Introduction
Small bowel tumors are rare and represent only 3 % -6 % of gastrointestinal neoplasms. The incidence in Spain is low, from 0.4 to 1 per 100,000 inhabitants/year: 2/3 are malignant, and only 1/3 are benign1. In the United States, the estimated annual incidence is 0.3 to 2 cases per 100,000 people, representing 0.4 % of gastrointestinal cancers and 0.2 % of deaths associated with malignancy, with prevalence rates higher in the black population than in the white; this has increased recently. It is estimated for less than 1 % in Panama and falls within the causes of malignancy in general2. In Mexico, a histopathological registry of malignant neoplasias in 2006 registered 106,238 malignant tumors, where only 0.32 % were located in the small intestine3. In Ecuador, there is no small bowel tumor data.
Not much is known about its natural history; the highest incidence occurs after 60-70 years. According to data from the Surveillance, Epidemiology, and End Results (SEER) Program from 1992-2006, the incidence rate for men and women is 1.45 and 1.00, respectively. In the black population, its presentation is not specific, asymptomatic in the early stages for long periods, and symptoms develop as the disease progresses. Therefore, there is a delay in diagnosis for approximately 6 to 8 months. Surgical treatment is limited to the size of the lesion and the degree of invasion4.
Case presentation
This is a 46-year-old female patient of mixed race, with no significant pathological history and surgical history of hysterectomy 8 years ago due to uterine fibroids, who presents a clinical picture of onset of 3 months of evolution due to abdominal pain widespread. On the visual analog scale (VAS), the pain is 6/10 points, accompanied by vomiting of food type 2 to 3 times a day and weight loss of approximately 15 kg. The patient denies the presence of thermal rise (fever) and gastrointestinal bleeding. She refers to having seen a private doctor, where she was evaluated for nonspecific abdominal pain. They performed an abdominal ultrasound in which they found a mass of approximately 78 x 68 mm between the epigastrium and the left hypochondrium. For this reason, the patient goes to this health clinic and is assessed in the outpatient services with admission tests, which report hemoglobin of 10.20 mg/dL, hematocrit of 33.10 %, leukocytes 6300/mm3, platelets 631,000, negative tumor markers, positive occult blood, albumin 2 mg/dL, total proteins 3.90 mg/dL. Based on this, endoscopic studies were indicated in search of a neoplastic lesion to be ruled out. When performing upper gastrointestinal endoscopy, the presence of extrinsic compression at the level of the distal body with erythematous gastropathy in the antrum was visualized, while the ileocolonoscopy was normal (Figure 1).
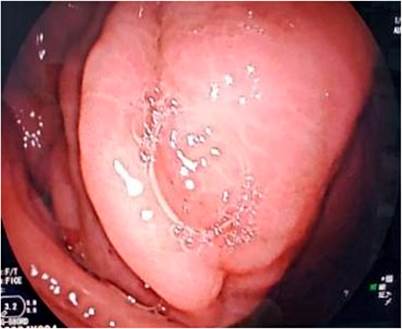
Figure 1 Video of upper gastrointestinal endoscopy (UGIE) where extrinsic compression is observed at the level of the distal body.
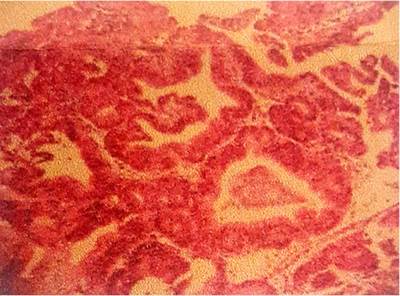
In addition, an abdominal and pelvic double contrast computed tomography (CT) is performed, where a circumferential mass is presented at the level of the proximal jejunum, which allows the passage of contrast medium and respects the cleavage planes in stage I (Figures 2 and 3). A procedure is performed with an endoscopic capsule, which is retained at the level of the jejunum, which allows us to observe a raised exophytic lesion in the proximal jejunum (Figure 4). Then, an antegrade enteroscopy is performed at the level of the proximal jejunum, where a raised, exophytic, ulcerated, friable lesion is observed, which occupies more than 90 % of the circumference but allows the passage of the equipment to the distal region (Figures 5 and 6). From there, the retained capsule is visualized and removed with mesh forceps. Finally, a biopsy is taken, which reports a tubulopapillary moderately differentiated adenocarcinoma of the proximal jejunum in the histopathological record (Figure 7).
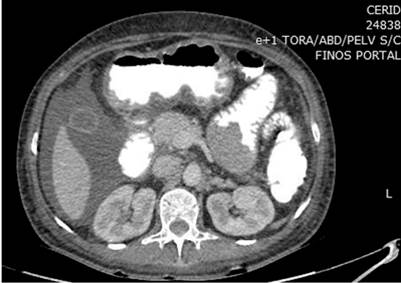
Figure 2 Cross-sectional abdominal contrast computed tomography (CT) shows a circumferential mass at the level of the proximal jejunum.
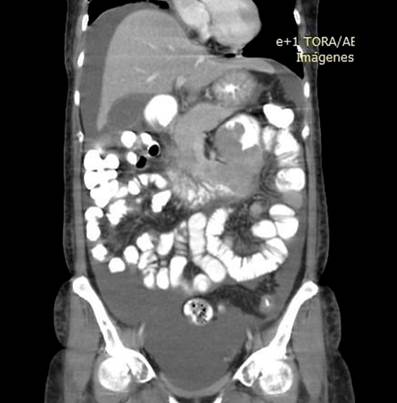
Figure 3 Coronal section abdominal contrast computed tomography shows a circumferential mass at the level of the proximal jejunum.
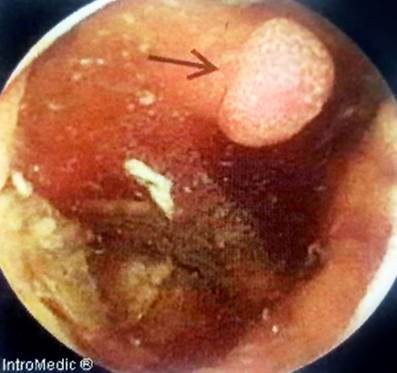
Figure 4 Video capsule endoscopy (VCE) that visualizes the presence of a raised exophytic lesion in the jejunum.
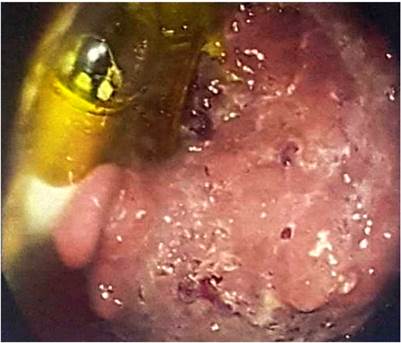
Figure 5 Enteroscopy in which a raised, exophytic, ulcerated, friable mass is observed, occupying more than 90 % of the circumference and showing the presence of the retained endoscopic capsule.
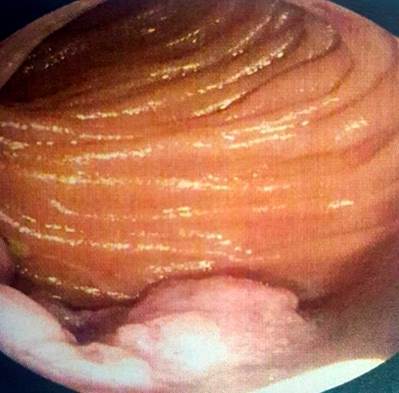
Figure 6 Enteroscopy shows the elevated exophytic lesion, which occupies part of the intestinal circumference of the jejunum.
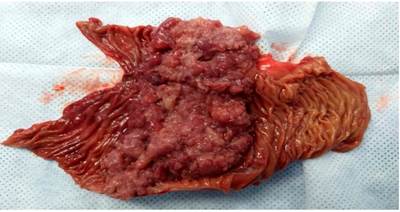
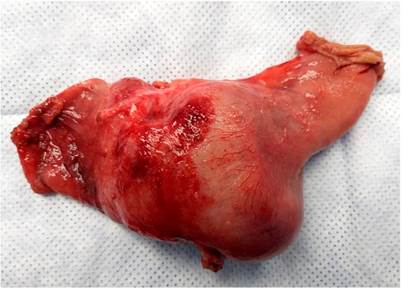
The surgery department evaluates the patient, where an exploratory laparotomy is performed with an intestinal tumor resection of the jejunum of approximately 15 x 10 cm (Figures 8 and 9) plus end-to-side anastomosis between the duodenum and the jejunum, without signs of peritoneal carcinomatosis. The histopathological report of the macrobiopsy is tumor size 11.5 x 7 x 3 cm; histopathological type; moderately differentiated adenocarcinoma G2; villous adenoma, whose tumor extension invades the muscularis propria. Margins: surgical margins closest to the distal lesion at 5 mm by microscopy, surgical border furthest from the lesion-free neoplasia, radial surgical edge free of neoplasia, lymph nodes with sinusoidal hyperplasia free of neoplasia. This resulted in R 0 margins free of neoplastic lesions.
During hospitalization, the patient was treated by the nutrition service with parenteral nutritional support of 35 kcal/kg/day for three days, then mixed nutrition with oral tolerance until diet progression without presenting postoperative complications and, finally, was discharged with outpatient follow-up. Currently, the patient is under multidisciplinary follow-up by gastroenterology, surgery, oncology, psychology, and nutrition, and improved prognosis with weight gain, nutritional indications, and a negative abdominal contrast computed tomography after one year.
Discussion
The small intestine comprises three parts: the duodenum, jejunum, and ileum. In total, it represents approximately 75 % of the digestive system and is about 15 to 20 feet long, that is, 6 to 8 meters. Only 6 % -25 % of gastrointestinal neoplasms occur in the small intestine. The jejunum follows the duodenum and is mainly found in the umbilical region of the abdomen5.
Five types of cancer can occur in the small intestine: adenocarcinomas, sarcomas, gastrointestinal stromal tumors (GIST), neuroendocrine tumors, and lymphomas. The most common is adenocarcinoma, representing between 30 % -40 % of those mentioned. Of the adenocarcinomas, 57 % are in the duodenum, 29 % in the jejunum, and 10 % in the ileum4,6. Furthermore, it has been mentioned that bile should be studied as a possible carcinogenic agent to explain the higher prevalence of adenocarcinomas in the duodenum7. Risk factors for malignant lesions are celiac disease, familial adenomatous polyposis, Peutz-Jeghers syndrome, Crohn’s disease, among other pathologies8.
The anatomy of the jejunum is the place where the greatest absorption of nutrients, amino acids, vitamins, and minerals occurs. This section of the intestine has valvulae conniventes, which make its wall thicker than in the ileum. Together with the villi, these valves represent why the absorptive capacity of the jejunum is high. It also contains Brunner’s glands, which are mucus-producing submucosal glands; however, they are found in greater quantities in the duodenum9. Approximately 80 % of jejunal adenocarcinomas cases have been reported to develop from 50-60 cm from the Treitz ligament10.
Jejunal adenocarcinoma can initially present with nonspecific symptoms, such as abdominal discomfort, pain, abdominal distension, or nausea that could be confused with other pathologies. Finally, the cases are presented as an obstruction in 40 % or gastrointestinal bleeding in 24 % of them4,11. It is also asymptomatic in the early stages, delaying diagnosis for approximately 6 to 8 months; however, as the disease progresses, symptoms manifest as abdominal pain, weight loss (which are the most common symptoms), gastrointestinal bleeding, vomiting, nausea, and obstruction (which are the least common). On physical examination, a palpable mass in the abdomen and signs of peritoneal irritation can rarely be found, which could be due to obstruction or perforation12.
In the data of the laboratory tests, mild anemia can be evidenced due to chronic blood loss and of unidentified etiology; given this, a neoplastic process should always be suspected. Liver function tests can reveal hyperbilirubinemia in duodenal tumors and elevated transaminases with liver metastases13.
For the diagnosis, it is essential to perform intestinal transit, abdominal ultrasound, abdominal computed tomography, tomographic enteroclysis, resonance enteroclysis, upper gastrointestinal endoscopy, antegrade enteroscopy, and capsule endoscopy. Barium intestinal transit is the standard test for intraluminal lesions or mucosal abnormalities beyond the jejunal duodenum, but it has limited sensitivity (53 %- 83 %) for small bowel cancers. Abdominal computed tomography with oral and intravenous contrast reveals the exact site and extent of the local disease, with intense peripheral enhancement by intravenous contrast and the presence of liver metastases. However, it is deficient in detecting small primary tumors with a specificity and sensitivity of 57 %; it is helpful for staging, but other imaging examinations should be used for small bowel pathologies.
For the study and investigation of these lesions, tomography enteroclysis and resonance enteroclysis are used since they are more sensitive (85 %- 95 %), with a specificity of 90 %- 96 %, and the tumor can be observed as a stenosing annular lesion of at least 3 mm in length13. Upper gastrointestinal endoscopy cannot pass beyond the Treitz ligament. Although it identifies around 93 % of duodenal tumors, the sensitivity is generally only 31 %10.
Antegrade enteroscopy can only visualize 30 % of the small intestine’s length. It can diagnose more cases of small bowel adenocarcinoma because most adenocarcinomas are found in the proximal 60 cm of the jejunum. Enteroscopy has a sensitivity of 100 % but a specificity of only 45 % in diagnosing small bowel tumors; 60 % of tumors identified are adenocarcinomas15.
The diagnostic rate for small bowel tumors is increasing due to the use of methods such as capsule endoscopy, which is the most sensitive and specific diagnostic method for small bowel disease. The capsule detects obscure gastrointestinal bleeding, with which most of these tumors are diagnosed, and 50 % to 60 % of them are malignant. This method has overcome the low sensitivity of barium studies.
Regarding treatment, the only curative option is surgical. Total resection of the lesion with a margin greater than 2 cm in length is recommended together with an extended lymphadenectomy16. The 2016 French Guidelines also recommend performing a thoracoabdominal and pelvic tomography to rule out distant metastases and a high and low endoscopy if other tumors suggest a triggering genetic pathology17. Curative resection of small bowel tumors is achieved in about 50 %; for example, a recent review by Agrawal et al. showed that curative resection for adenocarcinoma was 54.5 % of cases, with a postoperative mortality rate of 3.6 %18.
There are poor prognostic factors for patients with adenocarcinomas that include: male sex, over 55 years of age, distal metastases, poorly differentiated tumors, T4 tumors, among others. However, duodenal and/or ileal tumors have a worse prognosis compared to jejunal tumors12,19, and the prognosis of small bowel tumors is poor. The 5-year survival rate of patients depends on the stage of the tumor: stage I is 50 % to 60 %; in stage II, from 39 % to 55 %, in stage III, from 10 % to 40 %, and stage IV, from 3 % to 5 %19. Most series report a 5-year survival of 15 % to 35 % after curative surgery increases from 40 % to 65 % in most studies. A study by Halfdanarson et al with 491 patients with adenocarcinomas in the small intestine showed that the 5-year survival rate was 26 % in unresected tumors20. On the other hand, a 5-year survival rate of 40 % -65 % is reported in resected tumors, with a postoperative mortality rate of 3.6 %.
Due to the recurrence of lesions even after surgery, chemotherapy cycles are recommended as adjuvant treatment21. The 5-fluorouracil is the most widely used pharmacological agent in chemotherapy for patients who have already undergone surgery and presented extensive disease10,22.
Patient follow-up is given by imaging tests, such as positron emission tomography and double contrast computed tomography after treatment to determine whether it was effective or recurrent depending on the stage and the treatment performed24.
Conclusion
Jejunal adenocarcinoma is one of the rarest and difficult to diagnose small intestine tumors due to its nonspecific symptoms. That is why the doctor requires a high index of suspicion to carry out an early investigation and avoid a delayed diagnosis due to a poor prognosis. The survival of these patients is related to the clinical stage of the disease at the time of diagnosis. In our case, due to the suspicion of a neoplastic lesion of the small intestine, the diagnosis was made using methods, such as abdominal double contrast tomography, which revealed the exact site and extension of the tumor; the endoscopic capsule, which helped us with the type of injury and the site; and antegrade double balloon enteroscopy, which helped us with the diagnosis and taking a biopsy. Therefore, it was possible to have the patient surgically treated to improve the prognosis to 5 years.











 texto en
texto en