Introduction
The most common gastric oncological pathology is of malignant origin, the third leading cause of cancer death worldwide, with an estimated incidence of 950,000 cases per year1. However, a small percentage of patients, representing 0.005%-4%, suffer from gastric neoplasms of benign origin, such as schwannomas, leiomyomas, and glomus tumors. Most of these lesions are often confused with stromal tumors, for which the immunohistochemical study is essential to identify their negativity for synaptophysin and chromogranin A2.
Among these infrequent pathologies is the ectopic pancreas or atypical location of the pancreatic tissue, i.e., outside its anatomical limits or regular vascular connections. It is frequently located in the upper third of the stomach and predominantly along the greater curvature. This condition is usually found only in 0.6%-13% of clinical autopsies3.
These gastric tumors are commonly detected between the fourth and sixth decades of life and usually present with nonspecific symptoms, ranging from insidious abdominal pain to intestinal obstruction1-16.
This paper describes the experience of a quaternary care hospital in the diagnostic and therapeutic approach to unusual gastric tumors and a review of the literature regarding each type of tumor.
Patients and methods
The present study describes the clinical conditions and treatment of four patients diagnosed with a benign gastric tumor between January 2012 and April 2017. The cases presented herein were identified in the databases of the Clínica Colombia general surgery service; subsequently, we reviewed the medical records and images available for each particular case.
Regarding the selected patients, on the one hand, all of them reported abdominal pain at the time of the first medical examination, and the tests requested at the beginning included tomographic and endoscopic imaging. On the other hand, all the patients were invited to a multidisciplinary meeting, at which gastrointestinal stromal tumor (GIST, due to the characteristics in the image) and gastric adenocarcinoma were considered the primary differential diagnoses after reviewing the tests. The decision in all cases was laparoscopic subtotal gastrectomy.
The four cases referred to are described in detail below.
Patient 1
A 35-year-old man presented with a six-month history of epigastric pain, poor modulation with conventional analgesics and proton pump inhibitors (PPIs), associated with episodes of lipothymia on several occasions since the onset of symptoms, and history of morbid obesity and sleep apnea. His physical examination was within normal limits. An esophagogastroduodenoscopy (EGD) and a computed axial tomography (CAT) scan were initially performed, which revealed a tumor-like lesion of 2 cm at the level of the posterior surface of the antrum (Figure 1). Then, a biopsy by endoscopic ultrasonography (EUS) was requested, noting a subepithelial lesion dependent on the muscle layer that could be a GIST.
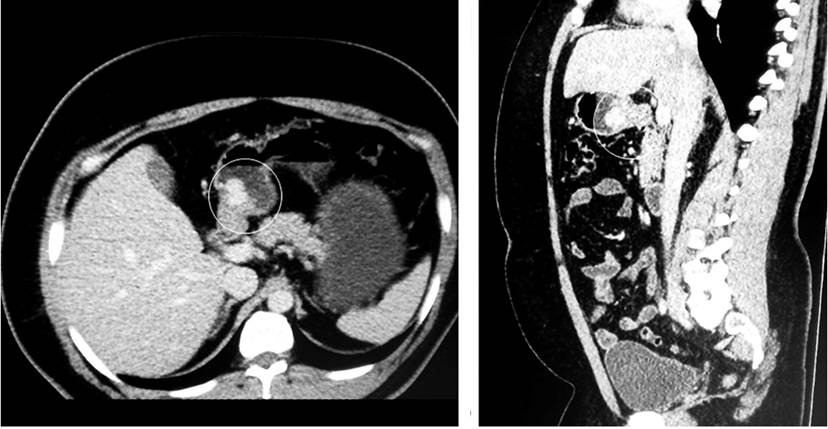
Figure 1 In the antral region, the CAT image shows a solid exophytic lesion with lobulated contours that captures the contrast and a 17 x 20 mm diameter, which could be related to a GIST.
With these results, the patient was taken to a laparoscopic Roux-en-Y reconstruction antrectomy without lymph node dissection (since the intraoperative characteristics more likely suggested a GIST). An anthropyloric tumor was described as an intraoperative finding.
He presented with upper digestive tract bleeding of spontaneous resolution during the postoperative period, no evidence of ulcers, and active bleeding in the control digestive tract endoscopy. Then, adequate tolerance to the oral route was determined in the medical evolution with subsequent discharge and no complications.
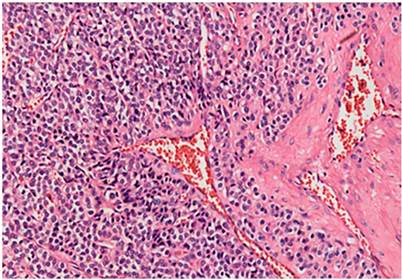
The histopathological analysis of the surgical specimen showed a glomuvenous malformation (glomus tumor) originating in the muscularis propria, positive for smooth muscle actin and negative for chromogranin-synaptophysin and S100 (Figure 2). Without further interventions, the patient is asymptomatic at present but has lost 10 kg, most likely associated with the type of surgery performed.
Patient 2
A 68-year-old woman presented with one-year pain in the epigastrium and right upper quadrant, associated with decreased food intake and, consequently, a loss of approximately 6 kg in eight months. She had a history of atrial fibrillation (AF) and hypertension (HTN), both managed with metoprolol and warfarin. There was a sensation of a mass in the epigastrium on physical examination, for which an (EGD) was indicated. It reported a 10 cm lesion with a depressed and ulcerated center with fibrin. The pathology report was not conclusive. Additionally, extension imaging was requested, revealing a large mass in the lesser curvature of the stomach (Figure 3). With a suspected GIST, she underwent a non-radical subtotal gastrectomy with laparoscopic Roux-en-Y reconstruction.
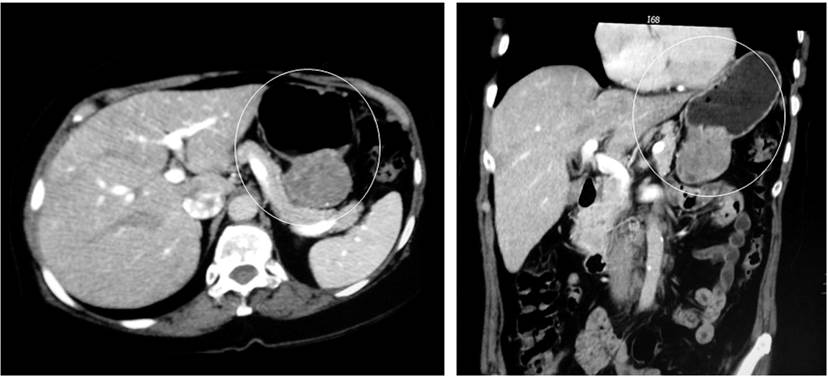
Figure 3 Abdominal CAT scan showing a mass in the lesser curvature of the stomach projected towards the mesentery of approximately 50 mm, GIST.
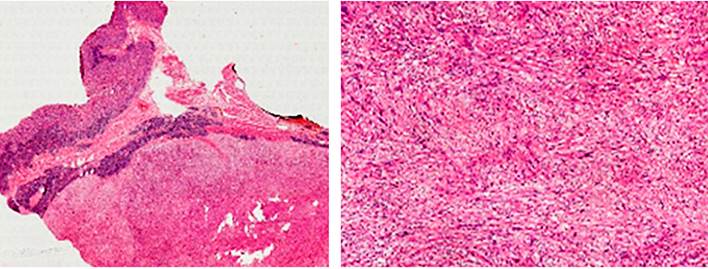
Intraoperatively, the presence of an exophytic tumor of approximately 7 cm in the lesser curvature, a pedicle, and not adhered to neighboring structures was observed. The postoperative evolution was adequate, so the patient was discharged on the fourth postoperative day. The pathology report revealed a schwannoma with negative section margins for tumor involvement (Figure 4). With this pathology, she was discharged from general surgery.
Patient 3
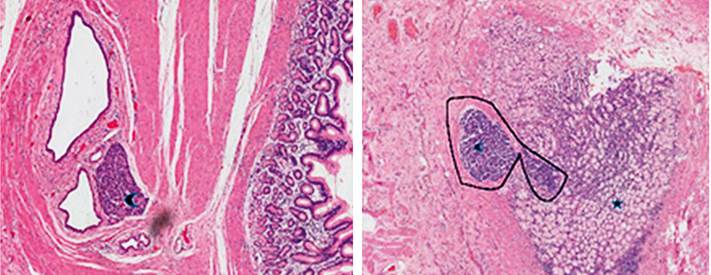
A 75-year-old male patient was a chronic PPI user. In control EGD studies, a polyp was noted in the antral region towards the lesser curvature under endoscopic follow-up since 2014 by taking an endoscopy plus biopsies-all negative for malignancy so far. In 2016, a new polypoid lesion appeared, for which a EUS was performed. The result showed an 8 mm prepyloric sessile lesion that compromised the submucosa and a 25 mm sessile hyperplastic polyp on the posterior wall of the antrum compromising the submucosa (Figure 5). During this study, biopsies were taken that reported hyperplastic polyps in the lesser curvature without ruling out tumor involvement at the base of the polyps. Thus, he underwent a laparoscopic subtotal gastrectomy with D2 lymph node dissection, reporting no postoperative complications.
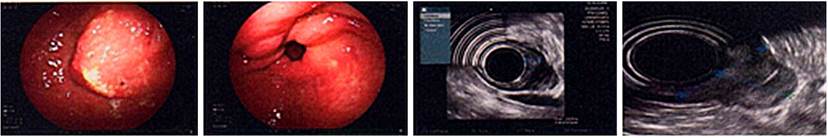
Figure 5 Sessile hyperplastic polyp in the prepyloric region measuring 6 mm x 8 mm in diameter compromising the submucosal layer, respecting the muscularis propria. Sessile hyperplastic polyp of 25 mm in diameter in the posterior wall of the antrum that involves the submucosal layer while respecting the muscularis propria.
The patient was examined ten days after surgery. The histopathological analysis of the surgical specimen reported two submucosal leiomyomas with no dysplastic or metaplastic changes, free section borders, fibrofatty tissue with vascular congestion, and three lymph nodes with sinus histiocytosis. Given the findings of the pathology, immunohistochemical studies were not performed. With these results, the patient was discharged from the surgery service.
Patient 4
A 26-year-old woman presented with epigastric pain, a history of two previous hospitalizations for upper digestive tract bleeding, and an EGD of the last hospitalization that reported a 2.5 cm lesion in the gastric antrum (with a GIST-compliant biopsy report). Tomographic imaging was requested in which a 3 cm cystic lesion was described on the lesser curvature (Figure 6). A laparoscopic radical antrectomy was carried out, detecting a 4-cm prepyloric lesion intraoperatively with up to 1 cm lymph nodes toward the lesser curvature. She had ileus during the postoperative period, which got resolved after a few days with medical treatment and was subsequently discharged.
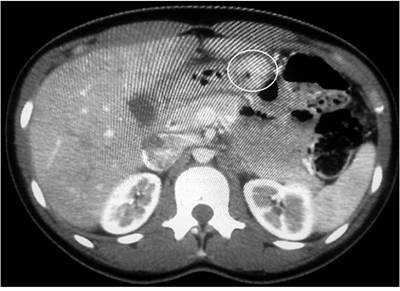
Figure 6 An abdominal CAT reports a multilocular exophytic cystic mass with 3-cm thick and irregular walls and partitions, apparently originating in the hepatogastric ligament, with alteration of the adjacent fat.
During control by surgery outpatient, the report of the surgical specimen revealed a mucinous cystadenoma originating in ectopic pancreatic tissue (Figure 7). Since malignant transformation is extremely rare, the patient was discharged from the surgery service.
Results
Of the four cases, half were female, and half were male, with a mean age of 51 years (26-75 years old). The predominant symptoms were epigastric pain, and only one of the cases was an incidental finding (Table 1); 100% of the patients had a lesion in the antral region, and only one of them had a second lesion at the prepyloric level. All the patients underwent subtotal gastrectomy with laparoscopic Roux-en-Y reconstruction, with no associated complications either intraoperatively or postoperatively; the mean hospital stay was 3.5 days (2-6 days). All were discharged from the specialty.
Table 1 Characteristics of the patients
| Case | Sex | Age | Clinical picture | Endosonographic peculiarities | Endoscopic peculiarities | Histopathology |
|---|---|---|---|---|---|---|
| Patient 1 | Male | 35 years old | Epigastralgia + lipothymia | Subepithelial lesion dependent on the muscle layer of approximately 2 cm | Glomus tumor Smooth muscle actin (+), negative for CD117-CD34, CKAE1 / AE3, chromogranin, synaptophysin, and S100 Ki-67: 8% | |
| Patient 2 | Female | 68 years old | Epigastralgia + weight loss | A 10 cm lesion with a depressed and ulcerated center and fibrin | Schwannoma S100 (+), desmin (-), CD117 (-), CD34 with focal positivity | |
| Patient 3 | Male | 75 years old | Asymptomatic | An 8 mm prepyloric sessile lesion involving as far as the submucosa and a 25 mm sessile hyperplastic polyp on the posterior wall of the antrum compromising as far as the submucosa | Leiomyoma* | |
| Patient 4 | Female | 26 years old | Epigastralgia + UGIB | A 2.5 cm lesion in the gastric antrum | Mucinous cystadenoma in the ectopic pancreas Cytokeratin 7-8 and 18 (+), epithelial membrane antigen (+) |
*Immunohistochemistry was not performed. UGIB: Upper gastrointestinal bleeding.
Discussion
It is tough to determine the etiology of gastric lesions based solely on clinical findings2. Considering that benign lesions are rare, they are frequently confused with tumors of mesenchymal origin, as is the case with GIST, which originates predominantly in the stomach4.
This paper describes four unusual cases of gastric tumors. The first is a glomus tumor, a condition first reported by Masson in 1924. It is of neuromyoarterial origin, composed of modified smooth muscle cells, and most commonly found in the soft tissues of the periphery, such as the extremities5,17.
Gastric glomus tumors are rare, accounting for only 1% of stromal tumors. Until a few years ago, only 130 cases had been reported in the literature. They are located predominantly in the gastric antrum, being more common in women between 50 and 60. Its typical clinical manifestation is upper gastrointestinal bleeding6,18.
CAT imaging is characterized by solitary hypervascularized lesions in the arterial phase that persist in the venous phase19. As Chabowski et al described, these tumors are usually in the lesser or greater curvature and the prepyloric region with an average size of 1-4 cm. The histology identifies a vascularized endothelium surrounded by tumor cells with an undefined nucleus and abundant cytoplasm. They differ from carcinoid tumors for being positive for vimentin and smooth muscle actin and negative for chromogranin7, which correlate with the histological findings of the surgical specimen from the first patient.
Schwannoma is a rare tumor developed in Schwann cells whose most common location at the gastrointestinal level is usually the colon8. Those of gastric location represent 0.2% of stomach neoplasms and 4% of all benign gastric tumors; only 8% of patients present with abdominal pain as the primary clinical manifestation, while the rest usually exhibit digestive tract bleeding or an incidental finding in imaging requested for another pathology9.
As of May 2017, only 221 cases of gastric schwannoma have been reported in the world literature, as mentioned by Bao-Guang Hu in his publication. It predominantly affects women between their 50s and 80s. It is characterized by a solitary lesion located predominantly in the body of the stomach in up to 59.3% of patients, followed by the antral region in 26.7%; malignant transformation is reported in only 4.5% of cases10.
These tumors are spindle cell proliferations, the clusters of which are known as Verocay bodies. Inside are hyalinized and thickened blood vessels20, with lymphocytic infiltrate and nuclei with a low degree of atypia; mitotic figures are rarely identifiable. They are usually positive for S-100 and negative for smooth muscle actin and desmin, among others, the gold standard for the histopathological diagnosis of these tumors21.
Diagnostic imaging is usually characterized by being well-circumscribed lesions, with minimal changes in the arterial phase on CAT and magnetic resonance imaging (MRI). They are usually hyperintense on T222.
In the patient of the present study, a GIST was suggested as the initial differential diagnosis, given the symptoms and endoscopic findings; despite an inconclusive initial biopsy, it required non-radical surgical management (due to the rarity of lymphatic involvement in most GISTs). Lastly, the final pathology, which showed a tumor originating in the muscularis propria and extended to the subserous fat, was compatible with a schwannoma.
Until 1983, GISTs were classified as smooth muscle tumors and included leiomyomas, leiomyoblastomas, and leiomyosarcomas; it was until the development of immunohistochemical techniques that true leiomyomas were shown to be negative for CD117 and CD34, unlike GISTs11.
In the third case, lesions were incidentally documented, exhibiting an increase in size during endoscopic follow-up, so surgical management was contemplated. The histological report of the surgical specimen was compatible with a corporal-antral leiomyoma for which immunohistochemical studies were not conducted. Tumors of this type, located at the gastric level, are frequently found near the cardia and are more common in men between 30 and 35. Additionally, Hui Zhu also described tomographic findings that allow differentiating leiomyomas from stromal tumors; some of the latter feature a round or oval contour, intraluminal growth pattern, and absence of intralesional necrosis12.
Histologically, they are paucicellular spindle cell tumors with low to moderate cellularity and little mitotic activity. Furthermore, the suppression of genes that code for the α5 and α6 chains of type IV collagen could explain the origin of these tumors23.
Gastric tumors are classified into three large groups: malignant, benign, and tumor-like lesions, including the ectopic pancreas2. In 1727, Shultz was the first to describe a heterotopic pancreas, but it was not until 1859 that Klob reported his histological findings. For his part, Heinrich classified these lesions into three types in 1909, according to their histological structure: the first is a typical pancreatic tissue with acini, ducts, and islets of Langerhans; the second contains acini and ducts but lacks islet cells; and the third, called adenomyoma, is made up of a large number of ducts. It is common to see smooth muscle accompanying the lesion13,24.
The ectopic pancreas is usually found in 0.5% of laparotomies, and 70% of cases are gastric, mainly at the prepyloric level and on the greater curvature15. Usually, endoscopic ultrasounds can show extramural growth with irregular margins16.
Unlike the cases reported in the literature, the patient in the fourth case was female and, like a small percentage of patients with this pathology, presented with abdominal pain and gastrointestinal bleeding2,13-15. Ura et al informed that only seven cases of malignant transformation of this type of lesion had been recorded in the literature; most of them were located in the anthropyloric region16. Nonetheless, due to the suspicion of malignancy, management should be surgical with frozen biopsy during the intraoperative period13.
Of the total of laparoscopic gastrectomies performed in recent years in our hospital, 3.5% were benign gastric tumors, matching the records found in the world literature, which mention that more than 95% of gastric lesions are of the adenocarcinoma type25. The findings in the immunohistochemistry of the surgical specimen were decisive in differentiating the origin of some lesions.
Following the recommendations in the literature, an algorithm for the diagnostic and therapeutic approach of gastric lesions was designed (Figure 8). It states that preferably all suspicious gastric lesions should be taken to endoscopic biopsy and endosonography to determine significant characteristics of the tumor, such as the degree of depth of the submucosa. Thus, like the latter, other prognostic factors can help decide the therapeutic approach25.
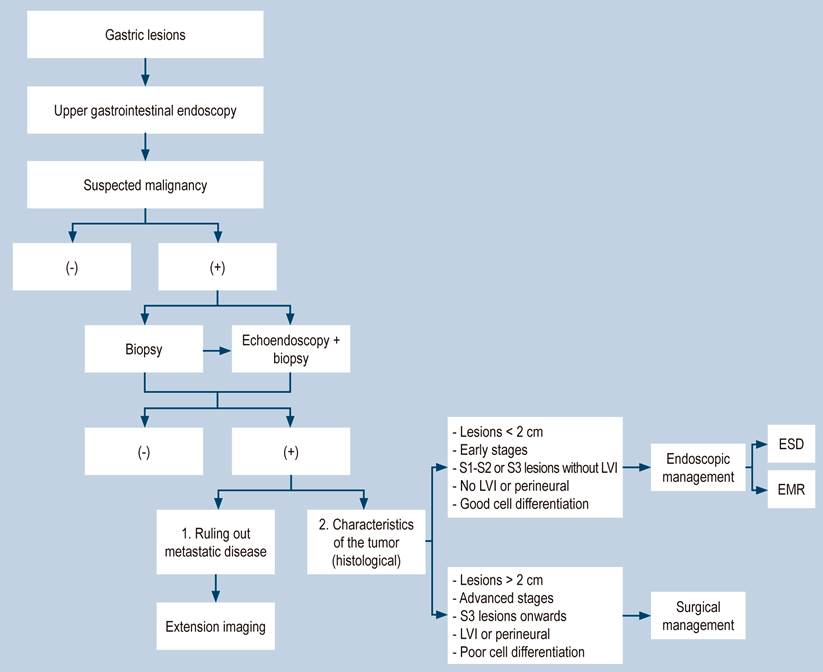
Figure 8 Algorithm for the diagnostic and therapeutic approach for gastric lesions. ESD: Endoscopic submucosal dissection; EMR: Endoscopic mucosal resection.
Accordingly, the main lessons are as follows:
First, although gastric cancer is the third leading cause of cancer death worldwide and should always be considered within the differential diagnosis of gastric lesions, the characteristics of preliminary imaging may also strongly suggest non-epithelial tumors (such as GIST) to propose the most convenient surgical management options.
Second, although GISTs represent a small percentage of gastric lesions, usually the infantile variant is the one that causes lymph node metastasis; therefore, lymph node dissection is dismissed for the surgical management of these tumors in adults. Moreover, this was one of the considerations when handling the cases above. Even though GISTs only require management with a gastric wedge, both the fact that some of the cases reported herein demonstrated a technically demanding location for performing organ-sparing surgery (such as a gastric wedge) and the possible epithelial origin of the lesions resulted in more aggressive surgical management. In two of the four cases described, lymph node dissection was performed given the high suspicion of a lesion of epithelial origin (adenocarcinoma).
Third, GISTs are usually dependent on the fourth echolayer in endosonography and are usually greater than 5 cm; given the high potential for malignancy, surgical management is always indicated in these cases as the first option. Now, even though the patient in the first case had a lesion of 2 cm in diameter, the endoscopic ultrasound findings suggested muscle involvement (fourth echolayer), which is why initial endoscopic management was not considered. Additionally, even though the patient in the third clinical case had two lesions between 1 and 2 cm, the endosonography report did not establish the compromise of the submucosa. Besides, the biopsies taken could not rule out tumor involvement; we think they were highly suspicious malignancy lesions with a high risk of metastasis. So, initial surgical management was also recommended for this patient.
Fourth, despite international guidelines advising endosonographic fine needle biopsy for non-epithelial lesions, this procedure brings about the risk of bleeding and intra-abdominal tumor spread or perforation. For these reasons, after discussing different options, the multidisciplinary board considered that due to the imaging peculiarities of the lesions and the fact that the biopsies were not conclusive in some patients, immediate surgical management was perhaps more relevant.
Fifth, it should be considered that, according to the pathology findings, immunohistochemistry is necessary for most of these rare lesions, making it possible to rule out gastrointestinal stromal lesions, among others. Nevertheless, studies of this type are costly to perform in patients with negative pathologies for malignancy, as in the case of patient 3.
Finally, we believe there was overtreatment in some cases; perhaps a second set of endoscopic ultrasound biopsies would have modified the surgical behavior in approximately half of them.
It is striking that most of the atypical lesions described in this research are usually located in the distal third of the stomach, suggesting that the origin of most of them could be related to levels of gastric acid production. No limitations are described in the conduct of the present study.











 text in
text in