Introduction
Biliary atresia is a condition that affects the hepatobiliary system by causing a progressive inflammatory obliteration of the intra- or extrahepatic ducts. Its incidence varies from 1:3000 to 1:20 000 live births1-3 and It occurs more frequently in females1,3. The onset of the disease occurs during the first weeks of life when the baby presents with progressive jaundice associated with acholia and choluria4. Kasai biliodigestive shunt is the treatment of choice. Despite being treated, 50% of patients require a liver transplant before turning 2 years old1.
Biliary atresia associated with positive cytomegalovirus (CMV) IgM is one type of biliary atresia5. It accounts for 10-20% of biliary atresia cases in Europe and 50% in China5. Its diagnosis tends to be late and is associated with a worse prognosis1,4.
The aim of this paper is to present the clinical case of a patient diagnosed with biliary atresia associated with positive CMV IgM, as well as discuss aspects related to this disease, its diagnosis and the current management suggested by the relevant literature.
Clinical case
This is the case of an 82-day-old patient who was born through vaginal delivery without complications and without any significant history of disease who was referred to our health institution due to having had the following clinical signs and symptoms for two months: generalized and progressive jaundice, acholia, choluria and fever episodes. The following findings were reported on physical examination: patient in a general good condition, with jaundice, distended abdomen and painless hepatomegaly on palpation. Elevated aspartate aminotransferase (AST: 430 U/L), alanine aminotransferase (ALT: 191 U/L), alkaline phosphatase (ALP: 531 mg/dL) and total bilirubin (10.41 mg/dL) and direct bilirubin (7.28 mg/dL) levels were reported in the remission laboratory tests results. In addition, complete blood count test values and blood sugar levels were normal. Given the clinical signs of cholestasis, biliary atresia and Alagille syndrome were suspected, so a liver panel and infectious disease tests were requested, as well as diagnostic imaging studies.
Decreased total protein (5.37 mg/dL) and albumin (3.5 mg/dL) levels were reported in the liver panel; normal coagulation times were observed in the complete blood count test. Immunoglobulin G (IgG) and immunoglobulin M (IgM) antibodies were positive for CMV. In view of this, treatment with ganciclovir at a 12 mg/kg/day dose was started and further tests were performed. Viral load was positive (372 copies/mL), thus active CMV infection was confirmed. Since Alagille syndrome was also suspected, the following imaging tests were requested: abdominal, cervical, thoracic and lumbosacral spine X-rays, in which no abnormalities were evidenced. Likewise, no calcifications were observed in the cranial CT scan, but moderate right hearing loss was evidenced in auditory tests, so it was suspected that the CMV infection was probably congenital. Bearing this in mind, a liver biopsy was required for confirmation purposes.
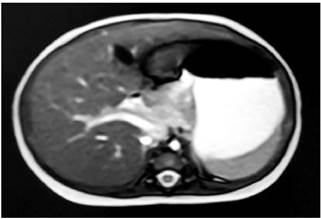
A 2.6 mm common bile duct was observed in an abdominal ultrasound; no other relevant findings were reported. A magnetic resonance cholangiopancreatography showed a 21 mm long and 3 mm diameter collapsed gallbladder, without dilatation of the intrahepatic duct; the extrahepatic duct could not be characterized due to a technical limitation (Figure 1). An intraoperative cholangiography was performed, in which biliary atresia was confirmed, and a Kasai biliodigestive shunt was performed when the patient was 95 days old.
The following intraoperative findings were evidenced: a cirrhotic and stone-like liver, with scarce bleeding on section, type III biliary atresia, without evidence of hepatic ducts or hilum dilatation, and absence of bile outflow after the section of the hepatic portal vein. Samples from the segment V were taken in order to perform a biopsy. A hepatic parenchyma with severe architectural distortion and bile ducts with mesenchyme and surrounding fibrosis showing giant cell neovascularization were described in the biopsy report. Proliferated bile ducts altered by fibrosis were seen in the portal spaces, which allowed confirming the diagnosis of biliary atresia associated with positive CMV IgM. Due to the absence of CMV deoxyribonucleic acid (DNA) samples or positive CMV IgM samples during the first 3 weeks of life, it was not possible to confirm whether the infection was neonatally acquired or congenital. After undergoing the procedure, jaundice significantly decreased and the patient was discharged. In subsequent follow-up assessments the need for liver transplant requirement was established.
Discussion
Biliary atresia is a disease in which inflammatory obliteration of the intrahepatic or extrahepatic ducts is produced1. Although its specific cause is unknown, it can be said that it is a multifactorial disease6. Genetic, inflammatory and toxic factors have been described6. 20% of all cases of biliary atresia are associated with other congenital malformations, in such cases it is known as biliary atresia with splenic malformation syndrome6. The factor responsible for causing this syndrome occurs during embryogenesis. On the contrary, in the remaining 80% of the cases, where there is only hepatic involvement, it is believed that the causal factor occurs during embryogenesis. Within this group, Lakshminarayana et al. established three different conditions with shared similarities. First, cystic biliary atresia, in which cystic changes associated with obliteration of the hepatic ducts occur; biliary atresia associated with positive CMV IgM results, the focus of this article, and finally, isolated biliary atresia, which does not share characteristics with the first two5.
CMV is a DNA virus that belongs to the Herpesviridae family. It causes a common unnoticed infection in infants or adults with a prevalence of 60%-90% worldwide. It can lead to a fatal outcome in neonates3. In the latter, its clinical manifestations vary from asymptomatic viremia to CMV syndrome or tissue-invasive CMV disease, which occurs when a specific organ is affected (pneumonitis, colitis, hepatitis)7. Liver involvement is common in congenital and perinatal infection cases8. Liver involvement may be mild and produce hepatomegaly or increased transaminases, or it can be moderate to severe (hepatitis, cholestatic liver disease and cirrhosis), although the latter are very rare8.
In particular, CMV-IgM positive results have been described in patients with biliary atresia. To prove a causal relationship between the virus and biliary atresia, it has been shown that, compared to patients with biliary atresia but negative CMV IgM results, viral DNA is detected in 60% of liver biopsies in patients with biliary atresia associated with positive CMV IgM results, which is associated with a greater number of histological characteristics typical of biliary atresia3,9,10. Therefore, CMV is currently considered a causative agent of biliary atresia.
It has been established that viral infection activates the immature immune system and triggers an autoimmune pattern secondary to tolerance breakdown or impaired immune regulation. It is characterized by Th1/Th2 cell differentiation imbalance and defects in the number and function of regulatory T cells leading to epithelial and ductal cell injury, fibrosis and liver cirrhosis3,4,11.
The timing in which the CMV infection that leads to biliary atresia is acquired is still under study, although it is believed to occur in the third trimester of pregnancy or during the neonatal period6. Zani et al. suggest the presence of prenatal infection if CMV DNA or positive CMV IgM are detected in the first 21 days of life. However, obtaining these laboratory results is not a usual finding10.
Biliary atresia associated with positive CMV IgM results differs from other types of biliary atresia: its clinical manifestations have a late onset. The patient is born apparently healthy, but after the second week of life obstructive cholestasis is developed, which in turn prolongs over time5. Given this clinical spectrum, other neonatal cholestasis etiologies must be ruled out12. The most important complementary tests for reaching a diagnosis are liver function tests, in which elevated liver enzymes and hyperbilirubinemia resulting from elevated direct bilirubin levels, are evidenced; viral serology with CMV IgM positivity; imaging findings confirming the presence of biliary atresia, and a liver biopsy5.
Regarding diagnostic imaging findings, a meta-analysis reported that ultrasound showed a sensitivity and a specificity of 74.9 % and 93.4 %, respectively, for the diagnosis of biliary atresia. This low sensitivity was attributed to the varying expertise of the operator13. Magnetic resonance cholangiopancreatography has a sensitivity of 87.7% and specificity of 64.7%13. Liver biopsy continues to be one of the most reliable methods to reach a biliary atresia diagnosis14. Specifically, expanded portal ducts, proliferation of the bile ducts, fibrosis and marked hepatic inflammation are evidenced in cases of biliary atresia associated with positive CMV IgM results1.
Treatment consists of Kasai biliodigestive shunt, a procedure that, when performed within the first 45 days of life, is associated with better prognosis and survival rates15. However, in patients with biliary atresia associated with positive CMV IgM results, its late clinical presentation and late diagnosis postpones surgical management, which, according to several studies, is performed on average when the patient is 70-75 days-old10,16, which decreases postoperative jaundice clearance and increases the probability of requiring liver transplant in the short term4.
Post-surgical treatment is performed using ursodeoxycholic acid, antibiotics and fat-soluble vitamins4. So far, several studies have assessed the efficacy of antivirals in the treatment of biliary atresia associated with CMV IgM positivity1,17. For example, Parolini et al. showed that treatment with ganciclovir or valganciclovir improved resolution of jaundice, increased the native liver survival rate and reduced the need for liver transplant in patients who underwent Kasai biliodigestive shunt1,17.
Conclusion
Biliary atresia associated with CMV IgM positivity has distinctive features that allow differentiating it from other causes of biliary atresia. Regarding its clinical manifestations, it has a late onset and it is diagnosed based on CMV IgM positivity and liver biopsy findings, where increased fibrosis and histologic features compatible with biliary atresia are evidenced. Treatment consists of performing a Kasai biliodigestive shunt and the postoperative administration of ursodeoxycholic acid, antibiotics and fat-soluble vitamins. At present, the effect of antivirals in the treatment of this disease is being evaluated.











 text in
text in