Introduction
Crohn’s disease (CD) can affect any segment of the digestive tract. It is characterized by segmental and transmural involvement. The most common histopathological findings in patients with CD are the presence of cryptitis, lymphoid aggregates and granulomas1,2. Granuloma is considered the pathognomonic finding of CD microscopic diagnosis, but it can only be detected in 40%-60% of intestinal segments resected as surgical specimens and in 15%-36% of endoscopic biopsies in 15%-36%3.
Regarding its location, CD most commonly affects the terminal ileum (two thirds of patients), followed by the ileocolonic region (40-50 %), the isolated small intestine (30-40 %) and the colon (15-25 %)4. Involvement of the upper digestive tract is less frequent (0.4 %-16 % of cases)4; in addition, esophageal involvement occurs only in 0.2 % of patients, followed by stomach and duodenum involvement (between 1 % and 4 % of cases)4-7. Furthermore, extraintestinal manifestations such as primary sclerosing cholangitis (PSC) and pulmonary manifestations are very rare in patients with CD8-11.
The case of a patient with CD who first presented with pulmonary manifestations and years later experienced varied manifestations affecting the whole digestive tract, as well as the biliary tract is presented here.
Clinical case
This is the case of a 43-year-old patient with a history of recurrent aphthous ulcers in the oral cavity, general malaise, fever episodes during the night, dry cough, and involuntary and significant weight loss. On physical examination the patient was cachectic, he had fever, and abnormal breath sounds. For this reason, multiple studies were performed. A CT scan of the chest showed a 6 mm scar area with cavitation in the left upper lobe, peribronchial thickening towards the left lower lobe, a small nodular lesion in the right base and large perihilar adenopathies. Furthermore, severe nodular endobronchitis was identified by means of a fibrobronchoscopy, with biopsies showing chronic active inflammation without granulomas; Ziech-Nielsen (ZN) and methenamine silver stains were negative; bronchoalveolar lavage (BAL) cultures for bacteria, fungi and mycobacteria were negative; acid-fast bacillus test, potassium hydroxide (KOH) and polymerase chain reaction (PCR) for tuberculosis (TB) were negative. In addition, antinuclear antibodies, perinuclear staining anti- neutrophil cytoplasmic antibodies (p-ANCA) and cytoplasmic staining anti-neutrophil antibodies (c-ANCA) tests were negative; enzyme-linked immunosorbent assay (ELISA) and human immunodeficiency virus (HIV) tests were also negative. Given these negative findings, a thoracoscopic segmental lobectomy was performed, with whitish lumpy areas reminiscent of caseating granulomas being described in the macroscopic findings. According to the pathology report, none of these specimens was positive for granulomas or granulomatous infections, but acute fibrinous and organizing pneumonia with microabscesses was reported. Parenteral antibiotic treatment was started and the patient was discharged with both a diagnostic impression of granulomatosis with polyangiitis and the following indications: use of prednisolone 50 mg/day and attend outpatient evaluation in the rheumatology service.
Granulomatosis with polyangiitis diagnosis was ruled out in the outpatient visit in the rheumatology service, given that there was no vasculitis, the ANCA test was negative, and histologic findings were not compatible with this disease. The patient reported experiencing occasional headaches, so a magnetic resonance imaging (MRI) of the brain was performed, which allowed observing a small aneurysm and, based on these data, a diagnostic impression of Behçet’s disease was made, so he was prescribed with methotrexate 10 mg/week and prednisolone 50 mg/day treatment was continued.
General and respiratory symptoms improved completely and the patient was asymptomatic for more than 1 year, when he began experiencing polydipsia, polyphagia and polyuria. During that year the patient did not attend any follow-up visit in the rheumatology service. Diabetes mellitus was diagnosed with glycosylated hemoglobin (HbA1c): 8.5%, so management with basal insulin and steroids tapering was indicated. Due to the notable improvement of the patient’s clinical condition, the administration of oral steroids was gradually suspended. When they were suspended, the patient presented with diarrhea and colicky abdominal pain, so different treatment regimens consisting of metronidazole, oral antibiotics and antidiarrheals were prescribed, which allowed for the occasional control of these symptoms. Over time, the number of bowel movements frequency increased to 25-30, and sometimes there was blood or mucus in the stools, or straining and tenesmus was experienced; besides, the patient experienced a 20 kg weight loss in 6 months. Given these clinical signs and symptoms, the patient was hospitalized. The following findings were reported on the physical examination on admission to the hospital: A pale and underweight patient (weight 41 kg, vs. a usual weight of 61 kg), with tachycardia (heart rate [HR]: 122 beats per minute [bpm]) with diffuse pain on abdominal palpation, without peritoneal irritation or visceromegaly. The results of laboratory tests on admission are shown in Table 1.
Table 1 Laboratory tests on hospitalization admission.
| Tests | Results | Reference values |
|---|---|---|
| Hemoglobin | 8.1 g/dL | 13-17 g/dL |
| Hematocrit | 25.6% | 42%-52% |
| Mean Corpuscular Volume | 74.7 fL | 78-98 fL |
| Leukocytes | 24500 mm3 | 4500-11 000 mm3 |
| Neutrophils | 21900 mm3, 89.4 % | 1500-6000 mm3, 50 %-70 |
| ESR | 90 | Up to 20 |
| CRP | 21.79 mg/dL | 0.01-0.82 mg/dL |
| Creatinine | 0.61 mg/dL | 0.60-1.10 mg/dL |
| ALP | 202 U/L | 40-150 U/L |
| GGT | 165 U/L | 12-64 U/L |
| ALT and AST | 6 and 10 U/L | 5-34 U/L |
| Glycemia | 130 mg/dL | Up to 100 mg/dL |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; ALP: alkaline phosphatase; GGT: Gamma-glutamyl transferase; ESR: erythrocyte sedimentation rate.
Endoscopic studies were performed at our institution which showed lesions reminiscent of punch pouch-like pseudodiverticula in the distal esophagus (Figure 1A); cobblestone-like gastric mucosa of granular appearance, mainly in the distal body and the antrum; an inflammatory ulcer in the pyloric region (Figure 1B), and pseudodiverticular formations of chronic inflammatory appearance in the duodenum (Figure 1C). Furthermore, a colonoscopy showed severe mucosal inflammation of the entire colon, with inflammatory stricture at the ileocecal valve and multiple inflammatory ulcers in the distal ileum (Figure 1D-F). In addition, a magnetic resonance enterography (MR enterography) showed extensive involvement of the entire small bowel by diffuse, concentric, asymmetric and irregular thickening from the stomach until the rectum (Figure 2A). Due to the elevated ALP and GGT levels, a magnetic resonance cholangiopancreatography was performed, which allowed detecting smooth and short stenotic segments alternating with larger caliber segments in the right and left intrahepatic bile duct associated with diffuse enhancement of the walls due to a nonspecific inflammatory phenomenon (Figure 2B). Colonic biopsies reported the presence of ulcerated mucosa with crypt distortion, decreased goblet cells, lymphoplasmacytic infiltrate in the lamina propria, polymorphonuclear exocytosis and microabscess formation, with negative results for ZN staining, modified ZN staining, PAS (periodic Acid-Schiff) staining and cytomegalovirus (CMV).
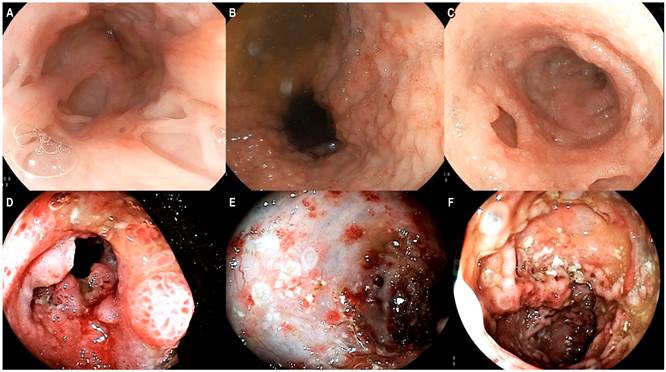
Figure 1 A. Endoscopic findings in the distal esophagus, punch pouch like pseudodiverticula. B. Endoscopic view of the antrum: cobblestone-like nodular mucosa. C. There is evidence of punch-like Kerckring’s notches in the duodenum. D. Severe involvement of the ileocecal valve. E and F. Inflammatory changes with multiple ulcers of variable size in the colon.
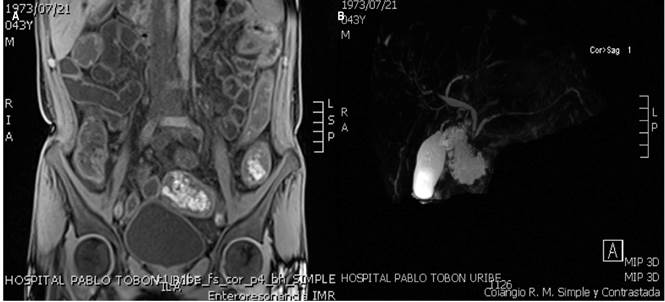
Figure 2 A. MR enterography: inflammatory changes caused by diffuse thickening throughout the digestive tract. B. Magnetic resonance cholangiopancreatography showing short, smooth strictures with enhancement of the intrahepatic biliary tract; findings compatible with PSC.
The clinical and radiological findings, as well as the biopsy reports were compatible with Crohn’s disease with extensive involvement of the digestive tract, associated with PSC. A Crohn’s Disease Activity Index (CDAI) of 623 was obtained, so treatment consisting of intravenous hydrocortisone 100 mg every 8 hours, oral mesalazine 1 gram every 8 hours and azathioprine 2 mg/kg/day was started. Infectious disease screening studies were negative, so inpatient biological therapy (infliximab) was prescribed at 10 mg/kg/dose in a shortened induction schedule (weeks 0, 1 and 4). The patient’s condition started to improve from day 3 after starting this treatment, experiencing a progressive decrease in fecal output and acute phase reactants. At 1 week, intravenous steroids were replaced by oral steroids and the patient was instructed to keep using prednisolone 40 mg/day for 1 month and to gradually suspend the use of steroids. After nearly 2 weeks, the patient reported having experienced almost complete resolution of abdominal pain, improvement in stool consistency, and absence of gastrointestinal bleeding.
Four months later, in a follow-up visit in the Inflammatory Bowel Disease Clinic, the patient reported having experienced a marked improvement of symptoms: he referred having three bowel movements per day, Bristol 4 consistency, and occasional non-incapacitating colicky abdominal pain that improved with antispasmodics, without rectal bleeding. Weight gain (52 kg), absence of abdominal pain or visceromegaly, and a CDAI score of 213 were reported on physical examination. Combination therapy (azathioprine 100 mg/day plus infliximab 5 mg/kg/every 2 months) was prescribed. Due to administrative issues with the patient’s health insurance company, no further controls were possible.
Discussion
The case of a young patient with CD who presented with respiratory manifestations long before gastrointestinal manifestations took place is reported here. In this case, multiple studies were performed, which allowed ruling out infectious diseases such as TB and deep mycosis; chronic granulomatous diseases such as sarcoidosis and granulomatosis with polyangiitis were also ruled out due to negative ANCA test results, and negative histopathology even for the surgical specimen obtained in the segmental lobectomy. Additionally, the patient had a good clinical response after undergoing a treatment consisting of steroids and immunomodulators for a long period.
IBD-related pulmonary involvement was first described in 1976 by Kraft et al12. Pulmonary involvement by IBD is the rarest extraintestinal manifestation, but its actual prevalence is probably unknown8-10. Pulmonary manifestations are variable and at least 4 forms of presentation have been described: small airway disease, parenchymal involvement, vascular involvement and, rarely, serosa13. There are several hypotheses about the lung-intestine connection or axis. The lungs and intestines originate from the same embryonic cell line in the anterior region of the endoderm. These organs have in common the columnar lining cells, the goblet cells and the submucosal lymphoid tissue, so it has been proposed that the lung-intestine axis may develop similar inflammatory reactions due to these shared anatomic-histologic features13,14. A second hypothesis is the shared antigen theory, which states that the epithelia of the intestine and the lungs are exposed to the same antigens, and said shared exposure may induce a similar inflammatory response in both systems. Other theories adduce that, during the activation of the cellular immune response, lymphocytes have a chemokine and chemokine receptor-based homing system that allows their proper migration to the appropriate tissue. In IBD patients, this migration system may be abnormal and less specific, so that lymphocytes can invade the intestine and other organs such as the lungs13.
The difficulty in diagnosing pulmonary manifestations of CD is likely due to the fact that pulmonary manifestations may occur before the onset of intestinal disease, and therefore the physician does not make an association between both situations (as it happened in this case), or that subclinical findings are present and the patient does not experience any symptom, and, thus, such association is not investigated by the physician9.
Sarcoidosis and granulomatosis with polyangiitis are the conditions that represent the greatest diagnostic challenge of pulmonary involvement in CD10. Even some cases of CD/sarcoidosis overlap have been reported. On the contrary, although granulomatosis with polyangiitis has pulmonary findings similar to those described in CD, gastrointestinal involvement is very rare10.
PSC is a chronic inflammatory disease of the bile ducts that causes stenosis and recurrent cholangitis. It has a high correlation with IBD (especially ulcerative colitis [UC]), in which it is estimated that 50-80% of patients have concomitant IBD11. Patients with PSC have a 2-fold increased risk of cancer and a 40-fold increased risk of primary hepatobiliary cancer15. Provided that a large number of patients are asymptomatic, biliary manifestations associated with CD are underestimated. Individuals with concomitant IBD and PSC have a worse prognosis, in particular for they have an increased risk of colorectal cancer. The exact mechanisms are unclear, but there is clear evidence that the risk of colorectal cancer is higher in patients with IBD and PSC than in those with IBD or PSC alone15,16.
As for the pharmacological treatment of patients with PSC with or without IBD, treatment options do not differ much at present. Anti-TNF agents have failed to control inflammatory activity in the biliary tract, regardless of their efficacy in treating inflammation in the intestine. Currently, the use of vedolizumab, a humanized monoclonal antibody selectively targeting the a4b7 integrin, has been proposed to be included in the treatment of these patients. The safety profile of videlizumab is higher than that of the existing anti-TNF-α agents17. The theoretical foundation of this proposal is that it has been successfully shown that intestinal adhesion molecules such as chemokine ligand 25 (CCL25) and mucosal adresin cell adhesion molecule 1 (MAdCAM-1) are abnormally expressed in the liver of patients with PSC, favoring the recruitment of gut-derived effector T cells expressing chemokine receptor 9 (CCR9) and a4b7. Although this mechanism sounds appealing, so far the clinical impact of vedolizumab on PSC has not been determined. A recent multicenter study conducted in 102 patients with PSC and IBD found that there were no significant differences in biochemical response to vedolizumab, although alkaline phosphatase level decreased by 20% or more in a subset of patients18. Vedolizumab appears to be well tolerated and the overall IBD response was the same as the one expected in patients without PSC18.
Although it has been described that the entire digestive tract can be affected by CD, upper digestive tract involvement is less frequent1; however, some studies have reported data suggesting that the prognosis of the disease is usually worse when there is upper digestive tract involvement5,19,20. The first and only prospective study known to date conducted in patients with CD that has actively searched for findings in the upper digestive tract was carried out in Italy. In said study, all patients underwent endoscopy in which biopsy samples were obtained for histopathological analysis and the detection of Helicobacter pylori at weeks 0 and 12 after receiving a treatment regimen. In this series, 119 patients with CD were recruited, and gastroduodenal involvement attributable to CD was found in 19 (16 %). Of these 19 patients, 11 were treated with anti-TNF drugs (10 with infliximab and 1 with adalimumab), and 8, with proton pump inhibitors (PPI) and other therapies (5-ASA, immunomodulators and, in one of them, steroids). According to these authors, mucosal healing was observed in 8 of the 11 patients (72.7 %) in the anti-TNF biological therapy group compared to 1 of 8 patients (12.5 %) in the PPIs and other therapies groups, with a statistically significant difference (p < 0.001). In addition, this study suggests that an EGD with biopsy should be performed in patients with CD in order to correctly determine the distribution of the disease7.
Although CD is considered a disease that can affect any part of the digestive tract, extensive gastrointestinal and multiorgan involvement are very rare. Thus, there is no information in the existing literature on how the long-term follow-up and treatment of these patients should be. However, there are recent data regarding the follow-up or colon cancer screening in patients with PSC and IBD, in which performing a colonoscopy plus ileoscopy every year is recommended15.











 text in
text in 



