Introduction
Tuberculosis (TB) is a disease with high worldwide morbidity and mortality; its main manifestation is the pulmonary form. However, extrapulmonary forms occur in 10 %- 42 % of cases, of which 25 %- 50 % correspond to abdominal location1,2,3.
Peritoneal TB develops from latent pulmonary TB by hematogenous spread, direct ingestion of the bacillus, consumption of pasteurized milk, etc.2,4. The main risk groups are individuals with human immunodeficiency virus (HIV) infection, immunosuppression states, close contact with infected people, life extremes, prisoners, illicit drug users, health personnel, patients with diabetes, chronic kidney failure (CKF), alcoholic liver disease and low socioeconomic status2,5. The main symptoms are abdominal pain, fever, ascites, weight loss, abdominal tenderness, and abdominal mass. Sweet syndrome has been described as an unusual manifestation6, a rare and acute febrile neutrophilic reactive dermatosis. It is characterized by the sudden appearance of painful, shiny erythematous plaques or nodules presented in the form of papules, pustules, or vesicles. They are located on the face, neck, and upper limbs. It may be accompanied by fever or low-grade fever, migratory arthralgia, leukocytosis, neutrophilia, and elevated erythrocyte sedimentation rate (ESR). Generally, it improves with a systemic corticosteroids treatment7,8.
Regarding the clinical approach, the abdominal computed tomography (CT) offers an important finding called omental cake, which requires a differential diagnosis with peritoneal carcinomatosis9. Ascites fluid study by paracentesis in patients with peritoneal TB characteristically shows a leukocyte count > 500/uL, lactate dehydrogenase (LDH) > 90 u/L, a serum-ascites albumin gradient (SAAG) of < 1.1 g/dL and adenosine deaminase (ADA) levels > 39 IU/L2,10,11,12. The gold standard for diagnosing peritoneal TB is laparoscopy with peritoneal biopsy and subsequent pathological or microbiological confirmation2,13. Pharmacological treatment consists of the same scheme used for pulmonary TB14. An increase in the levels of the tumor marker CA-125 > 35 U/mL has been reported in active peritoneal TB cases due to the anti-TB treatment’s good response, showing as a follow-up marker to the activity of the disease.
Next, we present the clinical case of a patient with peritoneal TB associated with a secondary Sweet syndrome. Ascites fluid studies suggestive of malignancy were taken, with an ADA false negative, possibly due to the administration of a carbapenem; a follow-up for treatment with cancer antigen 125 (CA-125) was done. The ADA false negatives reports and Sweet syndrome secondary to TB are exceptional, which we contribute to the literature by reporting our case.
Case report
A 46-year-old man has a medical history of stage V chronic kidney failure (CKF) of hypertensive etiology. He has been under management with hemodialysis-type renal replacement therapy every other day for 2 years. He has had arterial hypertension since the age of 16 and is in pharmacological management with enalapril and spironolactone. He was a convict for 9 years and was released 3 months ago. He was admitted from a less complex level due to an approximately 15-day clinical picture of evolution consisting of distension, diffuse abdominal pain, fever, asthenia, adynamic, and hyperoxia. Additionally, he mentions an involuntary weight loss of approximately 7 kg in the last 2 months. Upon admission, his vital signs are within normal ranges, with findings of decreased breath sounds in the left lung base and abdominal distention with positive-ascite wave without signs of peritoneal irritation. Paraclinical tests were taken, which showed thrombocytosis in the hemogram, anemia of normal volumes in the transfusion range, elevated ferritine, elevated nitrogen, hyperkalemia, hyponatremia and hypoalbuminemia, negative HIV, negative surface antigen for hepatitis B, a negative antibody for hepatitis C, positive procalcitonin, and normal thyroid stimulating hormone (TSH). Transfusion support was started with two units of blood cells without complications.
The transthoracic echocardiogram showed left ventricular hypertrophy, left ventricular ejection fraction (LVEF) 62 %, and mild pericardial effusion. Abdominal ultrasound showed no evidence of liver or biliary structural abnormalities with a moderate amount of ascites fluid. The upper gastrointestinal endoscopy UGIE did not reveal esophageal varices, thus ruling out chronic heart or liver disease due to ascites. Finally, the chest x-ray showed findings of consolidation at the level of the lingula and left pleural effusion (Figure 1).
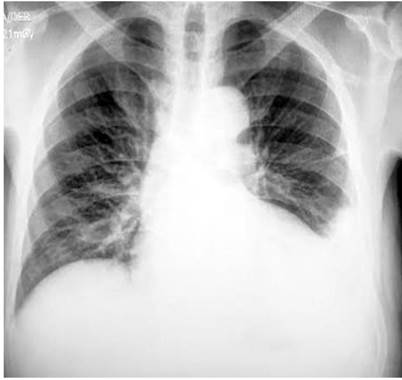
Figure 1 Chest x-ray in anteroposterior projection with findings of cardiomegaly, aortic atheromatosis, and obliteration of the left costophrenic angle.
Due to the radiological findings, the study with chest CT was expanded, where a moderate left pleural effusion was documented with passive atelectasis of the adjacent lung parenchyma and consolidation in the lingula (Figure 2). In the context of febrile syndrome with findings in the lung parenchyma, community-acquired pneumonia was considered. Treatment with meropenem was started on day 2 at the hospital since the patient referred previous antibiotic therapy at an unknown facility. Then, a pleural fluid study is performed, which documents an exudated effusion, with negative ADA, negative potassium hydroxide (KOH), negative cultures, and negative PCR for Mycobacterium tuberculosis. The cell block reported abundant lymphocytes, reactive mesothelial cells, the presence of large-nucleus cells, and abundant cytoplasm, with negative Ziehl-Nielsen (ZN) (Table 1).
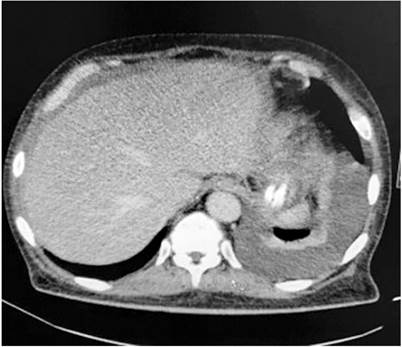
Figure 2 Simple chest tomography in the mediastinum’s window with findings of a left pleural effusion with adjacent passive pulmonary atelectasis and consolidation of the lingula.
Table 1 Pleural fluid study
| Pleural fluid study | |
|---|---|
| Color | Yellow |
| Appearance | Slightly turbid |
| Cellularity | No |
| pH | 8 |
| Glucose | 101 mg/dL |
| LDH | 154 IU/mL |
| Total proteins | 3.8 g/dL |
| ADA | 19.67 U/L |
| KOH | Negative |
| Culture | Negative |
On the other hand, an abdominal CT was performed for the study of ascitic syndrome, where omental cake-type lesions were observed. Tumor markers with a report of CA-125 of 210 U/mL (positive) are requested. Diagnostic paracentesis was performed, whose peritoneal fluid showed a non-hypertensive pattern SAAG 0.7, with ADA 19.92 (negative), culture, and negative PCR for Mycobacterium tuberculosis (Table 2). The peritoneal fluid cell block reported lymphocytes with reactive mesothelial cells, the presence of large-nucleus cells, and abundant cytoplasm. All the above suggests an underlying malignant process as the first possibility.
Table 2 Peritoneal fluid study
| Peritoneal fluid study | |
|---|---|
| Color | Yellow |
| Appearance | Slightly turbid |
| Red blood cells | 3452/mm3 |
| Fresh | 40 % |
| Crenocytes | 60 % |
| pH | 8 |
| Glucose | 75 mg/dL |
| LDH | 335 IU/mL |
| Albumin | 1.8 g/dL |
| Protein | 3.94 g/dL |
| ADA | 19.92 U/L |
| Gram’s stain | Negative |
| Culture | Negative |
| PCR Mycobacterium | Negative |
| Serum proteins | |
| Total proteins | 5.8 g/dL |
| LDH | 271 IU/mL |
| Glucose | 134 mg/dL |
| PCR Mycobacterium | Negative |
The patient was unaccompanied during his hospital stay; however, on day 6 of hospitalization, his ex-wife appears and provides the clinical history of the institution he was referred from. It contains information that had been ignored by the patient and provides information about generalized pustular cutaneous lesions, which the patient drained with pointed objects and was associated with febrile episodes. In this way, erythematous cutaneous lesions are evidenced by papules, nodules, and plaques on the upper and lower extremities and on the trunk of symmetrical distribution. Also, he has purplish erythematous nodules painful on palpation, with a local increase in temperature. All the above is characteristic of Sweet syndrome.
Given the findings of ascitic fluid, omental cake documented on abdominal CT, positive CA-125, and Sweet syndrome as a possible paraneoplastic manifestation with high suspicion of malignancy, it was decided to start the administration of a steroid while treatment with meropenem was suspended. All this led to a clinical improvement of skin lesions.
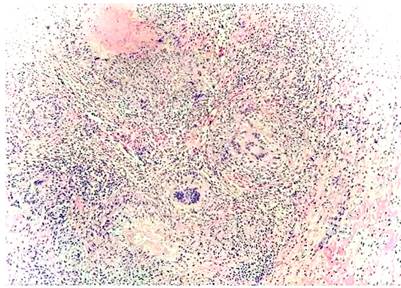
The clinical history of the previous hospitalization of the patient was reviewed, where diagnostic paracentesis was performed with an ADA report of peritoneal fluid of 36 U/L (positive). Given the lack of clarity of the current clinical picture, it was decided to perform a biopsy of the omentum, peritoneum, and round ligament by laparoscopy. This report confirms the presence of the chronic granulomatous inflammatory necrotizing process of infectious etiology, negative for malignancy (Figure 3). Therefore, it is concluded that the diagnosis is peritoneal TB. Thus, steroid management was suspended, and antituberculous control was started, which resulted in an improved prognosis. Follow-up was done with levels of CA-125, probing those levels negative ten days after starting the antituberculous treatment (initial CA-125 210, control of 115). For this reason, Sweet syndrome was considered as a manifestation of peritoneal TB, with an ADA false negative possibly due to the previous administration of carbapenem, with negative CA-125 as a marker of good response to anti-TB treatment.
Discussion
TB is a disease with a high morbidity and mortality rate. It is estimated that, in 2017, 10 million people got it, and 1.6 million died. The main study manifestation is the pulmonary form. However, extrapulmonary conditions occur in 10 %- 42 %: 25 %- 50 % of cases were abdominal, with mortality data reaching 35 % when there is no adequate treatment and up to 73 % in patients with cirrhosis2,3. The main risk groups are individuals with HIV, immunosuppressive states, close contact with infected people, under 5-year children, the elderly, prisoners, illicit drug users, health personnel, patients with diabetes, CKF, alcoholic liver disease, and low socioeconomic status2,5. The main symptoms presented are abdominal pain (75 %), fever (69 %), ascites (62 %), abdominal distention (60 %), weight loss (53 %), abdominal tenderness (49 %), and abdominal mass (34 %). Due to the nonspecific symptoms, diagnosis becomes difficult and delayed from weeks to months2,3.
Sweet syndrome has been described as a rare manifestation of peritoneal TB6; it is a rare and acute febrile neutrophilic reactive dermatosis. It is characterized by the sudden appearance of painful, shiny erythematous plaques or nodules presented in the form of papules, pustules, or vesicles. They are located on the face, neck, and upper limbs. Its paraneoplastic form is more serious, affecting the trunk and lower limbs. It can present other symptoms such as fever or low-grade fever, migratory arthralgia of large joints, leukocytosis, neutrophilia, and elevated ESR; it improves with systemic corticosteroids. Most of the causes are benign, autoimmune diseases, medications, and infections, including those caused by mycobacteria7,8.
Generally, peritoneal TB develops from a latent TB, whose mechanism involves activating a tuberculosis focus located in the peritoneum, which originates during the primary infection through hematogenous spread from the primary pulmonary focus to the lymphoid mesenteric nodes. Other mechanisms are the ingestion of the bacillus that goes to the lymphoid mesenteric nodes through Peyer’s patches in the intestinal mucosa. Acquisition of infection through consumption of pasteurized milk has been observed most commonly by Mycobacterium bovis2,4.
The diagnosis of peritoneal TB is often difficult. Paraclinical findings are observed at the hematological level, such as normocytic and normochromic anemia, thrombocytosis, and normal white blood cell count. The first-line imaging method is abdominal ultrasound, which shows the presence of ascites fluid and rules out portal etiology as the cause of ascites, which additionally offers a guide for the drainage of peritoneal fluid2,17. Another imaging aid is CT, which offers some findings, such as ascites, lymphadenopathy, intestinal thickening, peritoneal nodules, adherences, fibrinous septa, and a finding called omental cake, which requires a differential diagnosis with peritoneal carcinomatosis9.
In our case, the finding of the omental cake was documented, a rare condition due to infectious diseases. Generally, these diseases result from the hematogenous, lymphatic, or direct spread of infectious microorganisms to the peritoneum. Peritoneal TB usually exhibits these manifestations, and the appearance is similar to that of peritoneal carcinomatosis. Findings that can help distinguish it from peritoneal carcinomatosis include mesenteric macronodules, irregularity in the infiltrate of the omentum, fibrous wall covering the infiltrated omentum, splenomegaly, or splenic calcification9.
In the study of ascitic fluid when suspecting peritoneal TB, the stains for acid and alcohol fast bacilli (AAFB) and ZN have very low sensitivity17, and the cultures have a sensitivity of 21 %- 35 %18. Generally, the leukocyte count in ascites fluid is greater than 500 uL2. LDH is one of the tests with the highest sensitivity since it reaches 77 %; however, it has a similar sensitivity for other diseases such as pancreatic ascites and peritoneal carcinomatosis, which does not discriminate between these pathologies. Some authors propose a cutoff point greater than 90 U/L2,10. The SAAG index < 1.1 g/dL orients to a non-portal etiology, such as malignancy, pancreatitis, or peritoneal tuberculosis infections. Nevertheless, in cases where the diagnosis of peritoneal TB is accompanied by chronic liver disease, sensitivity decreases to 28 %- 88 %11,17. Increased levels of tumor markers such as CA-125 have also been reported in peritoneal TB; cut-off points of 35 U/mL have been proposed with a sensitivity of 83.33 % and a specificity of 50 % for active peritoneal TB, and studies have linked a decrease in these levels with a good response to anti-TB treatment4,6,9,15,16,17,18.
Among the other unusual findings in our case, there are peritoneal fluid abnormalities with an ADA false negative. ADA is a purine metabolism enzyme and a potent modulator of T cell differentiation, showing high sensitivity (100 %) and specificity (97 %) for the diagnosis of TB using cut-off points of 36-40 IU/L, with optimal cutoff > 39 IU/L2,12. Genomic amplification techniques such as PCR have shown sensitivity and specificity as high as 93.7 % and 91.7 %, respectively, in cases of pulmonary TB. It is faster than Mycobacterium culture techniques-an advantage. Some studies have estimated sensitivity for extrapulmonary TB of up to 94.5 %19,20. The ascitic fluid cytology study shows a sensitivity of up to 97 % when paracentesis is performed with three separate samples for diagnoses of malignancy with high specificity. However, low sensitivity is observed in staining for TB21. The gold standard for diagnosing peritoneal TB is laparoscopy with peritoneal biopsy and subsequent pathological or microbiological confirmation2,13.
Pharmacological treatment for peritoneal TB consists of the same scheme used for pulmonary TB. A response is generally observed within the first 3 months with the resolution of symptoms and normalization of paraclinics22. Surgical intervention is reserved for cases showing signs of intestinal perforation, intestinal obstruction, fistulas, abscesses, and hemorrhage4,9,12,13,16,18,19,20,21,22,23. As observed, our patient previously received a carbapenem, with an alteration in ADA reports; as it is known, these drugs are used to treat drug-resistant Mycobacterium tuberculosis14, which was the alteration cause for ADA reporting in ascites fluid.
Conclusion
We present a clinical case of a patient with peritoneal TB associated with a secondary Sweet syndrome; ascites fluid studies suggestive of malignancy were taken with an ADA false negative possibly due to carbapenem administration. Additionally, a CA-125 treatment follow-up was carried out. The reports of false negatives of ADA and Sweet secondary to TB are exceptional, which we contribute to the literature by reporting our case.











 text in
text in