Introduction
Neuroendocrine tumors (NETs) are a heterogeneous group of neoplasms originating from neuroendocrine cells. Most NETs overexpress somatostatin receptors, mainly types 2 and 51. The European Neuroendocrine Tumor Society (ENETS) recommends, when feasible, curative surgery with removal of the primary tumor, regional lymph nodes and liver metastases2.
However, recurrent laparotomies lead to multiple adhesions and anatomical alterations, making it difficult for surgeons to differentiate scar or inflammatory tissue from malignant tissue. The successful use of radioguided surgery (RGS) in other surgical procedures, such as sentinel node, thyroid cancer and parathyroid adenoma detection, has led to propose the use of RGS in the management of NETs3.
Surgical intervention in patients with gastroenteropancreatic NETs can be challenging in several clinical settings. On the one hand, some patients may have small tumors that are difficult to localize during surgical exploration4. On the other, preoperative localization may be based only on functional imaging findings, without these tumors being localized in conventional imaging studies (computed tomography [CT], ultrasound, magnetic resonance imaging [MRI]). Localization of lesions can be difficult in sites such as the mesenteric root and the retroperitoneum5.
However, the introduction of preoperative hybrid imaging techniques (single photon-emission computed tomography/computed tomography [SPECT/CT] or positron emission tomography/computed tomography [PET/CT]) has further improved the accuracy of CRG techniques, leading to the resection of small primary tumors, residual tumors, loco or regional relapses and distant recurrences6.
Since this is an innovative technique, the clinical case of a patient with a NET treated at the Instituto Nacional de Cancerología (National Cancer Institute) in Bogota, Colombia, is described (Figure 1). Most of the CARE guidelines were followed in the description of this case report7.
Clinical case
The following is the case of a 59-year-old patient who experienced two digestive bleeding episodes, one in June 2015 and the other in February 2017. In both episodes, an ulcer in the third part of the duodenum was observed by means of an upper gastrointestinal endoscopy; however, performing a biopsy was not possible in both procedures. In February 2017, a 44 x 32 mm cystic mass without contrast enhancement and partially surrounding the neck of the pancreas, and a 26 x 15 mass with arterial phase mild enhancement in the lumen of the first part of the duodenum were identified in an abdominal CT scan performed in another health institution. In March 2017, the patient underwent a partial pancreatectomy, a partial duodenectomy, an antrectomy and a lymphadenectomy in another health institution. The following findings were informed in the pathology report: a 3 x 1.2 cm well differentiated grade 2 (World Health Organization [WHO], 2017), unifocal duodenal NET with involvement up to the muscularis propria, without lymphovascular or perineural invasion, with a 1 x 10 cap mitotic index; negative resection margins, immunohistochemistry (IHC): Ki-67 of 10 % and positive for synaptophysin and cytokeratin (CK) AE1/AE3; negative for chromogranin.
In September 2017, the patient presented with another digestive bleeding episode (melena), so a new upper GI endoscopy was performed in which patchy erythematous mucosa in the fundus and the body of the stomach, remnant of the gastric antrum and normal intestinal lumen were observed. In October 2017, the patient underwent a somatostatin receptor scintigraphy in another health institution and in which an uptake in the right paramedian mesogastrium, Krenning 3/4, positive for somatostatin receptor overexpression, and suggestive of tumor recurrence was reported. Therefore, a 68Ga-DOTANOC (3.5 mCi) PET/CT was performed (Figure 2) in April 2018, which allowed finding a 20 x 22 x 18 lymph node with somatostatin receptor overexpression in the mesentery, above the third part of the duodenum, and maximum standardized uptake value (SUVmax) of 57.6 (Krenning 4/4), compatible with tumor persistence. No other lesions with somatostatin receptor overexpression were observed, whether distant or in the surgical site.
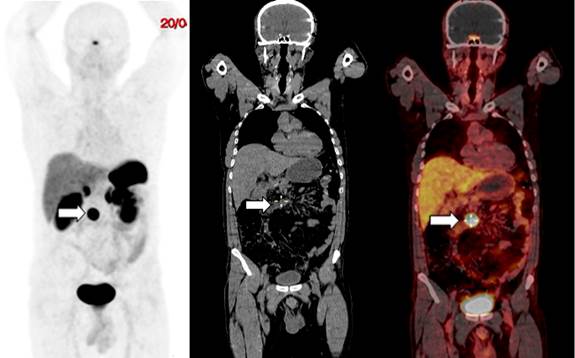
Figure 2 59-year-old patient with a duodenal NET. The 68Ga-DOTANOC PET/CT showed a 20 x 22 x 18 lymph node with somatostatin receptor overexpression located in the mesentery (arrows), above the third part of the duodenum with a SUVmax of 57.6 (Krenning 4/4). A. Maximal intensity image. B. Low-dose CT coronal view. C. PET/CT fusion image.
Since the CT scan of the abdomen performed in September 2017 did not show the node as it was not readily visible and given the uncertainty of the postoperative anatomy or the type of digestive reconstruction the patient had, a RGS was scheduled after discussing the case with the NETs multidisciplinary board.
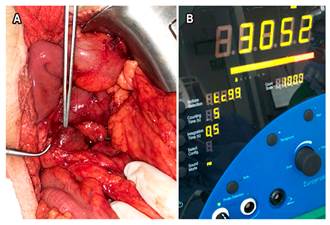
In August 2018, the patient underwent a laparotomy in which a mesenteric lymph node was resected. The patient was intravenously administered 15 mCi of metastable technetium-99-hydrazinonicotinyl-Tyr3-octreotide (99mTc-HYNIC-TOC) 5 hours before surgery (Figure 3) and the lesion was localized using a gamma probe. The following intraoperative findings were reported: a 2 cm mesenteric lymph node with an activity of 3052 counts, as recorded by the gamma probe. The activity in the tissue adjacent to the lesion was less than 700 counts (Figure 4). The pathology report confirmed the presence of a lymph node affected by a grade 2 (WHO, 2017) NET, mitotic count: 2 mitoses in 10 cap; cell proliferation index (Ki-67): 10%.
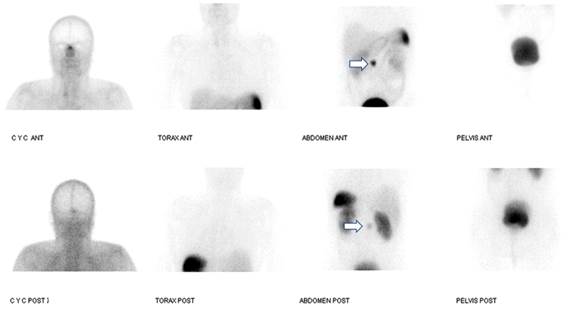
Figure 3 99mTc-HYNIC-TOC somatostatin receptor scintigraphy performed on the day of the surgery. Uptake in the mesogastrium (arrows), which corresponds to a lymph node with somatostatin receptor overexpression (Krenning 4/4) and that had already been identified in a 68Ga-DOTANOC PET/CT.
After the RGS, the patient continued to attend follow-up visits in the oncologic endocrinology and gastroenterology services of the institution. The patient was last assessed, at the time of writing this report, in January 2020, where no evidence of lesions suggesting tumor recurrence was observed in a MRI of the abdomen performed in the same month. Currently, the patient is asymptomatic.
Discussion
The incidence of gastroenteropancreatic NETs has increased to approximately 7.8 cases per 100 000 persons each year, while a prevalence of approximately 35 cases per 100 000 persons has been described8.
Surgical resection is the best curative treatment option for patients with early stage NETs. Complete removal of the tumor is an important prognostic factor in patients with gastroenteropancreatic NETs9, as it improves their quality of life and reduces the incidence of metastases. For this reason, achieving R0 or R1 resection has been associated with better survival outcomes3.
Likewise, determining both the extent of the tumor (localization and metastasis) and the location of the primary tumor is essential in the management of NETs. Certain locations, such as the small bowel, may be associated with multicentricity and special attention must be paid to ensure an adequate resection10. Intraoperative localization can be achieved using traditional surgical, radiological and endoscopic techniques, including palpation, endoscopic marking and intraoperative ultrasound11.
In addition, NETs can also be detected with RGS techniques using radiotracers and a gamma detection probe11. The radiopharmaceutical is administered preoperatively or intraoperatively, and the gamma probe is used to detect the primary tumor, lymph node involvement or metastatic disease12.
CRG has shown to be useful in the management of NETs in the detection of both occult and small tumors1. This technique allows optimizing the identification and complete surgical resection of all possible areas affected by the tumor13. In the context of tumor or lymph node relapse, cancer staging, using functional imaging with 68Ga-DOTA peptide PET/CT, must be performed in all patients prior to conducting RGS, as it assesses somatostatin receptor expression, the extent of the disease, and whether it is localized or it is a case of distant metastases. Patients with small tumors or with tumors located in difficult access sites are considered to be ideal candidates for RGS resection. In the case of our patient, in which the primary tumor had already been resected and in which no involvement by other lesion was evidenced on 99Tc-HYNIC-TOC scintigraphy and 68Ga-DOTA peptide PET/CT scintigraphy, the affected lymph node was resected, taking into account its location in the root of the mesentery, which makes it difficult to perform a lymphadenectomy. Lymphadenectomies of periduodenal lymph nodes are indicated during resection of the primary tumor.
RGS, together with PET/CT, are nuclear medicine procedures that had experienced a significant growth in the last 25 years. The term RGS comprises a set of pre-, intra- and postoperative techniques, whose main feature is the injection of a radiopharmaceutical associated with the intraoperative use of a portable radioactivity counting probe (known as gamma probe) that allows surgeons to identify and remove target tissues that accumulate radioactivity6. This gamma probe provides visual and audible feedback of the radioactivity count rate (range: 0-25 000 counts per second [cps]) as the tumor is approached by the surgeon9.
A tumor cps/background cps ratio of at least 1.5 is required to confirm the location of the lesion using an intraoperative gamma probe5. In our case, this ratio was 4.3, so the lymph node was successfully located.
Gamma probe scanning has identified 57% more NET lesions compared to manual scanning by the surgeon; likewise, it allows identifying lesions between 0.5 and 1 cm with high efficiency5. Therefore, gamma probes have a high degree of specificity and they can be used to help surgeons locate small tumor lesions that are difficult to detect with the naked eye9.
One of the largest studies, conducted in 44 patients (22 of gastrointestinal origin) and in which Gallium Ga 68-DOTATATE was used, found a sensitivity of 90 % and specificity of 25 % with a tumor/neighboring tissue ratio of 2.5, while with a ratio of 16, the sensitivity decreased to 54 %, but the specificity increased to 81 %4.
RGS with radiolabeled somatostatin analogues is available with different radiotracers such as 111In-pentetreotide, 99mTc-somatostatin analogues and 68Ga-somatostatin analogues13; the latter with a higher detection rate because it has higher emission energy. The detection rate is 94%3, which is higher compared to the other radiotracers. However, 68Ga-somatostatin analogues are not yet available in our country.
99mTc-HYNICTOC, a somatostatin analogue, was used in the case described here. This radiopharmaceutical was developed by Behé & Maecke14 in 2000; it has appropriate clinical characteristics such as high and specific affinity for somatostatin receptors, good biodistribution, renal excretion, low radiation exposure, availability and cost-effectiveness. Besides high quality images, this radiotracer provides the possibility of an earlier diagnosis (images at 10 minutes-4 hours)1. The use of this radiopharmaceutical is scarcely reported in the literature, with only 9 cases in which it was used in the small intestine1, so it is worth noting its usefulness.
RGS radiolabeled somatostatin receptor analogs allowed for the successful resection of a mesenteric adenomegaly in our patient. However, there are other factors associated with successful resection, including appropriate preoperative diagnosis to localize tumor sites, adequate exposure of the lesion during surgery, and the performance of the gamma probe in detecting the lesion13.
In cases where performing RGS is not possible due to is unavailability, surgeons use manual palpation or intraoperative ultrasound to identify small lesions; however, proper detection can be challenging even if skilled surgeons are in charge, due to the millimeter size of such lesions and the fact they are found in multiple locations in a single patient. Although many more studies are still required, further analysis is needed to shed light on the performance of RGS, whether it increases the number of resections or not, and how it affects the surgical procedure, as well as the survival of these patients15.
In the future, prospective studies conducted with larger cohorts will address the efficacy of RGS in minimizing symptoms, its impact on quality of life and overall survival, as well as its intra- or perioperative risk3. In the long term, follow-up and comparison with patients with similar characteristics and in which RGS was performed are required to determine if this therapeutic approach reduces the rate of persistent or recurrent disease in patients with NETs4. Given the low frequency of these NET presentations, conducting a randomized clinical trial is impractical.
In patients with these diseases, multidisciplinary discussion of the cases is important to look for the best diagnostic and therapeutic options; likewise, this type of treatments must be ensured in health centers with the expertise and resources necessary for their implementation, as it is the case of our institution. In the case described here, the interaction between different services was of great importance to achieve the complete resection of the lesion.
Conclusion
RGS is a feasible technique that allows locating NETs; besides it can detect more lesions and of smaller size compared to preoperative imaging tests and palpation by the surgeon. 99mTc-HYNICTOC is a useful radiopharmaceutical in the intraoperative localization of intestinal NETs that, despite being scarcely described in the literature, becomes a good alternative to identify the location of these tumors.











 text in
text in