female) | Chavart Score (Male) |
| Age (years) | 40-44 | 0 | 0 | 0 | 1 |
| 45-49 | 0 | 0 | 1 | 3 | |
| 50-54 | 2 | 4 | 2 | 4 | |
| 55-59 | 2 | 4 | 3 | 6 | |
| 60-64 | 3 | 6 | 4 | 8 | |
| 65-69 | 3 | 6 | 5 | 10 | |
| >70 | 2 | 9 | |||
| Sex | Male | 3 | 4 | ||
| Female | 0 | 0 | |||
| HP | HP (-) AG (-) | 0 | 0 | 0 | 0 |
| AG | HP (+) AG (-) | 2 | 1 | 8 | 8 |
| HP (+) AG (+) | 5 | 3 | 11 | 11 | |
| HP (-) AG (+) | 5 | 3 | 11 | 11 | |
| Smoking | Yes | 1 | 1 | ||
| No | 0 | ||||
| Salt | Yes | 2 | 1 | 1 | |
| No | 0 | 0 | 0 | ||
| Score | > 8 | 0-11: low 12-16: medium 17-25: high | > 20 | > 20 | |
Adapted from: Charvat H, et al. Int J Cancer. 2016;138 (2):320-31; Iida M, et al. Gastric Cancer. 2018;21 (3):383-90; Cai Q, et al. Gut. 2019; 68:1576-87.
Eom et al. (29 also developed a prediction model using a follow-up and a validation cohort. The model included age, body mass index (BMI), family history of cancer, salt intake, alcohol consumption and smoking. This prediction model showed good accuracy in both the study and validation cohorts. This paper proposed that the general population with risk factors is referred to a regular medical check-up. HP infection was neglected.
Several studies conducted in Asia and, particularly, in China mention the high-risk population for GC as subjects living in areas of high incidence for more than three years, with a family history of GC and risk factors such as high salt intake, smoking or heavy alcohol intake. Because of such a large population, UDE is not easy to apply massively and it is expensive; for this reason, they have established a stratification tool as pre-endoscopy screening, which is predominantly applied in those population groups globally considered high risk in order to identify individuals at higher actual risk of developing GC (30.
Cai et al. (30 published a multicenter study of 115 hospitals (from June 2015 to March 2017) with high-risk population (incidence of more than 30 per 100,000 inhabitants) due to factors such as age, sex, family history of cancer, high salt intake diet, AG, HP infection, geographical origin, smoking and alcohol intake. Using a study cohort and a validation cohort, they developed a tool to predict GC risk. A questionnaire recorded the following data: age, sex, body weight, BMI, smoking defined as more than one cigarette a day for one year (if the answer was yes, the number of cigarettes and duration were recorded), alcohol consumption (any type of alcohol once a week during the last year; if the answer is yes, alcohol class and frequency of consumption), dietary habits (salt consumption of more than 10 g/day), consumption of pickles, fried food, smoked foods, red meat, green vegetables, fresh fruits (frequent consumption considered 3 or more per week) and family history of GC.
In all patients, the serum levels of pepsinogen I and II, gastrin and immunoglobulin G (IgG) antibodies against HP was determined. Finally, the chosen patients were taken to an endoscopy with white light and underwent biopsies of the antrum, incisura and corpus, as well as endoscopic examination with improved NBI (Narrow Band Imaging) images.
The four procedures: questionnaire, laboratories, gastroscopy and pathology were performed by the researchers. Two-thirds of participants were randomized for the study cohort and one-third for the validation cohort.
In the study cohort (9838 participants) 267 subjects with GC (2.7%) were found and 138 (2.7%) in the validation cohort (5091 participants). In the univariate analysis, 17 variables were found as potentially associated with GC (p <0.25). Those with the most statistical power were age, sex, pepsinogen I/II ratio, gastrin and antibodies against HP, consumption of salted food and fried foods (all with a value of p <0.05).
Scores ranged from 0 to 25. Risk groups were established in the low, medium and high categories when scores ranged from 0 to 11, 12 to 16 and 17 to 25, respectively. 66.7% were considered low risk, 27.6% medium risk and 5.7% high risk. The prevalence of GC was 1.2%, 4.4% and 12.3%, respectively (p <0.001) (Table 2).
70.8% of patients with advanced gastric cancer (AGC) and 70.3% of patients with early gastric cancer (EGC) were diagnosed when endoscopy was performed in the groups considered as medium and high risk by the aforementioned score.
This group of factors (age, sex, pepsinogen I/II ratio, gastrin, serological status versus HP, salted food consumption and fried food) is interesting, because all have been identified as independent predictors of risk for GC and the results are consistent along with other studies.
Another paper by Chavart et al. (11 developed a prediction model to estimate the probability of occurrence of GC at 10 years based on a cohort of 19,028 individuals. To do so, they combined demographic and clinical variables (age, sex, smoking, salt consumption and family history) with IgG antibody status against HP and serum levels of pepsinogen I and II.
Consistent with previous studies, the authors reported that HP infection and AG were significant risk factors for the occurrence of GC. In this study, no difference in risk was found between categories C and D (of the ABC method when serology for HP is related to pepsinogen I and II levels). They also found an important weight for age, especially in men, smoking and salt intake as independent predictors of the GC occurrence. The probability of GC at 10 years was from 0.04% to 14.87% for men and from 0.03% to 4.91% for women (11 (Table 2).
Age had an important effect on men, increasing more than 5 times the risk at age 60 and more than 10 times over age 70 compared to men at age 40 (1.58% and 3.56% vs. 0.31%) (11.
The score ranged from 0 to 24. Individuals with a score of 10 or less had a cumulative probability of GC at age 10 of less than 0.4%, while this probability is >5% for individuals with a score of 20 or >20.
All of these studies (11,28,30 address the problem of GC risk prediction using tools involving clinical, lifestyle, and serological factors (which may express the functionality of the gastric mucosa), in order to identify individuals who may benefit most from the UDE.
A meta-analysis (27 was performed to evaluate the prediction of GC development by measurement of serum pepsinogen levels, HP antibody test, and a risk prediction model based on these two tests. This model is categorized into 4 groups: low risk A (Ac HP - and PG -), moderate risk B (Ac HP + and PG -) and high risk C (Ac HP + and PG +) and D (Ac HP - and PG +). This study included 9 prospective cohorts from Eastern countries with a total of 33,741 asymptomatic participants in GC screening programs. The mean age of the participants ranged from 45 to 57, while follow-up ranged from 3.9 to 14 years of age. This study found that adults with PG+ had about 4 times the risk of developing GC than those with negative tests (27,31.
As mentioned above, the low serum levels of pepsinogen I and the pepsinogen I/II ratio reflect the severity of gastric atrophy. These data in combination with the presence of antibodies against HP and gastrin have been used to identify individuals at high risk for GC (28.
Japanese early diagnosis guidelines for GC recommend the use of endoscopy or upper gastrointestinal series with contrast medium for opportunity screening, emphasizing that the former is more sensitive than the latter. Currently, serological studies of pepsinogen and antibodies are not recommended as screening in this same guideline due to the lack of evidence in reducing GC mortality (32.
The MAPS guideline recommends endoscopy to be considered in individuals over the age of 50 with multiple risk factors (men, smokers, pernicious anemia), and particularly for those with first-degree relatives suffering from GC (10. In this same guideline, the authors consider that there is not enough evidence to support the use of serological studies of pepsinogen and HP antibodies in screening programs, and even less in areas of low GC incidence (10.
Endoscopic input
Compared to other risk factors, AG and intestinal metaplasia (IM) exponentially increase the risk of GC. For this reason, individuals with these findings can be considered high-risk (7.
Chronic Atrophic Gastritis
The hypothesis stating that GC develops through a cascade of precursor lesions after HP infection is well known. A Dutch study (33 showed that the risk of GC increases with each step of this cascade according to the severity of premalignant gastric lesions. Annual incidence of GC at 5 years after diagnosis: 0.1% for AG, 0.25% for IM, 0.6% for moderate dysplasia, and 6% for severe dysplasia. On the other hand, the risk of GC in individuals with AG varies according to their severity; in addition, a high adjusted rate ratio (RR) of GC was reported for patients with severe body atrophy, of 5.76, compared to patients who had little or no atrophy (34.
Intestinal Metaplasia
In the Dutch study, the annual incidence of GC at 5 years of IM diagnosis is 0.25%. Another epidemiological study (35 suggests that patients with IM have 10 times the risk of developing GC. In a Chinese study (36 conducted in a high GC risk area, residents with precancerous lesions were followed for 5 years, and the OR for GC in subjects with IM was from 17.1 to 29.3.
The risk of GC also depends on the IM extent and phenotype. Complete IM is referred to when the mucosa of the stomach histologically resembles the mucosa of the small intestine. On the other hand, incomplete IM is referred to when the epithelium of the gastric mucosa resembles the colonic mucosa. IM can be classified as type I, II and III according to the mucin phenotype. Complete type I IM (only expresses sialomucins) and incomplete type III (expresses sulfomucins). Incomplete type II IM is a hybrid that expresses a mixture of gastric and intestinal mucins. Several reports talk about the risk of GC being higher in type III IM; however, other studies have reported otherwise. It seems that the IM phenotype is not decisive in the prediction or development of GC; however, other studies have shown that the prevalence of incomplete IM was significantly higher in patients with GC than in other gastric lesions. In addition, it is reported that over a half of the studies find a statistical relationship between IM and subsequent development of GC (relative risk for GC 4 to 11 times higher with the presence of incomplete IM compared to complete). The authors conclude that phenotype subclassification is useful as a predictor of GC risk (7.
IM tends to appear first in the angularis incisura and extends to the neighboring mucosa in both directions towards the antrum and the corpus. A study reviewing IM distribution models shows that IM extension is significantly associated with an increased risk of GC. In conclusion, it has been proposed that IM distribution, rather than the subtype, may be of greater risk predictive value for GC (7.
High-risk groups
The risk factors identified for GC differ in their OR. Compared to other factors, AG and IM markedly increase the risk of GC. A key question in the treatment of these high-risk patients is how to select a higher-risk group among subjects with AG and IM (7.
There are several ways to identify high-risk individuals for the development of GC, such as non-invasive methods (pepsinogen and HP antibodies), endoscopy, and histology. A histological examination is necessary for the diagnosis of a precancerous gastric lesion. Endoscopy, especially with modern technology (improved imaging), has acceptable accuracy in diagnosing these lesions. Currently, the main approach in Western countries is endoscopy with histology, while in Eastern countries with high GC prevalence, only endoscopy is used (31.
Preendoscopic risk measurement based on clinical and demographic characteristics such as age, ethnicity, sex, smoking, and HP infection is useful for identifying individuals with high pretest probability for a possible cost-effective approach, especially in low- and intermediate-risk countries (31.
Histological stratification of GC risk involves measuring the extent and severity of AG and IM (4. In Asian countries, the extent of the atrophy is often measured endoscopically through mucosal observation, using the Kimura-Takemoto classification, as well as the presence of metaplasia and its location. In the West it is preferred to use the histological classification performed in systematic biopsies including corpus and antrum, and that are reported by the pathologist using the OLGA (Operative Link on Gastritis Assessment) or OLGIM system (Operative Link on Gastric Intestinal Metaplasia Assessment)5. Both approaches require endoscopy performed on a clean stomach and with sufficient observation time. In one case, site-specific biopsies are required; in the other, training in endoscopic classification (4.
OLGA is based on a combination of the atrophy score with samples obtained from the antral and oxyntic mucosa (Sydney protocol). The combined score values express the “mucosal state” and represent a message of the severity of the atrophy, which is parallel to GC risk (OLGA states O, I and II: low risk, OLGA III and IV: high risk of GC). Prospective studies have supported the prognostic value of AG staging and its usefulness in clinical practice (2,37.
The exact mechanism, contribution of environmental risk factors and host genetic susceptibility are all involved in the progression of gastric carcinogenesis and have not yet been fully clarified. 20% of patients with GC may have a family history of GC. The risk is 2 times higher in men than in women and usually occurs between the ages of 60 and 80. 18% of GC is attributed to smoking and when the individual consumes alcohol in addition to smoking, the risk of GC increases up to 5 times (38.
No more than 30% of patients with GC survive more than 5 years after diagnosis, because most are diagnosed in advanced stages (5. However, many patients consult at some point prior to the GC onset; most even go to an endoscopic study, but they rarely focus taking into account the risk factors associated with their particular history, which, added to very good endoscopic and histopathological information, would allow us not only to define their follow-up but to specify prevention programs in relation to their disease.
The incorporation of risk factors within prediction models could allow a more appropriate selection of patients at risk of GC for screening. A large number of prediction models have been developed, however, none of them is perfect; each has its limitations and requires validation in large populations before its clinical implementation can be recommended (39.
In geographic regions with high disease burden for GC, these work tools, which include risk factor assessment and endoscopic information, may have a place as monitoring and prevention programs (39.
Detection of premalignant gastric lesions usually requires endoscopy plus biopsies. However, there are demographic and clinical characteristics that are useful for predicting the presence of these lesions including place of origin, ethnicity, sex, age, family history of GC, HP infection, and serum pepsinogen levels (31.
One study found that individuals who immigrated to the United States from high-risk areas (Chinese and Latin American) have a higher risk of developing GC compared to natives, as do individuals infected with HP and with first-degree relatives with GC. Male sex, smoking, and older age are also associated with an increased risk of developing GC (31.
Risk measurement prior to endoscopy is possible through an understanding of the role of risk factors and is useful for selecting individuals with high pre-test probability, especially in regions with low and intermediate risk (11.
GC is a public health problem with more than one million new cases diagnosed each year around the world. Despite the decline in its incidence and mortality in the last 5 years, GC remains the third leading cause of cancer-related death in the world (38. Modifying diet and lifestyles is the most rational form of GC prevention. Fruit consumption, avoiding smoking and alcohol intake, maintaining an adequate body weight, physical activity and not excessive intake of salt or smoked foods help decrease the risk of this disease (14.
Conclusion
Primary and secondary prevention strategies such as dietary modifications and screening programs are very important measures to reduce the risk of GC. However, risk factors are not taken into account when establishing follow-up strategies. These factors, just like HP infection, may be at work for many years or even decades and we only care about eradicating HP. It is likely that the development of GC derives from the interrelation of all these factors, both modifiable and non-modifiable, and if we give them context within the clinical contribution, we could have a greater impact on the disease.
Therefore, evaluating the real weight of risk factors would help to define high and low risk groups, to determine the relevance of the endoscopic procedure and its frequency, as well as the different prevention strategies.
Identifying risk factors can provide insight into the etiology of the disease and may suggest prevention strategies. Knowledge of epidemiology, natural history, and risk factors should be essential in the practice of the gastroenterologist and surgeon to tailor decisions regarding risk stratification, screening, and prevention.
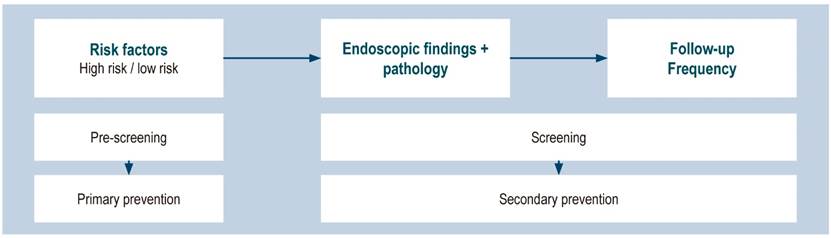
It is therefore necessary to develop a study tool based on risk factor identification for each individual, which can be added to endoscopic and histological findings and used in clinical practice for GC risk classification (11(Figure 2).
Every day we find more literature on chemotherapy tailored to each tumor, but little in relation to prevention and follow-up strategies tailored to each patient or each population group. We would like to foster a tailor-made risk assessment medical culture so that we actively research all the risk factors mentioned above, and consider them in clinical decisions in an active and permanent manner.











 text in
text in