Introduction
Parasites-protozoa, helminths, and ectoparasites-are microorganisms that live on or within a host organism and feed at its expense1. Infection in humans corresponds to the most frequent form, and it is estimated that one-third of the world’s population has had a parasite infection2.
Helminths are large multicellular organisms seen with the naked eye as adults. Within this group are Trichuris trichiura, Ascaris lumbricoides, Hookworms, and Strongyloides stercoralis (S. stercoralis)3. The S. stercoralis is a parasite first described in 1876 in France by the doctor Louis Normand when he discovered a parasite in the fecal matter of soldiers that was initially called Anguillula stercoralis4. It affects at least 100 million people in the world, being endemic in tropical and subtropical areas and regions with poor sanitary conditions5.
There are three clinical forms of strongyloidiasis: infection when there is no increase in the number of helminths in the duodenum or jejunum and no extraintestinal migration; self-infection when the parasite is able to start a new life cycle without going outside; and hyperinfection due to an increase in the maturation of filariform larvae disseminating outside the gastrointestinal tract6. The main risk factor for the development of hyperinfection is the development of a state of immunosuppression in a person with chronic S. stercoralis infection, either due to the use of steroids7, organ transplantation and immunosuppressive therapy8, and infection by human T-cell lymphotropic virus-I (HTLV-I)9. The latter factor predicts treatment failure10. The clinical manifestations depend on the immunity status of the host; they have an asymptomatic, oligosymptomatic course with chronic gastrointestinal manifestations, or severe due to larval dissemination that can compromise the life of the patient11.
The following is a description of the clinical case of a patient with a history of HTLV-I infection and ulcerative colitis (UC) who developed S. stercoralis hyperinfection, its diagnosis, and treatment.
Case report
A 27-year-old sweeper from a low socioeconomic stratum and living in the urban area consulted the emergency room after 7 days of having abundant foul diarrheal stools associated with abdominal pain in the epigastrium and vomiting of food content. In the review of symptoms by systems, he referred to 6-month intermittent diarrheal stools without mucus or blood and weight loss of 10 kg. Past medical history: history of tropical spastic paraparesis secondary to HTLV-I infection, and UC diagnosed 6 months ago by colonoscopy plus biopsy (Figure 1A and B), which was active given the persistence of diarrheal stools, sometimes with hematochezia. It was associated with colicky abdominal pain despite the use of mesalazine 500 mg orally every 12 hours; however, the patient had no gastroenterology control by the time he was diagnosed.
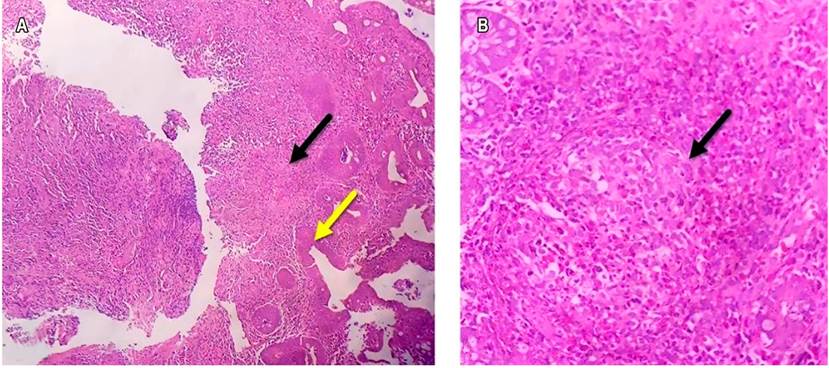
Figure 1 A. Histopathological study of the colonic mucosa with hematoxylin-eosin staining, 4X resolution, showing superficial erosions in the mucosa, fibrin, and cellular debris (yellow arrow) and non-caseating granuloma formation (black arrow). B. Histopathological study of the lamina propria, 40X resolution, with the presence of non-caseating granuloma (black arrow), associated with an increase in the number of inflammatory cells like lymphocytes, plasmocytes, polymorphonuclear neutrophils, and eosinophils, which permeate through the glandular epithelium. Dysplastic changes are not observed.
Upon admission, a blood pressure (BP) was recorded at 80/50 mm Hg; heart rate (HR) 106 beats per minute (bpm); respiratory rate (RR) 17 breaths per minute (bpm); oxygen saturation (So2) 95 %; temperature (T) 36 °C; weight 45 kg; body mass index (BMI) of 17. The physical examination revealed dry mucous membranes, tenderness in the epigastrium, and digital rectal examination without injuries or bleeding. Laboratory test results showed leukocytosis, moderate anemia of normal volumes, elevated C-reactive protein (CRP), hypoalbuminemia, stool culture with an absence of parasites, and a coproscopy with an inflammatory pattern (Table 1).
Table 1 Laboratory examinations
| Examination | Ranges | Outcome |
|---|---|---|
| Leukocytes (103) | 5-10 | 11 700 |
| Neutrophils (#) | 2.8-7 | 8600 |
| Lymphocytes (#) | 0.9-4.9 | 2090 |
| Eosinophils (#) | 0-0.5 | 120 |
| Hemoglobin (g/dL) | 12-15 | 9.1 |
| Hematocrit (%) | 36-45 | 29 |
| Mean corpuscular volume (fL) | 82-98 | 86.3 |
| Platelets (103) | 150-450 | 335 000 |
| D-dimer (ng/mL) | 0-500 | 3517 |
| PCR | 0-5 | 10 |
| VSG | 2-3 | 2 |
| Lactate | 0.4-2.2 | 0.8 |
| Albumin (g/dL) | 3.5-5.2 | 1.07 |
| Total proteins (g/L) | 6.4-8.3 | 3.61 |
| Creatinine (mg/dL) | 0.5-1.5 | 0.47 |
| BUN (mg/dL) | 7-20 | 8.1 |
| Sodium (mmol/L) | 136-145 | 135 |
| Potassium (mmol/L) | 3.6-5.1 | 4.91 |
| Chlorine (mmol/L) | 98-107 | 101 |
| Glucose (mg/dL) | 65-110 | 81 |
| HIV | - | Negative |
| Stool culture | - | Absence of parasitic structures |
| Coproscopy | - | 7 leukocytes per field Positive occult blood |
Blood urea nitrogen (BUN); human immunodeficiency virus (HIV); erythrocyte sedimentation rate (ESR).
Intravenous hydration with fluids was started, which normalized vital signs. An upper gastrointestinal endoscopy (UGIE) and biopsy were performed, which revealed areas of predominantly antral gastric mucosa ischemia and severe erosive pangastritis, consistent with the patient’s symptoms. A new colonoscopy was not performed since the patient did not accept another endoscopic study.
On the third day of hospitalization, the patient started with fever, dyspnea, increased RR at 30 rpm, subcostal pulls, decreased breath sounds in both lung fields, So2 80 %, and severe hypoxemia in arterial gases, which did not improve with oxygen support (fraction of inspired oxygen (FiO2) 50 %). For this reason, the patient required orotracheal intubation and was transferred to the intensive care unit (ICU), where empirical antimicrobial therapy with vancomycin + piperacillin/tazobactam was started. Extension studies were requested to identify the cause of the clinical worsening.
The chest radiography showed multiple alveolar opacities (acinar nodules) (Figure 2A), confirmed with the chest CT angiography associated with areas of consolidation in the posterior segment of the right lower lobe. Additionally, it confirmed the presence of bilateral ground glass, diffuse micronodules, bilateral pleural effusion, and absence of filling defects in the pulmonary artery or its branches (Figure 2B and C). Sputum studies reported the presence of S. stercoralis filariform larvae (Figure 3A), and both the tracheal secretion culture and the blood cultures were positive for multisensitive Klebsiella pneumoniae. Gastric mucosa biopsies revealed erosive gastritis with epithelial atypia of reactive nature and numerous parasite structures compatible with S. stercoralis (Figure 3B).
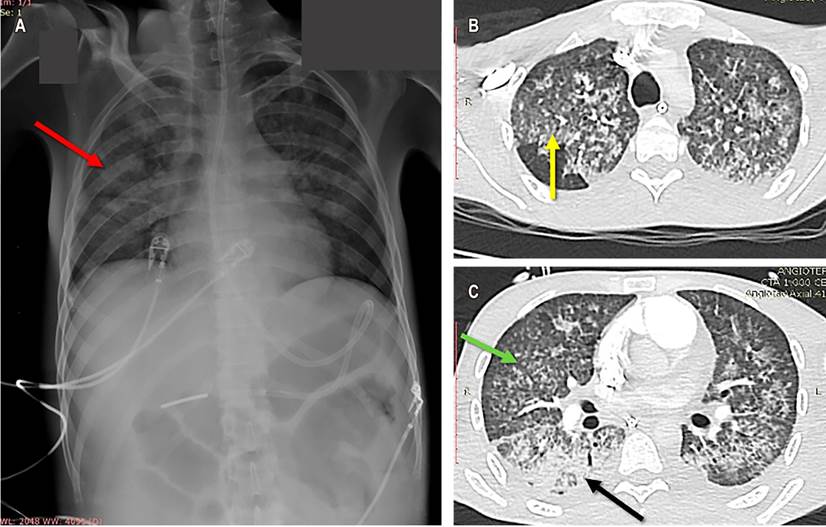
Figure 2 A. Portable PA chest radiography, showing diffuse alveolar opacities, acinar-nodule type (red arrow). B. Chest CT angiography with multilobar ground glass areas (yellow arrow). C. Micronodular opacities with a predominantly lobular distribution, with diffuse interstitial involvement (green arrow); and alveolar opacities predominantly in posterior segments with air bronchogram and pleural effusions (black arrow).
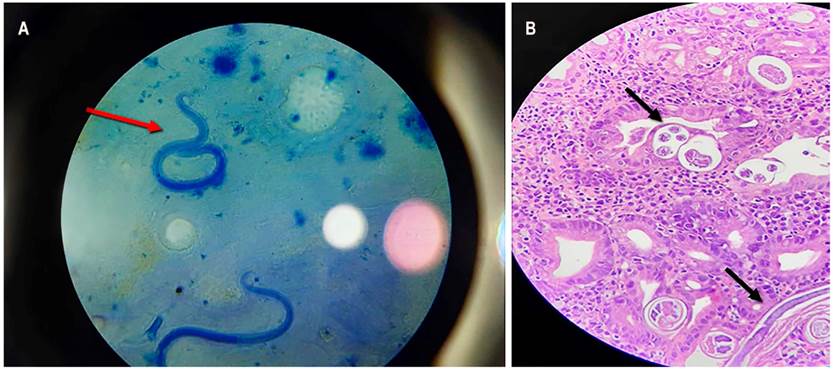
Figure 3 A. Filariform larvae of Strongyloides stercoralis in fresh sputum microscopy sample. B. Histopathological study of gastric mucosa with hematoxylin-eosin staining at 40x resolution, where the mucosa is invaded at the epithelium level and interstitium by larvae compatible with Strongyloides stercoralis, and a moderate to severe interstitial lymphoplasmacytic reaction.
Treatment was started with ivermectin at 200 mcg per kilo plus albendazole 400 mg per day. However, despite the management instituted, the patient remained feverish, with persistent hypotension and requiring vasoactive support with norepinephrine, which led to his death 2 days after admission to the ICU.
Discussion
The case of a young patient with a history of HTLV-I infection and UC was presented, who developed a hyperinfection syndrome caused by S. stercoralis and its clinical presentation, diagnosis, treatment, and complications.
S. stercoralis infection is highly prevalent in developing countries5. The predisposing risk factors are a low socioeconomic level, risk professions, immunosuppressive states, and HTLV-I infection, which directly correlates to the increased risk of acquiring the infection and worse clinical outcomes10. In this case, the patient met multiple factors that made him a susceptible host for S. stercoralis.
In individuals with HTLV-I infection, the presentation forms disseminated by excessive larval production are the most common. They are characterized by the involvement of different organs and systems, in addition to the gastrointestinal tract12. Although the patient’s gastroenteric symptoms began 6 months before the emergency consultation and worsened a week before admission, it raises the suspicion of active UC and, possibly, chronic infection by S. stercoralis, since this microorganism can survive for long periods in the host due to autoinfection processes, causing chronic diarrhea. Later, the infection manifested with the extraintestinal larval dissemination picture that compromised the respiratory tract, which led to the fatal outcomes described.
The diagnosis of S. stercoralis infection requires visualization of the larvae in a clinical sample. Even though the stool did not show parasites, the sensitivity of this type of examination is low both due to the intermittence in larval elimination and the different stool methods available13. However, larval imaging in sputum and gastric mucosa reveals increased maturation of filariform larvae disseminating outside the gastrointestinal tract, constituting a hyperinfection syndrome.
Among the differential diagnoses in this case, there is the possibility of undiagnosed parasitic colitis since many of the clinicopathological findings are similar to those found in UC, such as aphthoid ulceration, edema, and infiltration of the lamina propria by lymphocytes, plasma cells, and eosinophils in colonoscopy and/or peripheral eosinophilia14,15. And, on the other hand, Löffler’s syndrome, which is part of the pulmonary eosinophilia. However, the peripheral eosinophil count was normal in the case presented, making this diagnostic option impossible 16.
Treatment should be started promptly with ivermectin and albendazole; however, in patients with a history of HTLV-I infection, despite its early management, the clinical response is not favorable17. In the patient mentioned above, the medical management described in the literature was started with a broad-spectrum antibiotic therapy due to a bacterial coinfection. Still, despite this, there was no clinical response towards improvement, which his underlying medical condition of immunosuppression could explain by HTLV-I, the involvement of multiple organs and systems, and severe hypoalbuminemia caused by a redistribution phenomenon in critically ill patients18; altogether led to the fatal outcome.
The clinical case described reaffirms the need to consider parasitic infections within the differential diagnoses in individuals who meet the risk factors of a susceptible host and present gastrointestinal manifestations of a long evolution. A timely intervention such as deworming reduces morbidity and mortality.
Conclusion
Parasitic infections constitute a differential diagnosis of chronic intestinal diseases, especially in people with immunosuppression as the leading risk factor. Even though the diagnosis represents a clinical challenge, when it is carried out on time, it allows the initiation of a guided management therapy, which reduces the morbidity and mortality associated with infection by this group of microorganisms.











 texto en
texto en 



