Introduction
Leukocytoclastic vasculitis is defined as damage and inflammation of blood vessel walls. It consists of small-vessel vasculitides that anatomically and pathologically involve leukocytoclasia or hypersensitivity vasculitis1. Leukocyoclastic vasculitis is an extraintestinal manifestation of inflammatory bowel disease that occurs in 15% to 20% ulcerative colitis (UC) cases, since it shares an etiopathogenic mechanism with UC, and the causal relationship of the two conditions is caused by immune complexes produced in the intestinal mucosa due to the exposure of submucosal lymphoid tissue to fecal antigens; these vasculitides could precipitate in the walls of small vessels and clinically they can be synchronous at the time of onset. At least 20 cases have been reported up to 2014 in the English literature, with 40 years being the predominant age2,3. The association with Clostridium difficile can alter the course of the disease by activating the immune response towards the pathogen causing the infection, which is normally found in the environment and causes colitis, where the patient experiences diarrhea after being administered the antibiotics, since the latter disrupt the usual microbiota of this organ4.
Case presentation
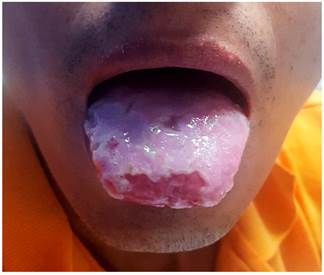
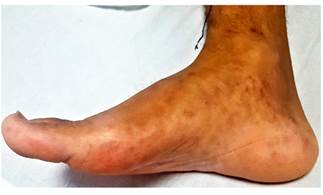
This is the case of a 36-year-old male with no relevant history of disease, but with a family history of colon cancer (his mother died from it) who had experienced watery diarrhea with presence of mucus and scarce bleeding for five years, and who had been treated with oral ciprofloxacin 500 mg for 7 days and probiotics on several occasions. The patient was admitted due to experiencing a worsening of his condition 20 days prior to admission consisting of colicky abdominal pain, abdominal distention, watery diarrhea with mucus and blood (4 to 6 stools per day), increased body temperature (unquantified), arthralgia without arthritis and an approximately 10 kg weight loss in 1 year. The following findings were described on the patient’s physical examination: soft, non-tender abdomen with erythema, edemas on both hands and feet, and presence of aphthous ulcers on the tongue (Figures 1 and 2).
The following results were reported on the patient’s admission tests: hemoglobin: 7.7 mg/dL; hematocrit: 26.5 %; leukocytes: 11 830/mm3; neutrophils 75.4 %; platelets: 544 000; CRP: 16.17 mg/dL; normal electrolytes values and kidney function; VDRL test: non-reactive; B12 vitamin > 2000; ferritin: 170.58; serum iron: 31; transferrin: 205; tumor markers within normal values; negative serology tests for cytomegalovirus, herpes virus, rubella, hepatitis B virus, hepatitis C virus and human immunodeficiency virus (HIV); positive Stool C difficile toxin test; negative stool culture; in addition, a negative result was obtained in all immunological markers tests, except for anti-neutrophil cytoplasmic antibody (c-ANCA). An upper gastrointestinal tract endoscopy allowed identifying ulcers in the distal esophagus (Figure 3) and performing a biopsy by removing tissue from both the edges and the center of the ulcers in order to make differential diagnoses (cytomegalovirus, herpes simplex, Crohn’s disease, among others). The biopsy report described the following results: mixed inflammatory cell infiltration with nuclear polymorphs in the epithelium, positive immunohistochemistry for cytomegalovirus and negative for herpes. A thickening of the folds in the prepyloric region was also observed (Figure 4) and a biopsy was performed to rule out the inflammatory bowel disease with Crohn’s disease differential diagnosis. In said biopsy, chronic active erosive gastritis with GIEMSA and hypodense colonization with Helicobacter pylori bacilli, which was associated with the incidental lesions, were reported. In addition, a colonoscopy allowed the identification of both an altered vascular, erythematous, granular, edematous and friable pattern in the colon and a pseudopolypoid lesion in the descending colon (Figure 5), while UC was described in the biopsy report.
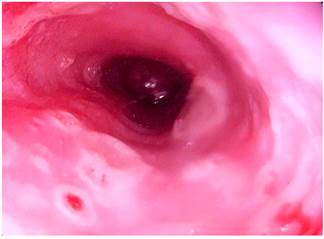
Figure 3 Upper gastrointestinal tract video endoscopy showing the presence of 5 mm ulcers with raised edges and erythematous background in the distal esophagus.
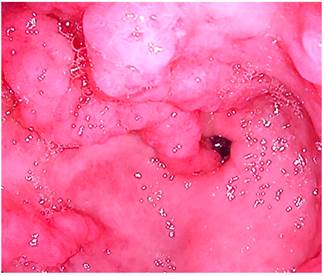
Figure 4 Upper gastrointestinal tract video endoscopy at the level of the antrum in which a thickening of the folds in the prepyloric region with erythemata and edemata are observed.
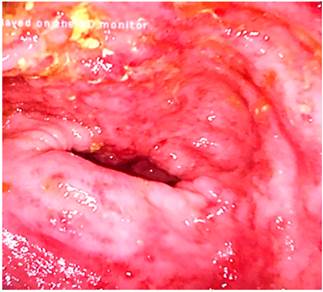
Figure 5 Colonoscopy showing an alteration of the vascular pattern, mucosa, granular erythema and friable edema and the presence of superficial ulcers in the sigmoid colon.
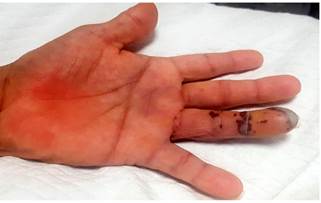
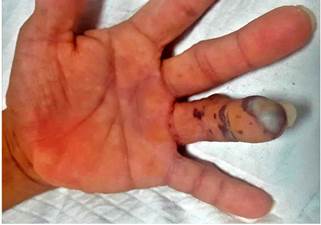
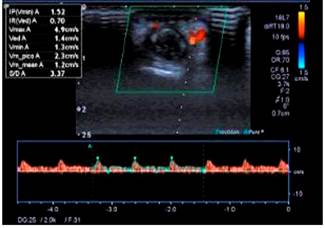
48 hours after the endoscopic procedure was performed, petechial and necrotic skin lesions appeared on the fourth finger of the left hand (Figures 6 and 7). Thus, an upper limb Doppler ultrasound that showed decreased fluids with parvus tardus waves in the palmar and dorsal digital arteries of the fourth finger of the left hand was performed (Figure 8). He was then assessed by the dermatology service and based on the results of a skin biopsy, a small-vessel vasculitis (septal panniculitis, perivascular inflammatory infiltration with predominance of neutrophils, fibrinoid damage and extravasated red blood cells) diagnosis was reached. The patient was then treated as follows: he was administered 3 pulses of intravenous methylprednisolone 500 mg, which was subsequently switched to oral prednisone 60 mg until corticosteroid administration was suspended; subsequently intravenous metronidazole 500 mg was administered for 14 days and oral mesalazine 3 g was prescribed, which he is currently using. At present, he is asymptomatic and does not show any sign of lesions on his hands or tongue, and UC is in clinical remission; corticosteroid therapy was suspended and the only drug he is taking is mesalazine 3 g/day; furthermore, the patient has reported he has not experienced any clinical manifestations in any of his follow-up visits in the outpatient service.
Discussion
Inflammatory bowel disease has multiple extraintestinal manifestations. The most frequent include osteoarticular involvement (osteopenia, osteoporosis), skin (psoriasis, Sweet’s syndrome, dermatitis herpetiformis, epidermolysis bullosa acquisita, necrotizing vasculitis), ophthalmologic (uveitis, dry eye, episcleritis, etc.), hepatobiliary and neurological manifestations; in addition, skin manifestations occur in 15% of cases5,6.
Leukocytoclastic vasculitis is a cutaneous vasculitis with varying clinical features that might include very rare systemic manifestations associated with inflammatory bowel disease1. Histologically, these lesions show neutrophilic infiltration around the affected vessels, fibrinoid necrosis (fibrin deposition in the blood vessel wall), red blood cell extravasation, and endothelial cells damage7. Its pathophysiology is based on the formation of antigen-antibody complexes in the vascular wall at the time the patient is exposed to the etiological factor, which triggers complement activation with subsequent occurrence of hemorrhage and ischemic thrombosis8.
Leukocytoclastic vasculitis has an annual incidence of 30-45 cases per 1 million people. It can occur at any age and its prevalence is similar in men and women. Etiologically, it can be idiopathic in 50% of cases or it can be caused by different factors including infections (Mycobacterium, hepatitis B, hepatitis C, Staphylococcus aureus, Chlamydia, Neisseria and HIV), the use of different drugs (beta-lactams, furosemide, allopurinol, non-steroidal anti-inflammatory drugs, amiodarone, clindamycin, vancomycin, sulfonamides, metformin) and systemic or neoplastic diseases9,10.
Leukocytoclastic vasculitis associated with systemic autoimmune diseases accounts for 10-15% of cutaneous vasculitis cases; most frequently, it is associated with rheumatoid arthritis, systemic lupus erythematosus, phospholipid syndrome, Sjögren’s syndrome and Behçet’s disease; it also shares a etiopathogenic mechanism with UC and, therefore, the causal relationship of the two conditions is caused by immune complexes produced in the intestinal mucosa by exposure of the submucosal lymphoid tissue to fecal antigens; these vasculitides could precipitate in the walls of small vessels and activate proinflammatory cytokines, such as interleukin 15 (IL-15) and its receptor; likewise, they could differentiate lamina propria B cells resulting in the formation of typical lesions in patients with UC, which has been established as an extraintestinal manifestation of UC (58%)1,3,10,11.
Leukocytoclastic vasculitis clinical presentation is characterized by palpable purpura, pain and burning sensation in multiple (58%) or a single area (42%). Lesions are predominantly found in the lower limbs (83%), followed by the upper limbs (42%), the trunk (25%) and the buttocks (25%)3,12,13. When leukocytoclastic vasculitis is suspected as an extraintestinal skin manifestation of UC, a skin biopsy must be performed in order to observe the histologic features of neutrophil and lymphocyte infiltration around dermal blood vessels showing leukocytoclasia, fibrin deposition in the vessels, fibrin thrombi, erythrocyte extravasation and the resultant epidermal necrosis10,14.
Symptoms in patients with leukocytoclastic vasculitis and UC include fever, myalgias, arthralgias, hematuria, abdominal pain, vomiting, bloody diarrhea, rectal incontinence, and tenesmus; other symptoms include anorexia, asthenia, and weight loss. Symptom onset is reported to occur approximately one week after exposure to the triggering factor9,15,16. Colonic involvement in patients with leukocytoclastic vasculitis and UC is observed in 50% of cases with pancolitis, 40% with distal colitis and 10% with the development of leukocytoclastic vasculitis after total colectomy2.
Other causes of dermatologic extraintestinal manifestations and inflammatory bowel disease must be considered as differential diagnoses and must be ruled out before a final diagnosis is reached. Dermatological causes include pyoderma gangrenosum, necrotizing vasculitis, cutaneous polyarthritis nodosa, and granulomatous vasculitis. On the other hand, infectious causes include C. difficile, Escherichia coli, Salmonella, Shigella, Yersinia and Campylobacter. Crohn’s disease, pseudomembranous colitis and irritable bowel syndrome are also other differential diagnoses9,16,17.
The association of C. difficile in patients with leukocytoclastic vasculitis and UC is due to the fact that it is a gram-positive, sporulating, strict anaerobe bacillus. It is normally found in the environment. When it causes an infection in humans, it generally produces colitis, which in turn manifests as diarrhea, fever, anorexia, leukemoid reaction, hypoalbuminemia and bleeding after the intake of antibiotics, since the latter alter the normal bacterial flora of this organ18. It is the most common cause of diarrhea in the hospital setting due to its etiological association with antibiotics. The risk of developing C. difficile infection also depends on other factors such as age, diet, and dose of the causative antibiotic, among others19.
Diagnosis is based on clinical and laboratory criteria. These criteria must include three sudden onset diarrhea episodes in which the cause is not identified, the presence of C. difficile toxins A or B in the stools, or the endoscopic presence of pseudomembranes in the colon18. In a study conducted in the United States of America, Zacharioudakis et al. assessed the factors that have been identified as triggers of C. difficile infection or the transition from colonization to infection in exposed patients who might require subsequent hospitalization. According to these authors, antibiotic use was associated with infection in 75% of these patients, being fluoroquinolones the most commonly prescribed class of antibiotics20.
Most leukocytoclastic vasculitis cases are self-limited, with 90% of them occurring in weeks to months of symptom onset10. Treatment depends on two factors: its etiology and its extent. In case of infectious causes, the underlying infection must be treated. When it occurs as part of a systemic vasculitis, treatment is based on the severity of organ involvement. Generally, it requires the use of steroids and immunosuppressants7.
Based on the activity and severity of the disease, there are several treatment regimens to be used in patients with leukocytoclastic vasculitis and UC. Mild to moderate disease is usually treated with oral or topical aminosalicylates or with glucocorticoids. These drugs inhibit the production of cytokines and other inflammatory mediators. Glucocorticoids have shown to be effective in inducing disease remission; however, patients may experience side effects such as weight gain, hyperglycemia, acne, hirsutism, high blood pressure and bone resorption, the latter requiring the use of supplemental calcium and vitamin D. Regarding aminosalicylates, mesalazine stands out, and side effects include headache, abdominal pain, nausea, vomiting, and diarrhea, among others.
Severe disease is characterized by having more than 6 bloody stools per day, anemia and hemodynamic decompensation. Generally, it requires the patient to be hospitalized for treatment and stabilization. When glucocorticoids treatment is not effective, salvage therapy consisting of infliximab or cyclosporine use for approximately 5 to 7 days until a positive response is obtained has been reported to be effective21.
Regarding the treatment of C. difficile-associated diarrhea or colitis, general measures include the suspension of medical treatments that use antiperistaltic and laxative agents, and assessing the possible suspension of antibiotic, opioids and proton pump inhibitors (PPIs) treatments22.
The severity of C. difficile infection must be evaluated. In a first episode, if the infection is mild to moderate, metronidazole is administered orally for 10 days. If the infection is severe, the oral administration of vancomycin 125-500 mg 4 times per day during 14 days or fidaxomicin 200 mg 2 times a day for 10 days is required. In the case of a severe and complicated infection, the administration of vancomycin 4 times a day, either orally, using a nasoenteral tube or rectally via a retention enema, together with intravenous metronidazole, both during a period of 14 days, is indicated. On the other hand, in case of recurrent infection, the same treatment regimen used for first episode C. difficile infections or tapered pulsed vancomycin treatment regimens are indicated. Fecal microbiota transplantation is also indicated in cases where three or more recurrences take place23. Fecal microbiota transplantation has shown a good clinical response in the treatment of patients with recurrent infection. In a randomized trial, symptom resolution was reported in 94% of patients who were administered vancomycin for 5 days, followed by one or two fecal microbiota transplantations24. According to several clinical trials, the aforementioned treatments are those recommended to treat C. difficile infection.
From the conditions already addressed, the incidence of C. difficile infection has been found to be 1.8% to 5.7% in UC patients25. In addition, this infection alters the course of UC by activating the immune response to the pathogen causing the infection25. Both concomitant diseases are associated with a higher mortality rate16.
Conclusions
Leukocytoclastic vasculitis and UC associated with C. difficile infection is uncommon. Clinically their onset is synchronous, although the occurrence of skin lesions is not always related to the activity of inflammatory bowel disease. In the case reported here, vasculitis onset coincided with the C. difficile infection-associated exacerbation of the undiagnosed inflammatory bowel disease. The occurrence of any dermatologic manifestations must be looked into, since reaching a diagnosis of vasculitis may have important implications for the evolution and clinical improvement of both inflammatory bowel disease and vasculitis itself.











 text in
text in