Introduction
Malignant biliary obstruction is usually a clinical finding in advanced stages of the disease when treatment is usually for palliative purposes. The most common causes are pancreatic adenocarcinoma, cholangiocarcinoma, duodenal/ampullary adenocarcinoma, gallbladder adenocarcinoma, lymphomas, and compression by metastatic nodules1.
At present, endoscopic retrograde cholangiopancreatography (ERCP) is the treatment of choice for jaundice due to malignant distal biliary obstruction in patients with advanced disease or for palliative purposes2,3. In cases where ERCP is not feasible or successful, without gaining access to the papilla, biliary drainage should be attempted using the safest and most effective technique based on institutional experience. Percutaneous transhepatic cholangiogram (PTC) is an effective alternative, but with non-negligible morbidity and mortality since potential risks such as bile leakage, biliary peritonitis, hemorrhage, and percutaneous drain displacement (which is variable, but some series reach up to 30%) are described4. The other data against percutaneous drainage is the need to have an external biliary drainage catheter that could significantly alter patients’ quality of life5.
Another method of biliary drainage in malignant obstruction is endoscopic ultrasound-guided biliary drainage (EUS-BD), which is considered an effective alternative treatment3,6-10. Many authors prefer EUS-guided choledochoduodenostomy (EUS-CDS) as the endoscopic technique when the biliary obstruction is distal and hepatogastrostomy in cases of proximal biliary obstruction or when the duodenum cannot be accessed3,6.
We describe our initial experience in EUS-BD carried out in a reference hospital in Colombia.
Materials and methods
A retrospective case series of consecutive patients at the Hospital Pablo Tobón Uribe in Medellín, Colombia, from January 2019 to December 2020. Patients were older than 18 years with a diagnosis of malignant biliary obstruction taken to ERCP, which failed or was not feasible due to anatomical alteration.
In all cases, the procedure was performed under general anesthesia by endosonographists with more than two years of experience (JJC, GMK) using linear endoscopic ultrasound (Fujifilm® 580L), Expect needleTM 19-gauge puncture/aspiration needles; 0.035-inch x 450 cm hydrophilic guidewire, 6 Fr cystotome, and partially and fully covered metal biliary prosthesis (WallflexTM 6 and 8 cm long). During the procedures, insufflation with carbon dioxide (CO2) was carried out.
Technical success was defined as the proper positioning of the prosthesis with its proximal end in the bile duct and its distal end in the duodenal lumen, with fluoroscopic and endoscopic verification. Adequate bile drainage is observed.
Clinical success was determined by improvement in jaundice and a decrease in serum bilirubin to at least 50% of the pre-week value or typical values at 30 days of follow-up.
Statistical analysis
Demographic variables are presented as percentages and frequencies. Quantitative variables are described as means with standard deviations (± SD) in cases of the normal distribution. Continuous variables and median with interquartile range (IQR) are found for the non-normal distribution variables. Analyses were performed using Epi Info™ 7 software.
Description of the technique performed
Under endosonographic guidance, the bile duct is identified, delimiting the level of biliary obstruction. The duct size and the distance from the transducer to the wall most proximal to the equipment are then measured; in all cases, Doppler is used to avoid blood vessels. Subsequently, the bile duct is punctured with a 19-G needle under endosonographic guidance and aspirated to verify the return of bile. Then, a water-soluble contrast medium is applied to perform a cholangiography verified fluoroscopically. Upon mapping the bile duct, the 0.035-inch hydrophilic guidewire is advanced; the needle is withdrawn in a coordinated fashion to avoid the exit of the bile duct guidewire. Under endosonographic and fluoroscopic guidance, the cystotome is passed with a pure cut at 70 W until accessing the bile duct and immediately removed. A covered metal biliary stent is moved forward until placing it under fluoroscopic and endoscopic guidance. Finally, adequate biliary drainage is checked (Figure 1).
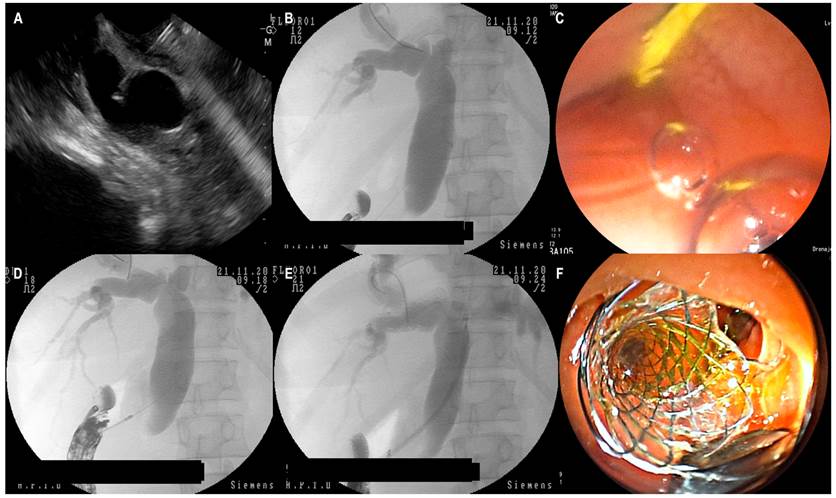
Figure 1 EUS-CDS. A. Transduodenal puncture of the common bile duct with a 19-G needle. B. Fluoroscopic view of EUS-guided cholangiography. C. Endoscopic view of the hydrophilic guidewire in the duodenum. Note the scant return of the contrast medium through the hole. D. Fluoroscopic view of the moment of cutting and passage from the 6 Fr cystotome to the common bile duct. E. Passage of the metal stent under fluoroscopic guidance. F. Endoscopic view of the biliary drainage, showing the distal (transduodenal) part of the stent. Images obtained at the Hospital Pablo Tobón Uribe.
Results
Between January 2019 and December 2020, 478 ERCPs were performed, of which 18 failed in the scenario of malignant biliary obstruction. Twelve transparietohepatic bypasses and six cases of biliary bypass were performed due to EUS-CDS in distal biliary obstructions at the institution. The mean age was 71.8 years (age range: 34-91 years), and the female sex represented two-thirds of the population under study. The average weight was 48.6 ± 4.9 kg, with a total body mass index (BMI) between 16 and 22. The leading cause of malignant biliary obstruction was adenocarcinoma of the head of the pancreas in 50% of the cases. The mean hospital stay after the procedure was 2.6 (SD ± 1.2) days. The leading causes of failed ERCP were secondary to tumor infiltration of the duodenal wall and critical duodenal stenosis; two cases (33.3%) required a duodenal stent placement in the same procedure (double biliary/duodenal bypass). The general characteristics of the cases are shown in Table 1.
Table 1 Demographic data, diagnosis, causes of failed ERCP, quality of life status, type of stent, and survival 30 days after the procedure
| Case | Age (years) | Diagnosis | ECOG | Cause of failed ERCP | Common bile duct diameter (mm) | Stent type | Stent size (mm) | Survival at 30 days |
|---|---|---|---|---|---|---|---|---|
| 1 | 34/F | Periampullary tumor | 1 | Duodenal wall deformed by infiltration | 19 | Coated metal | 60 x 10 | Yes |
| 2 | 75/M | Pancreatic adenocarcinoma | 4 | Duodenal stenosis | 15 | Coated metal | 60 x 10 | No |
| 3 | 78/F | Distal cholangiocarcinoma | 2 | Difficult cannulation, pinhole | 15 | Coated metal | 80 x 10 | Yes |
| 4 | 70/M | Pancreatic adenocarcinoma | 3 | Duodenal wall deformed by infiltration | 14 | Partially coated metal | 60 x 10 | Yes |
| 5 | 91/F | Periampullary tumor | 3 | Duodenal stenosis | 15 | Coated metal | 60 x 10 | Yes |
| 6 | 83/F | Pancreatic adenocarcinoma | 3 | Severe distal biliary stenosis | 10 | Coated metal | 80 x 10 | No |
ECOG: Eastern Cooperative Oncology Group; stent size (length [mm] x diameter [mm]).
The biliary bypass was performed in obstructive jaundice; Table 2 describes the mean values of the most relevant laboratories prior to the procedure. At three months of follow-up, paraclinical control was obtained from two patients with total bilirubin decreased to 0.87 ± 0.53 mg/dL and alkaline phosphatase of 378 ± 74 IU/L with adequate clinical evolution in all cases and minimum surveillance of 48 hours prior to discharge. Antibiotics were administered in all cases before the procedure. In three cases, intravenous (IV) piperacillin/tazobactam was used every six hours due to evidence of purulent cholangitis; in the other three, prophylactically prior to the procedure with ampicillin/sulbactam 3 g.
Table 2 Laboratory characteristics before the procedure
| Laboratory | Mean (SD) |
|---|---|
| Total bilirubin (mg/dL) | 9.7 ± 8.6 |
| Direct bilirubin (mg/dL) | 6.5 ± 6.4 |
| Alkaline phosphatase (IU/L) | 630 ± 256 |
| Platelets (109/L) | 296.3 ± 253 |
| INR | 1.18 ± 0.18 |
| Albumin (g/dL) | 2.05 ± 1.8 |
INR: International normalized ratio.
Technical success was achieved in 100% of the patients, while clinical success in five (83.3%). It was impossible to assess clinical success in the remaining case due to early death unrelated to the procedure (pulmonary embolism three days after the procedure). Self-limited bleeding occurred as an adverse event during a procedure in a patient with mild thrombocytopenia. This case did not require transfusion of blood products or additional interventions.
Delayed dysfunction was found in one case due to migration of the stent at six months. The average days of follow-ups were 111; the patient with the most extended follow-up has been jaundice-free for 365 days. Survival at 30 days from the procedure was 66.7%, and two patients died before 30 days due to causes unrelated to the procedures (pulmonary embolism and tumor progression).
Discussion
This case series in a third referral hospital shows our initial experience with EUS-BD in treating malignant biliary obstruction when the bypass by failed ERCP was not possible. In previous years, biliary drainage used to be done through PTC in all cases when the bypass was defined for palliative purposes.
Malignant biliary obstruction has a poor prognosis in the short term since the diagnosis is generally late. In this context, surgical management with curative intent is impossible, so we intend to make palliative interventions that improve patients’ quality of life.
Although percutaneous drainage using PTC is usually safe and effective, variable complication rates are described that are comparable to what has been described so far in EUS-BD11. The cons of percutaneous drainage may be the need to maintain an external catheter in many cases and perform reoperations more frequently (such as changing the catheter due to occlusion)4,12. In cases where internal biliary bypass with metal stents is performed, the complications described for this technique include cholangitis, bacteremia, bile leak, pleural puncture, empyema, bleeding, pain during or after the procedure, and dislocation of the catheter. Besides, stent occlusion is mentioned as a late sequela, which may result from obstruction by biliary debris or sludge, tumor growth through the stent, or excessive tumor growth above or below the stent.
When reviewing the operating characteristics of PTC in malignant distal obstructions, several studies of interest compare PTC with ERCP. In a first study published in 1987, Speer et al demonstrated significantly lower success rates for jaundice relief (61% vs. 81%, p = 0.017) relative to ERCP and significantly higher 30-day mortality (33% vs. 15%, p = 0.016). The highest mortality after percutaneous stents was due to bleeding and bile leakage13. Only plastic stents were used for PTC in this study, and the current technique of PTC with insertion of metal stents is likely to produce significantly different results. In subsequent studies, Piñol et al in 200214 and later Beissert et al12 compared PTC with self-expanding metal stents with polyethylene plastic endoprosthesis for ERCP, the latter little used in malignant pathology today. In Piñol et al’s work, the technical success rates of both procedures were similar (percutaneous: 75%, endoscopic: 58%, p = 0.29), while the therapeutic success was higher in the percutaneous group (71% vs. 42%; p = 0.03). Major complications were more frequent in the percutaneous group (61% vs. 35%; p = 0.09). In Beissert et al12, the complication rate was higher in the plastic endoprosthesis group (39% vs. 22%). There were no statistically significant differences between stent patency and clinical success. In a more recent multicenter study, internal biliary drainage with metal stents using PTC was compared with EUS-guided drainage in patients with malignant biliary obstruction in whom the initial ERCP was unsuccessful. EUS-guided drainage was an effective and safe alternative with similar clinical success, complication rates, costs, and quality of life11. Although stenting for PTC could be an attractive option, the bypass is usually done internally-externally in most centers.
For patients with palliative treatment, whose intention is to improve their quality of life, the EUS-BD may be superior and is a durable and safe method with high rates of technical and clinical success15-17, which can be performed when ERCP is unsuccessful. Furthermore, a recent systematic review and meta-analysis suggest that EUS-BD could have lower stent dysfunction rates than conventional ERCP18.
At present, several EUS-BD techniques have been developed, including various access routes and drainage methods3-5,19. Regarding the approach routes, two are used mainly: the transgastric intrahepatic approach or EUS-guided hepatogastrostomy and the transduodenal extrahepatic approach or EUS-CDS. Biliary drainage can generally be attained through transmural stent placement, antegrade stent placement, and the Rendezvous technique (EUS-RV)3.
Endoscopists often select one or two safe techniques with a high probability of success among the many EUS-BD techniques. The choice of technique or access route is usually individualized. The most relevant aspects for deciding on the approach are the operator’s preference (greater confidence in the method and experience), the availability of supplies in the institution, the patient’s anatomical characteristics, the underlying disease, the location of the biliary stricture, and the diameter of the intrahepatic bile duct. All these elements are considered essential in selecting access routes and drainage methods19, but the optimal treatment strategy for EUS-BD has not yet been established.
Many authors prefer the EUS-guided hepatogastrostomy approach when the stenosis is proximal or hilar, and EUS-CDS is preferred when the obstruction is distal3,4,6,7,9.
EUS-BD involves several steps, including puncturing the target, manipulating the guidewire, dilating the puncture tract, and placing a stent. Between these steps, technical difficulties may arise in each access route and drainage method. Furthermore, no consensus has been reached regarding problem-solving when the initial technique of EUS-BD is challenging. For these reasons, both technical problems and treatment algorithms have been poorly defined in EUS-BD, despite reporting overall technical success rates of 90%-96%3.
In our case series, EUS-CDS had a technical success of 100% and a clinical success of 83.3%, like that published by Villaverde et al20. When reviewing this last series, partially covered metal stents were used in all patients to prevent migration. In our series, the only case of late migration occurred in the patient in whom a partially covered metal stent was employed. There are still no comparative studies between different self-expanding metal stents in EUS-BD, but cholangitis, biliary peritonitis, and duodenal perforation with partially covered stents have been described more frequently21.
Concerning the type of prosthesis to be used in EUS-BD, there is a recent study in which lumen-apposing metal stents (LAMS) and covered self-expanding metallic stents were compared. From this study, it stands out that technical and clinical success was similar in the two groups. There were no significant differences in adverse events, 13.5% in the LAMS group and 20% in the self-expanding metal stent group (p = 0.71)7. The advantage of LAMS with a hot mechanism (hot Axios) is its insertion in a single step. It is technically more straightforward than self-expanding metal stents7,22, which require following the steps described in our paper. Despite these technical differences described, the costs associated with LAMS could limit their use in many institutions in Latin America and the world.
About plastic stents, there are higher rates of stent dysfunction and a higher risk of bile leakage; moreover, with current chemotherapy regimens, the overall survival of patients exceeds six months, which is why they are not recommended1.
In unresectable advanced pancreatic cancer cases, duodenal stenosis can present concurrently in 13-20% of patients, with a mean survival of 12 weeks23. Tonosuka et al described a series with 11 cases in which double bypass was performed using EUS-BD stents and duodenal stents with a technical and clinical success rate of 100%. In this study, the late complication rate was 27.3% (due to cholangitis and perforation in one case), and a better quality of life was accomplished in these patients. A double bypass (biliary/duodenal) was performed in two cases with technical and clinical success and no complications.
Our study’s limitations are the relatively small number of cases and their retrospective assessment.
We consider that multicenter studies should be conducted to compare this procedure’s technical and clinical success versus transparietohepatic bypass and describe patients’ quality of life.
It may be necessary to choose the initial cases well to achieve greater confidence in a relatively new method in our environment and increase the casuistry. In the future, it will be an alternative on the same level as a percutaneous bypass in malignant distal biliary obstructions.
In any case, the knowledge and progressive experience of this therapeutic alternative in managing malignant biliary obstruction will improve the perception of endoscopists about EUS-CDS24.
Conclusions
In cases of malignant distal biliary obstruction in which palliative bypass therapy is considered, ERCP should be attempted first. If ERCP is not feasible, EUS-CDS is an alternative method with a high effectiveness rate due to its clinical and technical success; besides, it is safe since few complications in expert hands are described.
EUS-BD may offer a better quality of life than PTC, as it would avoid the need for an external catheter and frequent drain changes in patients.











 text in
text in 



