Introduction
Worldwide, tuberculosis is one of the major diseases with the highest social and economic burden. By 2017, a global incidence of 10 million cases was estimated, being more frequent in Southeast Asia and Africa1, and about 1.6 million people died from it. In Latin America, an incidence of 263,000 cases and nearly 20,000 deaths were reported for the same year1. In Colombia, tuberculosis continues to be a prevalent disease, with an incidence of 26.5 cases per 100 000 inhabitants and a total of 14 480 new cases reported in 20172, where it mainly affects people older than 65 years, has a higher prevalence in males and pulmonary tuberculosis is predominant (83.3 %)2.
Risk factors of tuberculosis can be classified into two large groups: people who have been in contact with Mycobacterium tuberculosis or who have had the disease (immigrants, indigents, IV drug users, health care workers) and those who are immunocompromised (the elderly, people with human immunodeficiency virus [HIV], silicosis, diabetes mellitus [DM], chronic kidney disease [CKD], malnutrition, among others)3. Coinfection with HIV is the most important risk factor, for at least 40% of patients with the virus die from tuberculosis and in approximately half of the cases the disease is only diagnosed until an autopsy is made4.
Although tuberculosis mainly affects the lung, the frequency of patients in which an extrapulmonary diagnosis of the disease is made is higher, which is explained by the hematogenous or lymphatic dissemination of M. tuberculosis bacillus through the body and whose clinical presentation can be evidenced in later years. Risk factors of extrapulmonary tuberculosis include HIV infection, use of tumor necrosis factor (TNF) antagonists, use of corticosteroids, presence of malignancies, being female and having comorbidities such as DM or CKD5. Its prevalence varies from 10 % to 60 % depending on the geographical location. For example, in 2016, extrapulmonary tuberculosis and the mixed presentation form had a prevalence of 20 % and 10 %, respectively, in the United States6. In Colombia, out of the total number of new tuberculosis reported in 2017, about 17 % were extrapulmonary cases, and most of these patients had one of the abovementioned risk factors2.
The genitourinary tract, the lymph node, the bones, and the pleura are the extrapulmonary sites most frequently affected by the disease; meanwhile, the gastrointestinal tract ranks fifth or sixth in order of frequency depending on the local epidemiology5. Furthermore, within the gastrointestinal tract, tuberculosis is most commonly found in the ileocecal region and the terminal ileum, as it has been reported that 67% of gastrointestinal tuberculosis cases occur in these regions7. Likewise, identifying gastrointestinal tract involvement is a diagnostic challenge given that the disease presents with a very varied and non-specific symptomatology, which can sometimes include serious complications such as perforation, bleeding and obstruction. Secondly, it mimics other diseases like ulcerative colitis, lymphoma, amoebiasis or Crohn’s disease7. Its diagnosis requires clinical and laboratory findings, being microbiological culture and molecular analysis techniques the tests with the highest sensitivity and specificity. Finally, it can bet treated pharmacologically or surgically depending on its clinical presentation.
This is the case of a 75-year-old man with intestinal tuberculosis who had risk factors for developing this disease and in which management was complex from the time of hospitalization due to the presentation and location of the disease. Likewise, the diagnostic tools and differential diagnoses that were considered to reach the diagnosis and adequate treatment are described, as well as how, in spite of this, the patient died.
Clinical case
This is the case of a 75-year-old retired man from Bogota, who worked as a guard for more than 50 years. He also worked in the agriculture sector for 4 years in a rural area near the city. He had a history of smoking for more than 3 decades (pack-year index [PYI]= 12), exposure to wood smoke, arterial hypertension, CKD with a glomerular filtration rate (GFR) of 49 mL/min/1.73 m2 according to the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation, prostate cancer that required him undergoing a prostatectomy and subsequent anti-androgen therapy with gonadotropin-releasing hormone (GnRH) analogues with normal prostate antigen values at 3-year follow-up. In addition, the presence of anemia was under study and iron deficiency was documented in the anemia panel tests, which was treated on an outpatient basis with erythropoietin and vitamin B12 supplementation.
The patient was admitted due to having experienced a weight loss of approximately 12 kg, night sweats, asthenia, adynamia for 9 months, as well as occasional productive cough in the last month. Additionally, he reported having experienced 4 to 5 diarrhea episodes per day, without mucus or blood, for 4 days prior to admission, which resolved with hydration and general care measures. Fever (38.3 °C), general paleness and tachycardia were documented on admission; besides, leukopenia, microcytic anemia with criteria for blood transfusion, thrombocytopenia, mild hypovolemic hypoosmolar hyponatremia, hypomagnesemia and hypoalbuminemia were reported on admission laboratory tests (Figure 1 and Table 1). Findings compatible with silicoanthracosis and hypodense images in the vertebrae of unspecific nature were observed on a CT scan of the chest (Figure 2).
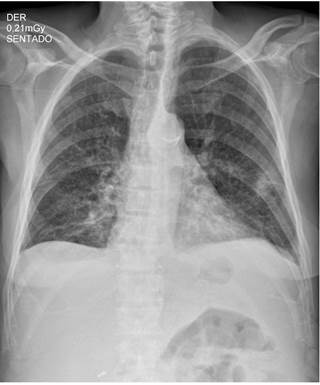
Figure 1 Anteroposterior (AP) view of chest X-ray showing thick reticular interstitial opacities in both lung fields and free costophrenic angles.
Table 1 Laboratory findings on admission and during hospitalization.
| Laboratory test | On admission | At week 1 | At week 2 | At week 3 |
|---|---|---|---|---|
| Total bilirubin (mg/dL) | 2720 | 3690 | 2040 | 2530 |
| Direct bilirubin (mg/dL) | 2067 | 2940 | 1428 | 2049 |
| Indirect bilirubin (mg/dL) | 260 | 350 | 208 | 254 |
| Albumin (g/dL) | 23 | 28.9 | 23.2 | 24.5 |
| Alkaline phosphatase (IU/L) | 6.9 | 9.5 | 7.1 | 7.7 |
| Amylase (IU/L) | 78 000 | 92 000 | 58 000 | 50 000 |
| AST (IU/L) | 1.4 | 1.06 | 1.02 | 0.97 |
| ALT (IU/L) | 28.8 | 30.8 | 31.7 | 35.3 |
| PT | 0.52 | 1.06 | ||
| INR | 0.30 | 0.85 | ||
| PTT | 0.22 | 0.21 | ||
| Sodium (mEq/L) | 2.3 | 2 | ||
| Potassium (mEq/L) | 142 | 138 | ||
| Chlorine (mEq/L) | 24 | 27 | ||
| Calcium (mEq/L) | 31 | 28 | ||
| Magnesium (mEq/L) | 30 | 21 | ||
| HIV | 16.4 | 16.1 | ||
| VDRL | 1.06 | 1.04 | ||
| Hepatitis C serology | 33 | 48.5 | ||
| Hepatitis B serology | 126 | 129 | 135 | 130 |
| Vitamin B12 | 4.2 | 3.07 | 5.03 | 4.4 |
| Folic acid | 94 | 99 | 101 | 99 |
| Prostate antigen | 8.1 | 9.2 | 9.3 | 10.3 |
| Antigen test | 1.23 | 1.41 | 2.24 | 1.5 |
| Histoplasma antibodies | Negative | |||
| Rheumatoid factor | Non-reactive | |||
| ANA | Negative | |||
| ENA | Negative | |||
| Anti-Ro antibodies | > 2000 | |||
| Anti-La antibodies | 19.2 | |||
| Anti-Sm antibodies | 0.463 | |||
| RNP | Negative | |||
| Anti-DNA antibodies | Negative | |||
| Total bilirubin (mg/dL) | 10.4 | |||
| Direct bilirubin (mg/dL) | Negative | |||
| Indirect bilirubin (mg/dL) | Negative | |||
| Albumin (g/dL) | 2.9 | |||
| Alkaline phosphatase (IU/L) | 3.6 | |||
| Amylase (IU/L) | 12.6 | |||
| AST (IU/L) | 2.0 | |||
| ALT (IU/L) | Positive 1/40 |
ANA: antinuclear antibodies; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BUN: blood urea nitrogen; ENA: extractable nuclear antigens; INR: international normalized ratio; RNP: ribonucleoprotein; PTT: partial thromboplastin time; PT: prothrombin time; VDRL: Venereal Disease Research Laboratory test.
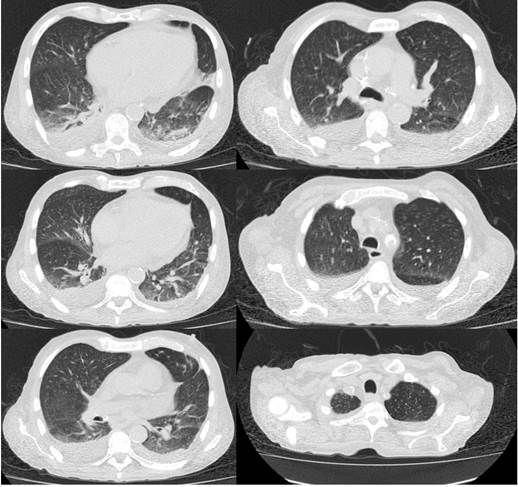
Figure 2 High-resolution computed axial tomography (HRCT) of the chest thorax in which silicoanthracosis, atelectasis of the lower lung lobes and altered density of the vertebral bodies and sternum.
Given the persistence of the fever episodes, antibiotic treatment with cefepime was started and an acid-fast bacilli smear was performed, in which negative results were obtained. Due to the altered hematological cell lines, further studies were carried out: peripheral blood smear (normal results), protein electrophoresis with a polyclonal component, flow cytometry for paroxysmal nocturnal hemoglobinuria (negative), and a bone marrow biopsy (negative for malignancy), with a hypocellularity of 10%, an expected result for a 75-year-old person. Bone marrow involvement was ruled out and intravenous iron administration was started. Regarding the febrile syndrome, the following findings were documented in additional studies: normal liver function tests, an abdominal ultrasound with evidence of hepatomegaly and splenomegaly with multiple focal lesions corresponding to prior infarcts, negative blood cultures, negative febrile antigens, negative sputum culture, normal prostate antigen and a thyroid ultrasound without any abnormal finding. A contrast-enhanced computed tomography of the abdomen was also performed, where a thickening of the ileocecal mucosa was evidenced, as well as the splenic lesions that had already been documented (Figure 3). Endocarditis was ruled out by means of transesophageal echocardiography.
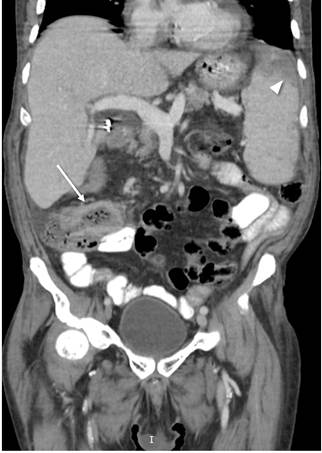
Figure 3 CT scan of the abdomen showing thickening of the distal ileum wall (white arrow) and subcapsular hypodense images corresponding to infarcts (arrowhead).
The following findings were documented in a contrast-enhanced MRI of the whole spine: lytic lesions located in the L2, L3 and L4 vertebrae and right iliac bone with signs of bone marrow reconversion (Figure 4). The patient’s condition worsened progressively while being clinically surveilled during the antibiotic treatment. On a colonoscopy an ulcer was evidenced in the ileocecal valve and cecum, so a biopsy was performed in order to analyze the sample using stains for detecting fungi and polymerase chain reaction (PCR) for tuberculosis. Given the worsening of the clinical condition of the patient, together with the findings evidenced in multiple organs, treatment with amphotericin B was started due to the suspicion of histoplasmosis, however such management was suspended since negative galactomannan levels were reported.
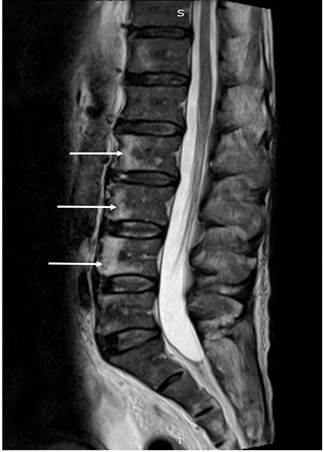
Figure 4 Magnetic resonance imaging (MRI) of the lumbar spine showing focal lytic lesions at L2, L3 and L4 (white arrows).
Despite the patient did not have any symptom related to autoimmunity, but he was suffering from prolonged fever, autoimmunity tests were requested, and the following findings were reported: negative rheumatoid factor, negative ENA, negative anti-dsDNA and positive ANA at a dilution of 1/40.
The patient’s clinical condition deteriorated as he experienced somnolence, anasarca and the fever worsened, especially in the evenings; then he presented with ventilatory failure and multiorgan dysfunction. A positive PCR report for tuberculosis from the sample taken from the ileocecal ulcer was received, a 4-drug regime treatment for TB consisting of pyrazinamide, ethambutol, rifampicin and isoniazid was immediately started. Despite the treatment, the patient presented with septic and distributive shock, acute hypoxemic respiratory failure and finally died 3 days after starting the 4-drug regimen.
Discussion
Extrapulmonary tuberculosis is an unusual type of tuberculosis. It accounts for 20% to 25% of TB cases8. HIV infection, use of tumor necrosis factor (TNF) antagonists, use of corticosteroids, the presence of malignancy, DM or CKD have been described as risk factors5. Regarding its pathophysiological mechanism, blood or lymphatic dissemination, with distribution to any region of the body, have been described; the prevalence of gastrointestinal tract (GIT) involvement in extrapulmonary tuberculosis cases ranges between 3 % and 5 %, raking sixth place in terms of frequency9. An up to 2 % mortality rate has been reported in patients undergoing treatment for tuberculosis10. Currently there are no local data in Colombia regarding the prevalence, morbidity and mortality of this type of extrapulmonary tuberculosis. Zoonotic tuberculosis caused by M. bovis represents < 1 % of the causes of GIT tuberculosis, and it is associated with the consumption of unpasteurized dairy products11.
The pathophysiology behind infection by the bacillus can occur by the ingestion of sputum from an active focus in the lung, hematogenous or lymphatic dissemination, and direct contact from an adjacent organ. The bacillus has a predilection for the ileocecal region and the terminal ileum due to their characteristics such as stasis, abundant lymphoid tissue, increased absorption rate and direct contact of the bacillus with the mucosa7. Additionally, in Peyer’s patches, the presence of α-cells, responsible for phagocytizing foreign agents, serve as a gateway for the microorganism11.
GIT tuberculosis has a larval, bizarre and nonspecific clinical presentation. Patel & Yagnik12, in a study conducted in 69 patients with GIT, 84% had ileocecal valve involvement, and the most common symptoms were abdominal pain (76%), fever (72%), weight loss (60%), chronic diarrhea (28%) and sensation of having an abdominal mass (10%). In some cases there may be complications such as intestinal perforation, fistula development, obstruction secondary to a mass (tuberculoma) or gastrointestinal tract bleeding11,13. Regarding macroscopic findings, transverse mucosal ulcers are the most frequent in GIT tuberculosis cases (60%), with a greater involvement of the jejunum, the ileum and the cecum; followed by hypertrophic ulcer (30%) and, finally, hypertrophic lesions (10%) with greater involvement of the ileum and the cecum13. Histological changes include confluent caseating granulomatous inflammation, epithelioid macrophages, Langhans giant cells and lymphocytes. However, none of these findings is pathognomonic of the disease, so additional aids such as staining, culture or molecular testing are required14.
Imaging findings are not very sensitive, with indirect signs such as obstruction, perforation or calcified mesenteric lymphoid nodules. Barium tests are useful to identify mucosal lesions, constriction, a deformed cecum or a dysfunctional and dilated ileocecal valve13. On the other hand, findings that may observed on CT scans include an asymmetric thickening of the intestinal wall with ganglionic growth, terminal ileum stricture, para-aortic and mesenteric adenopathy with extensive areas of central hypodensity and peripheral hyperattenuation15.
Abnormal laboratory findings are nonspecific, some of them include the presence of leukopenia, thrombocytopenia, anemia, increased erythrocyte sedimentation rate and elevated protein C levels16. Microscopy, mycobacterial culture and PCR assay can be used to diagnose the disease or isolate the bacillus. Mycobacterial culture has a sensitivity of about 60% with a time limit of 4 weeks to obtain results. PCR is the test with the highest performance to diagnose intestinal tuberculosis with a sensitivity and specificity of 87% and 96%, respectively, in the case of next-generation PCR assays17. However, Kivihya-Ndugga et al.18, in a study that compared the performance of chest X-ray, Ziehl-Neelsen staining and PCR in diagnosing tuberculosis, PCR had a specificity PCR of 84%, inferior to the 98% reported for Ziehl-Neelsen staining based microscopy. This proves that the use of PCR depends on the local prevalence of the disease and the available cost-effectiveness when tuberculosis is suspected.
Taking all of the above into account, the diagnosis of GIT tuberculosis is based on clinical suspicion and the presence of risk factors that can be confirmed in the anamnesis, supported with imaging and laboratory findings. However, the main differential diagnoses such as Crohn’s disease, ulcerative colitis, lymphoma, amoebiasis, histoplasmosis, among others, must always be ruled out first9. The treatment of tuberculosis of the GIT is similar, in terms of duration and drug regimen, to that of pulmonary tuberculosis, even 6-month treatments have a similar efficacy11. Treatment of people with tuberculosis and HIV coinfection is based on CD4 lymphocyte levels: if they are < 100,000 cells/µL, antiretroviral treatment with HAART must be started; if they are between 100,000 and 200,000 cells/µL, the first phase of treatment for tuberculosis diseases can be implemented with subsequent initiation of HAART; finally, if they are > 200,000 cells/µL, the two phases of antituberculosis treatment can be administered before starting HAART11. Response to treatment is usually confirmed by the disappearance of symptoms 2 weeks after starting the treatment regimen for tuberculosis and the disappearance of lesions, confirmed by means of endoscopic procedures, 3 months after starting the therapy13.
In this paper, the case of a patient with ileocecal tuberculosis was described. This is a rare disease that, when suspected, requires ruling out other conditions more likely to be present in patients with nonspecific symptoms, persistent fever, and multiple comorbidities such as the one presented here. In addition, the unspecific symptoms and signs of our patient, together with the negative results obtained in usual diagnostic tools, made it difficult a proper management of the disease, which in turn resulted in the late initiation of treatment. We present this case as a contribution to the literature addressing GIT tuberculosis, and in our opinion, the proper understanding of this type of tuberculosis, as well as of its management, favors a proper approach of future patients with this disease.











 text in
text in 



